Abstract
Nuclear factor kappa B (NF‐κB) signaling pathway is activated in many colorectal cancer (CRC) cells and in the tumor microenvironment, which plays a critical role in cancer initiation, development, and response to therapies. In the present study, we found that the widely used antimalarial drug mefloquine was a NF‐κB inhibitor that blocked the activation of IκBα kinase, leading to reduction of IκBα degradation, decrease of p65 phosphorylation, and suppressed expression of NF‐κB target genes in CRC cells. We also found that mefloquine induced growth arrest and apoptosis of CRC cells harboring phosphorylated p65 in culture and in mice. Furthermore, expression of constitutive active IKKβ kinase significantly attenuated the cytotoxic effect of the compound. These results showed that mefloquine could exert antitumor action through inhibiting the NF‐κB signaling pathway, and indicated that the antimalarial drug might be repurposed for anti‐CRC therapy in the clinic as a single agent or in combination with other anticancer drugs.
Keywords: colorectal cancer, drug repurpose, mefloquine, NF‐κB, therapy
1. INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies with more than 1.4 million new cases each year worldwide.1, 2 Although there is a marked downward trend in developed countries, the incidence rate of CRC has been increasing significantly in many developing regions, largely attributed to population aging, dietary change, and environment pollution.3 Additionally, the 5‐year survival rate of those diagnosed with the cancer at advanced stages is <30%, and ~700 000 patients die from CRC annually, posing a major challenge for the health‐care system.4, 5 Despite significant progress in drug development technologies, there are only a few new small molecular drugs and biologics that have markedly benefited patients with CRC.6 Intriguingly, breakthrough immunotherapies, including checkpoint blocking and adaptive T‐cell transfer, are not effective in the majority of CRC patients.7 Thus, it appears necessary to search for new agents on as many fronts as possible. One of the possible routes is to screen the FDA‐approved drug library for targeting essential pathways in CRC.8
Transcription factor nuclear factor kappa B (NF‐κB) is composed of the dimerized members of the Rel family proteins RelA(p65), RelB, p50/NFκB1 (p105), p52/NFκB2 (p100), and c‐Rel, among which the p65/p50 complex is the most common active heterodimer.9, 10 They normally stay inactive as a result of binding with IκB in the cytoplasm. Inflammatory and immunological stimuli are able to activate IκB kinase (IKK), which, in turn, phosphorylates IκBα and promotes its ubiquitination and proteasomal degradation, resulting in nuclear translocation of NF‐κB. Once in the nucleus, p65 often undergoes a series of post‐translational modifications including phosphorylation, acetylation, and methylation, which regulate both the strength and duration of the transactivation activity. The target genes of NF‐κB include cell survive‐related Bcl‐2 and XIAP, cell proliferation‐related Cyclin D1, CDK2 and MYC, and pro‐inflammatory cytokines. Not unexpectedly, the NF‐κB pathway is aberrantly activated in many tumors and is closely associated with their development and progress. In CRC, NF‐κB is activated in cancer cells as well as in the surrounding microenvironment to sustain inflammation and promote proliferation, angiogenesis, invasion and eventually metastasis. Retrospective analysis indicated that activated NF‐κB is associated with tumor metastasis and poor prognosis.11, 12 Thus, targeting the NF‐κB pathway has been an actively sought‐after strategy for novel anti‐CRC drug discovery, and a number of specific inhibitors have been developed for further clinical studies.13
Through screening a library of FDA‐approved drugs with a NF‐κB response element‐driven luciferase reporter, we found that one of the effective inhibitors of NF‐κB‐dependent transcription is mefloquine, an effective drug used to prevent and treat malaria.14 Our studies showed that mefloquine suppressed NF‐κB activity by inhibiting IKK activation, and induced apoptosis in CRC cells both in vitro and in vivo, which could be attenuated by expressing constitutively active IKK in the cells. These results indicated that mefloquine could potentially be repurposed as a chemotherapeutic for the treatment of CRC.
2. MATERIALS AND METHODS
2.1. Cells, culture and chemicals
Colorectal cancer cell lines HT‐29, HCT116, RKO, SW620 and Lovo were purchased from ATCC (Manassas, VA, USA). HEK293T cell line was kindly provided by Dr Huashun Li from Tongji University, Shanghai, China. All CRC cell lines and HEK293T were maintained in DMEM supplemented with 10% FBS, 100 μg/mL penicillin, and 100 units/mL streptomycin. The 104‐compound library and IKK‐16 were purchased from Selleck Chemicals (Houston, TX, USA). Doxorubicin (DOX), mefloquine (Mef), and tumor necrosis factor alpha (TNF‐α) were purchased from Sigma‐Aldrich (St Louis, MO, USA).
2.2. Plasmid construction and gene transfection
Human constitutively activated IKKβ (CA‐IKKβ) gene was generated and cloned into pcDNA3.1 vector with a Flag tag as previously described.15 A NF‐κB luciferase construct (pNF‐κB‐Luc) driven by specific NF‐κB response elements was purchased from Beyotime Biotechnology Institute (Nantong, China). siRNAs against IKKβ (siIKKβ) and the negative control (siNC) were purchased from Guangzhou Ribobio Co., Ltd (Guangzhou, China). Target sequences of siIKKβ#1 and siIKKβ#2 were 5′‐GGAACAGTGAAGTTCTCAA‐3′ and 5′‐GGAGAAGCCAAGAGACCAA‐3′, respectively.
Plasmids or siRNAs were transiently transfected into HEK293T or HCT116 cells by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions.
2.3. Luciferase assay
HEK293T cells were transfected with pNFκB‐Luc or empty vector along with the internal control Renilla luciferase by Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Twenty‐four hours later, cells were incubated with indicated drugs for 12 hours, and then cell lysates were prepared for luciferase assay using Dual‐Luciferase® Reporter Assay System (Promega, Madison, WI, USA) as described previously.16, 17
2.4. Immunoblotting
Whole cell lysates were prepared for immunoblotting as described previously.17, 18, 19 Equal amounts of total proteins (30 μg) were subjected to SDS‐PAGE separation, followed by immunoblotting analysis with specific antibodies. Primary antibodies against p‐p65 (Ser536), p65, p‐IκBα (Ser32), IκBα, p‐IKKα/β (Ser176/180), IKKα, IKKβ, PARP, Cleaved‐Caspase 3, Bcl‐2, MCL1, XIAP and Bim were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti‐GAPDH was purchased from Abgent (Suzhou, China). Anti‐mouse IgG and anti‐rabbit IgG HRP conjugated antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
2.5. Quantitative real‐time polymerase chain reaction (qRT‐PCR)
Total RNA was extracted using RNAiso Plus (Takara Bio Group, Otsu, Japan) according to the manufacturer's instructions. cDNA was synthesized from equal quantities of total RNA using the PrimeScriptTM RT reagent Kit with gDNA Eraser (Takara Bio Group). To determine mRNA levels of Cyclin D1, Bcl‐2 and XIAP, qRT‐PCR was carried out using SYBR Green qPCR Master Mix (Clontech Laboratories, Inc., Mountain View, CA, USA) with Roche LightCycler® 480II real‐time PCR system (Roche, Basel, Switzerland). The primers used were as follows: Cyclin D1, forward 5′‐AGCTGTGCATCTACACCGAC‐3′ and reverse 5′‐GAAATCGTGCGGGGTCATTG‐3′; Bcl‐2, forward 5′‐CTGGGAGAACAGGG TACGATAA‐3′ and reverse 5′‐GGCTGGGAGGAGAAGATGC‐3′; XIAP, forward 5′‐TGGCAGATTATGAAGCAC‐3′ and reverse 5′‐CTCCTCCACA GTGAAAGC‐3′; GAPDH, forward 5′‐GCACCGTCAAGGCTGAGAAC‐3′ and reverse 5′‐TGGTGAAGACGCCAGTGGA‐3′.
2.6. Cell growth and viability
Colorectal cancer cells were plated at a start density of 5000 cells per well into 96‐well plates 1 day before Mef or DOX treatment. Cells were incubated for 24 hour with Mef or DOX at indicated concentrations. The viable cells were evaluated by Cell Counting Kit‐8 (CCK‐8) staining according to the manufacturer's instructions (Biotool, Houston, TX, USA).
2.7. Kinase activity in cell‐free assay
Kinase activity in the presence of Mef or IKK‐16 was measured using a Kinase‐Glo Luminescent Kinase Assay (Promega) as described previously.20
2.8. Xenograft studies
Human CRC cells HCT116 were injected s.c. into the right flanks of female nude mice (5‐6 weeks old; Shanghai SLAC Laboratory Animal Co. Ltd, Shanghai, China) with a density of 5 million cells/site per mouse. When tumors were palpable, mice were randomly divided into 2 groups. One group orally received Mef (30 mg/kg) in PBS containing 10% Tween 80 and 10% DMSO, and the other group was given vehicle for 20 days. At the same time, tumor sizes were measured every other day. At the end of the experiment, tumors were excised for immunoblotting. This animal study was approved by the Review Board of Animal Care and Use of Suzhou Institute of Systems Medicine.
2.9. Immunofluorescence staining
HCT116 cells were cultured on sterile glass coverslips in 6‐well plates to 80% confluence in DMEM. HCT116 cells were then starved overnight, followed by incubation with mefloquine or DMSO for 2 hours. Cells were then treated with 50 ng/mL TNF‐α or DMSO for 20 minutes. Finally, the cells were subjected to confocal microscopic analysis. For confocal microscopic analysis, cells were fixed in 4% paraformaldehyde at room temperature for 10 minutes and permeabilized in 0.5% Triton‐100 for 5 minutes. Cells were then blocked in TBS containing 3% BSA for 1 hour at room temperature before being stained with anti‐p‐p65 overnight at 4°C. Cells were then gently washed and incubated with FITC‐conjugated goat anti‐rabbit IgG (Beyotime Biotechnology Ltd). After 3 brief washes with PBS, slides were stained with 5 μg/mL DAPI for 10 minutes. After 3 more washes with PBS, the slides were mounted with Slowfade gold anti‐fade reagent (Invitrogen). Protein expression was visualized on Leica confocal microscopy.
2.10. Statistical analysis
Student's t test was used for comparisons of the 2 groups. All statistical tests were 2‐sided and P‐value < .05 was considered statistically significant.
3. RESULTS
3.1. Mefloquine inhibits NF‐κB in colorectal cancer cells
To find novel inhibitors for the NF‐κB signaling pathway, a luciferase reporter system driven by the NF‐κB response elements was used to screen a library of 104 FDA‐approved anti‐infectious drugs. As shown in Figure 1A and Table S1, a number of compounds from the library decreased luciferase activity significantly in HEK293T cells transfected with the reporter. Among them, the antimalarial drug mefloquine reduced luciferase activity more than 2‐fold and also decreased cell viability, so was selected for further assessment (Figure 1A,B; Figure S1). As shown in Figure 1C, mefloquine inhibited both spontaneous and TNF‐α‐induced NF‐κB‐driven luciferase activities dose‐dependently, and was therefore an effective NF‐κB inhibitor.
Figure 1.
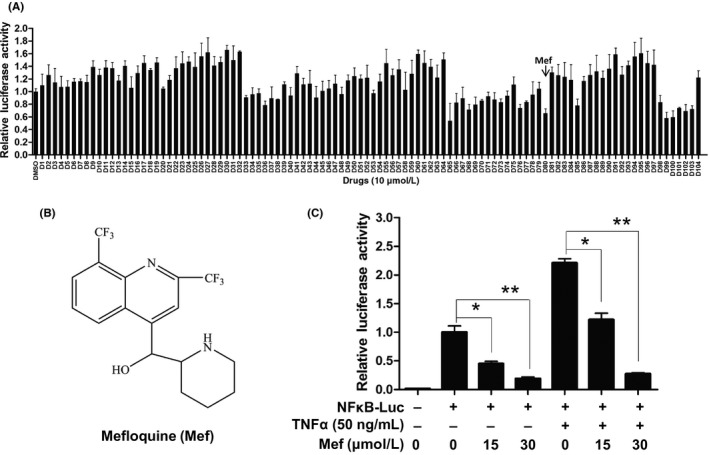
Antimalarial drug mefloquine inhibited nuclear factor kappa B (NF‐κB)‐driven transactivation. A, HEK293T cells were transfected with luciferase reporter driven by NF‐κB response elements for 24 h, and treated with 104 drugs or DMSO for 12 h. Luciferase activity was determined using the Dual‐Luciferase Reporter Assay System. B, Chemical structure of mefloquine (Mef). C, HEK293T cells were transfected with vector or NFκB‐luc by Lipofectamine 2000 (Invitrogen) for 24 h. Cells were then treated with 0, 15, or 30 μmol/L mefloquine for 12 h. Luciferase activity was measured followed by stimulation with tumor necrosis factor alpha (TNF‐α) or control vehicle for 20 min. *P < .05, **P < .01
We then examined the effects of mefloquine on the growth of the 5 lines of CRC cells by using the CCK‐8 assay. Although all the cells underwent a concentration‐dependent growth inhibition, HCT116, RKO, and Lovo cells were significantly more sensitive to the compound when compared with HT‐29 and SW620 cells (Figure 2A). Immunoblotting showed that NF‐κB p65 and IκBα were expressed in all the cells (Figure 2B). However, only HCT116, RKO, and Lovo cells contained high levels of phosphorylated p65 (p‐p65) and the IκB kinase components IKKα, IKKβ, IKKγ, (Figure 2B), indicative of NF‐κB activation in these cells. After treatment with mefloquine, phosphorylated p65 in HCT116 and RKO cells was decreased dose‐dependently (Figure 2C). Noteworthily, TNF‐α increased phosphorylated p65 in these cells, which was also abolished by pretreatment with mefloquine (Figure 2D; Figure S2). Furthermore, the expression of NF‐κB target genes Cyclin D1, Bcl‐2 and XIAP was assessed by RT‐qPCR. As shown in Figure 2E, their levels were downregulated significantly by mefloquine. These results indicated that mefloquine is a NF‐κB inhibitor in CRC cells.
Figure 2.
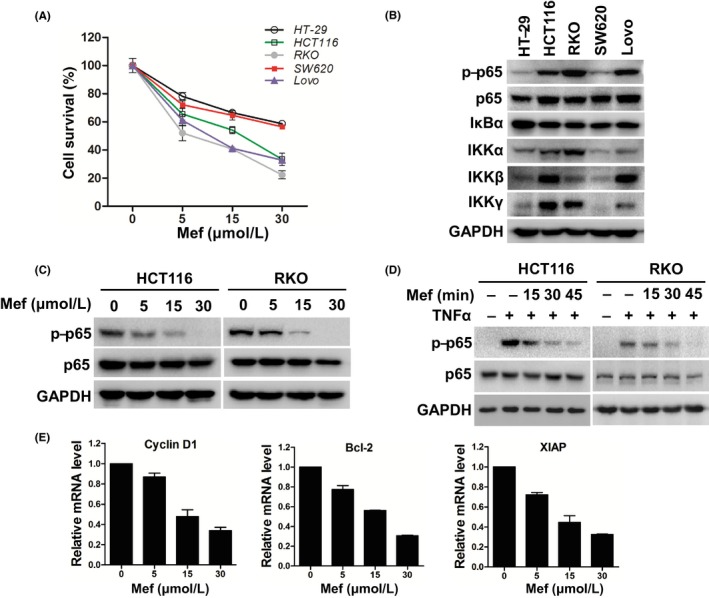
Mefloquine (Mef) suppresses nuclear factor kappa B (NF‐κB) activity in colorectal cancer cells. A, CRC cells HT‐29, HCT116, RKO, SW620 and Lovo were treated with the indicated concentrations of mefloquine for 24 h. Their viability was then measured by CCK‐8 assay. B, Immunoblotting analysis of NF‐κB signaling components p‐p65, p65, IKBα, IKKα, IKKβ, and IKKγ in the 5 lines of CRC cells. GAPDH was used as a loading control. C, After exposure to increased concentrations of mefloquine overnight, HCT116 and RKO cells were lysed and analyzed by immunoblotting against p‐p65 and p65. GAPDH was used as a loading control. D, Following starvation overnight in serum‐free medium, HCT116 and RKO cells were incubated with 30 μmol/L mefloquine for the indicated times, then stimulated with tumor necrosis factor alpha (TNF‐α; 50 ng/mL) for 20 min. The cells were lysed and analyzed by immunoblotting of p‐p65, p65 and GAPDH. E, mRNA levels of Cyclin D1, Bcl‐2 and XIAP were measured by qRT‐PCR in RKO cells treated with 0, 5, 15 or 30 μmol/L mefloquine overnight. IKK, IκB kinase
3.2. Mefloquine inhibits IKK activation
To understand the mechanisms of inhibiting p65 phosphorylation and NF‐κB transactivation by mefloquine, we examined the statuses of upstream regulatory molecules IκBα, IKKα and IKKβ in RKO and HCT116 cells.21 As shown in Figure 3A, exposure to mefloquine for 15 minutes resulted in a significant decrease of phosphorylated IκBα, which is required for its ubiquitination and proteasomal degradation22 and, by 30 minutes, the decrease reached a plateau. Mefloquine‐induced reduction of p‐IκBα was also dose‐dependent and accompanied by an increase of total IκBα (Figure 3B). Interestingly, although the levels of total IKKα and IKKβ were not affected, phosphorylated IKK was mostly diminished upon mefloquine treatment (Figure 3B). We also assessed IKK activity from HCT 116 cells by anti‐IKKβ antibody immunoprecipitation and in vitro kinase assay. As shown in Figure 3C, although 60 nmol/L IKK inhibitor IKK‐16 decreased kinase activity by ~50%, 30 μmol/L mefloquine did not markedly reduce IKK activity. Taken together, these data indicated that the drug acted by blocking IKK activation.
Figure 3.
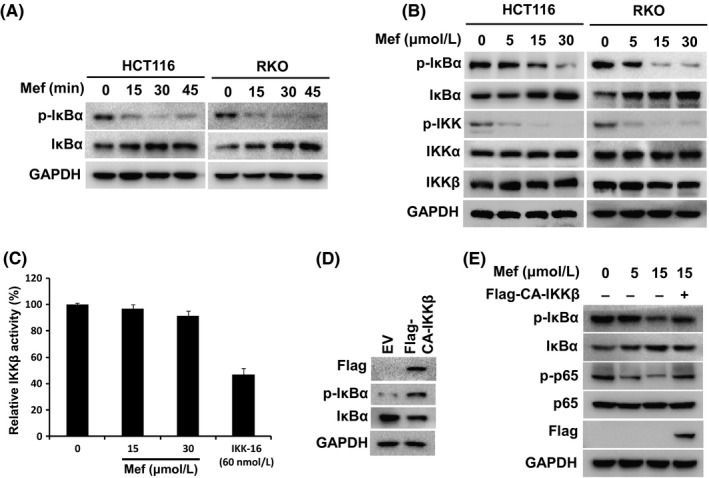
Mefloquine (Mef) inhibits IKK activation. A, After treatment with 30 μmol/L mefloquine for different times, HCT116 and RKO cells were analyzed by immunoblotting of p‐IκBα, IκBα and GAPDH. B, HCT116 and RKO cells were treated with the indicated amounts of mefloquine overnight and then analyzed by immunoblotting of p‐IκBα, IκBα, p‐IKK, IKKα and IKKβ. GAPDH was used as a loading control. C, IKKβ activity analyses in a cell‐free system. Increasing concentrations of Mef or 60 nmol/L IKK‐16 were incubated with the recombinant IKKβ. Kinase activity of IKKβ was then determined. D, Empty vector (EV) or Flag‐CA‐IKKβ plasmids were transfected into HCT116 cells, which were lysed for immunoblotting against Flag, p‐IκBα, IκBα and GAPDH. E, After transfection with EV or Flag‐CA‐IKKβ plasmids for 24 h, HCT116 cells were treated with the indicated concentrations of mefloquine overnight and then analyzed by immunoblotting against p‐IκBα, IκBα, p‐p65, p65, Flag and GAPDH. IKK, IκB kinase
To further assess the role of IKK inhibition, a constitutively activated IKKβ (CA‐IKKβ) was generated and transfected into HCT116 cells.15 As shown in Figure 3D, transfection of CA‐IKKβ led to increase of phosphorylated IκBα and decrease of total IκBα. Moreover, mefloquine‐induced decrease of p‐IκBα and p‐p65 was markedly reversed in CA‐IKKβ‐expressing cells (Figure 3E). These results indicated that blocking IKK activation is critical for the inhibitory action of mefloquine on NF‐κB signaling.
3.3. Mefloquine induces apoptosis in CRC cells
The inhibitory action of mefloquine on IKK and NF‐κB activation prompted us to further examine its effects on the growth and survival of CRC cells. As shown in Figure 4A, exposing HCT116, RKO and Lovo cells to mefloquine for 12 hours led to a significant increase of annexin V+ population in all 3 lines of cells. We then assessed the statuses of caspase‐3 and caspase substrate PARP in these cells by immunoblotting. Mefloquine induced activation of caspase‐3 and cleavage of PARP dose‐dependently (Figure 4B). Immunoblotting also showed that the apoptosis‐inhibiting proteins Bcl‐2, MCl‐1, and XIAP were all downregulated, whereas the pro‐apoptotic BH3‐only protein Bim was elevated in mefloquine‐treated cells (Figure 4C). These data showed that mefloquine induced apoptosis of these CRC cells. Furthermore, when HCT116 cells were transfected with CA‐IKKβ, mefloquine‐induced cell death was significantly attenuated, suggesting the involvement of IKK inhibition in the process (Figure 4D,E).
Figure 4.
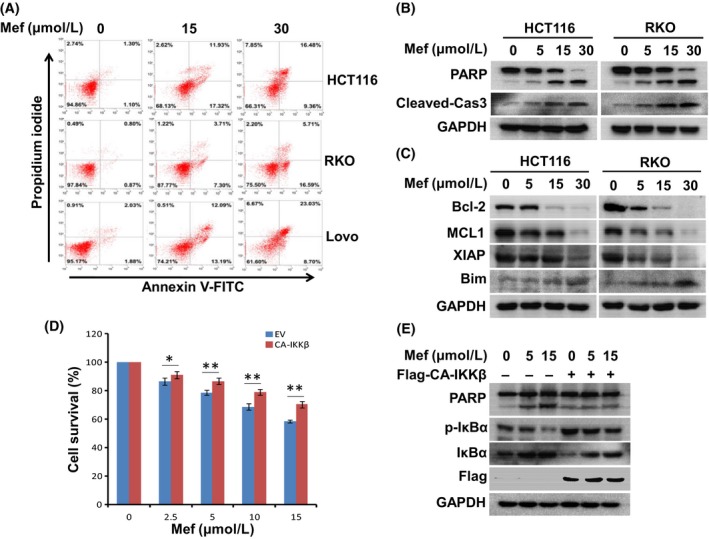
Mefloquine (Mef) induces colorectal cancer cell apoptosis. A, HCT116, RKO and Lovo cells were treated with mefloquine for 12 h and then stained with Annexin V‐FITC and propidium iodide. Percentages of annexin V+ are indicated in the scatterplot (right low and upper quadrants). B, HCT116 and RKO cells were incubated with 0, 5, 15, 30 μmol/L mefloquine overnight and then evaluated by immunoblotting against PARP, cleaved‐caspase 3 and GAPDH. C, HCT116 and RKO cells were treated with 0, 5, 15, 30 μmol/L mefloquine overnight and then analyzed by immunoblotting against anti‐apoptotic (Bcl‐2, Mcl1 and XIAP) and pro‐apoptotic (Bim) proteins. GAPDH was used as a loading control. D, Empty vector (EV) or CA‐IKKβ plasmids were transfected into HCT116 cells. Cells were treated with indicated concentrations of mefloquine overnight and then evaluated by CCK‐8 assay. IKK, IκB kinase. *P < .05; **P < .01, as compared with control. E, Empty vector (EV) or CA‐IKKβ plasmids were transfected into HCT116 cells. The cells were treated with indicated concentrations of mefloquine overnight and then evaluated by immunoblotting against PARP, p‐IκBα, IκBα, Flag and GAPDH
To evaluate the anti‐tumor effect of mefloquine in vivo, HCT116 cells were inoculated s.c. into the right flanks of nude mice. When the xenografts became palpable, mice were orally given mefloquine (30 mg/kg bodyweight) or vehicle for a continuous 20 days. As shown in Figure 5A, the drug markedly reduced tumor growth. Tumor volumes of the 2 groups showed a significant difference on day 9 (P < .05), and the difference became bigger thereafter. At the end of the experiment (day 20), tumors were excised and weighed. Average weight of tumors from the mefloquine‐treated group was reduced to 25% of that from the control group (Figure 5B,C). Furthermore, immunoblotting analysis of the excised tumors showed that tumors from the control animals contained high levels of phosphorylated p65, which was barely detectable in tumors from mefloquine‐treated mice (Figure 5D). Thus, mefloquine is a potent anti‐CRC agent that induces apoptotic cell death in vitro and in vivo through blocking activation of IKK.
Figure 5.
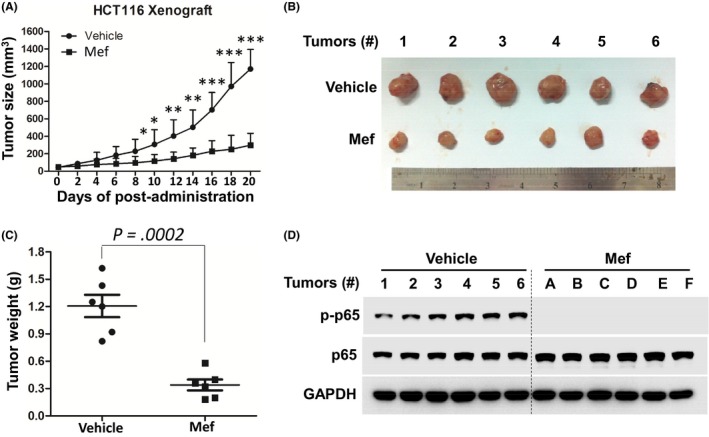
Mefloquine (Mef) inhibits the growth of colorectal cancer cell‐derived tumors in vivo. A, HCT116 cells (5 × 106) were inoculated s.c. into nude mice. When the tumors became palpable, mice were randomly divided into 2 groups (n = 6/group). One group was orally given mefloquine (30 mg/kg bodyweight) in PBS containing 10% Tween 80 and 10% DMSO daily for a continuous 20 days. Another group was given vehicle as a control. Tumor volumes were monitored every other day. *P < .05; **P < .01; ***P < .001. B, Tumors were excised at the end of the experiment and (C) their weights were measured. D, Tumor tissues were analyzed by immunoblotting of p‐p65 and p65. GAPDH was used as an internal control
3.4. Mefloquine enhances the cytotoxic action of DOX against CRC cells
Doxorubicin is one of the most commonly used chemotherapeutics for many cancers including CRC. Development of resistance to the drug has been frequently found in various experimental models and clinically.23 The finding that downregulation of NF‐κB sensitized cancer cells to anti‐cancer drugs led us to examine whether mefloquine affected the effect of doxorubicin on CRC cells.24, 25, 26 As shown in Figure 6A, doxorubicin or mefloquine alone led to ~25% and ~50% reduction of viability, respectively, in both HCT116 and RKO cells as measured by CCK‐8 assay. When the cells were treated with both drugs simultaneously, the viability reduction increased to ~80%. Mefloquine and doxorubicin also acted synergistically to inhibit p65 phosphorylation and to induce PARP cleavage (Figure 6B), showing that mefloquine significantly enhanced the cytotoxic action of doxorubicin.
Figure 6.
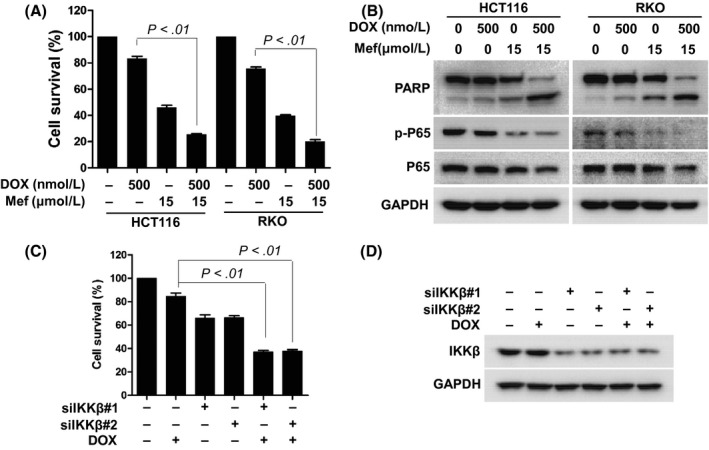
Mefloquine (Mef) enhances the cytotoxicity of doxorubicin against colorectal cancer cells. A, B, HCT116 and RKO cells were treated with 500 nmol/L doxorubicin overnight in the presence or absence of 15 μmol/L mefloquine. The cells were assessed by (A) CCK‐8 assay for viability or (B) immunoblotting against PARP, p‐p65, p65, and GAPDH. C, D, HCT116 cells were transfected with siNC, siIKKβ#1 or siIKKβ#2 for 48 h and then treated with doxorubicin overnight. Cells were evaluated with the CCK‐8 assay for viability, and immunoblotting against IKKβ. GAPDH was used as a loading control. IKK, IκB kinase
Use was also made of the genetic approach to explore the synergistic action. Two siRNAs were selected to knock down IKKβ in HCT116 cells (Figure 6C). Although cell viability assessed by CCK‐8 decreased to only ~80% when HCT116 cells were treated with doxorubicin alone overnight, it decreased markedly to ~40% with IKKβ knockdown in the system. Therefore, blocking the NF‐κB pathway enhances the cytotoxic activity of doxorubicin, which likely accounts for the synergistic action of mefloquine.
4. DISCUSSION
Colorectal cancer presents a specific challenge in the clinic because of its high incidence rate and demands long period of chemotherapy. It is not uncommon to have CRC patients with metastasized tumor and rising serum levels of carcinoembryonic antigen (CEA) seeking treatment. Thus, novel, effective and low toxicity drugs are urgently needed to treat CRC. It has been indicated that repurposing existing drugs will enable us to cut down the time and financial limitations of advancing an anti‐cancer program from hits and leads to clinical candidates. In fact, successful lists are growing impressively,27 including the discovery that the anti‐diabetic drug metformin has significant anti‐neoplastic action.28 We started with screening for compounds that inhibit NF‐κB‐mediated transactivation and found that the antimalarial drug mefloquine blocked NF‐κB activation and induced apoptosis in colorectal cells harboring constitutively activated p65. Thus, it is conceivable that the drug widely used for malaria prevention and treatment might be tried for CRC that are addicted to activated NF‐κB. Interestingly, it has been found that mefloquine induced breast cancer cell death by inhibiting autophagy, killed prostate cancer cells by reactive oxygen species (ROS)‐mediated mechanisms, and inhibited the proliferation of gastric cancer cells through blocking the PI3K/Akt/mTOR signaling pathway.29, 30, 31 Therefore, mefloquine appears to be an effective agent for many types of cancers, although it remains to be determined whether there is a common mechanism responsible for its action in different cancer cells. It was also shown that mefloquine may target the 80S ribosome of Plasmodium falciparum to inhibit protein synthesis.32 Whether similar action of mefloquine takes place in mammalian cells and contributes to its anti‐CRC action remains to be further investigated. Noteworthily, a number of anti‐cancer and anti‐inflammation agents have been found as effective anti‐parasite chemicals,33 whereas anti‐leishmanial thiadiazine derivatives and anti‐plasmodium are potent anti‐neoplastic agents,34, 35, 36 suggesting that we should cross‐examine more therapeutic agents in both parasites and cancer systems.
NF‐κB is activated in many different cancers, and has been reported to be overexpressed in 50%‐75% of CRC.37 Its activation is also related to resistance to chemotherapeutics and poor prognosis of patients with CRC.38 Hence, the NF‐κB pathway is widely regarded as an attractive therapeutic target in a broad range of malignancies including CRC. Strategies that target upstream receptors, IKK activation, IκBα phosphorylation and degradation, p65/p50 nuclear translocation, NF‐κB‐DNA binding, and NF‐κB transactivation have all been developed and reported. Besides antibodies and recombinant proteins that block receptor activation on the cell surface, it has been estimated that more than 1000 natural or synthetic compounds have been identified as inhibitors of the NF‐κB signaling pathway.39, 40 The IKK complex has been the focus of many inhibitors because of its critical role as the integrator of various signals that modulate NF‐κB activity.41 However, kinase inhibitors have not achieved any clinical success, presumably as a result of global suppression of NF‐κB and its various biological functions, leading to severe dose‐limiting toxicities. As mefloquine is a widely used antimalarial drug whose most notable side‐effects are neurological and/or psychiatric, it is conceivable that blocking the activation of IKK through certain pathways may avoid the toxicity associated with global inhibition of IKK and NF‐κB. Although a number of different mechanisms, such as inhibiting cholinesterases, interacting with adenosine A(2A) receptors, oxidative damage, and blocking non‐receptor tyrosine kinase 2, have been proposed for the antimalarial action of mefloquine,42 it is not clear how mefloquine affects IKK activation. As the concentrations of mefloquine we used in inhibiting NF‐κB in vitro and in vivo were in the same range as the clinical antimalarial dose (30 mg/kg bodyweight), the drug might exert anti‐CRC action without the side‐effects of global NF‐κB inhibition. The combination of mefloquine with other anti‐cancer agents, such as doxorubicin, might also be worth further investigation.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGMENTS
This work was supported by Natural Science Foundation of Jiangsu Province (BK20171231), the Innovative Research Group of Chinese Academy of Medical Sciences (2016‐12M‐1‐005), and the Non‐profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2016ZX310194).
Xu X, Wang J, Han K, Li S, Xu F, Yang Y. Antimalarial drug mefloquine inhibits nuclear factor kappa B signaling and induces apoptosis in colorectal cancer cells. Cancer Sci. 2018;109:1220–1229. https://doi.org/10.1111/cas.13540
Funding Information
Natural Science Foundation of Jiangsu Province (Grant/Award Number: BK20171231); the Innovative Research Group of Chinese Academy of Medical Sciences (2016‐12M‐1‐005), and the Non‐profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2016ZX310194).
X. Xu and J. Wang contributed equally to this study.
REFERENCES
- 1. Pinto A, Eveno C, Pocard M. Update on clinical trials in colorectal cancer peritoneal metastasis. Int J Hyperthermia. 2017;33:543‐547. [DOI] [PubMed] [Google Scholar]
- 2. Maida M, Macaluso FS, Ianiro G, et al. Screening of colorectal cancer: present and future. Expert Rev Anticancer Ther. 2017;17:1131‐1146. [DOI] [PubMed] [Google Scholar]
- 3. Fidler MM, Bray F, Vaccarella S, Soerjomataram I. Assessing global transitions in human development and colorectal cancer incidence. Int J Cancer. 2017;140:2709‐2715. [DOI] [PubMed] [Google Scholar]
- 4. Due SL, Wattchow DA, Sweeney JL, Milliken L, Luke CG. Colorectal cancer surgery 2000–2008: evaluation of a prospective database. ANZ J Surg. 2012;82:412‐419. [DOI] [PubMed] [Google Scholar]
- 5. Magaji BA, Moy FM, Roslani AC, Law CW. Survival rates and predictors of survival among colorectal cancer patients in a Malaysian tertiary hospital. BMC Cancer. 2017;17:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vogel A, Hofheinz RD, Kubicka S, Arnold D. Treatment decisions in metastatic colorectal cancer – beyond first and second line combination therapies. Cancer Treat Rev. 2017;59:54‐60. [DOI] [PubMed] [Google Scholar]
- 7. Kroemer G, Galluzzi L, Zitvogel L, Fridman WH. Colorectal cancer: the first neoplasia found to be under immunosurveillance and the last one to respond to immunotherapy? Oncoimmunology. 2015;4:e1058597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coussens NP, Braisted JC, Peryea T, Sittampalam GS, Simeonov A, Hall MD. Small‐molecule screens: a gateway to cancer therapeutic agents with case studies of food and drug administration‐approved drugs. Pharmacol Rev. 2017;69:479‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prasad S, Ravindran J, Aggarwal BB. NF‐kappaB and cancer: how intimate is this relationship. Mol Cell Biochem. 2010;336:25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maruyama W, Shirakawa K, Matsui H, et al. Classical NF‐kappaB pathway is responsible for APOBEC3B expression in cancer cells. Biochem Biophys Res Commun. 2016;478:1466‐1471. [DOI] [PubMed] [Google Scholar]
- 11. Shi W, Ye Z, Zhuang L, et al. Olfactomedin 1 negatively regulates NF‐kappaB signalling and suppresses the growth and metastasis of colorectal cancer cells. J Pathol. 2016;240:352‐365. [DOI] [PubMed] [Google Scholar]
- 12. Yi W, Xiao E, Ding R, Luo P, Yang Y. High expression of fibronectin is associated with poor prognosis, cell proliferation and malignancy via the NF‐kappaB/p53‐apoptosis signaling pathway in colorectal cancer. Oncol Rep. 2016;36:3145‐3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF‐kappaB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 2011;30:1615‐1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baud V, Karin M. Is NF‐kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309‐313. [DOI] [PubMed] [Google Scholar]
- 16. Xu X, Han K, Zhu J, et al. An inhibitor of cholesterol absorption displays anti‐myeloma activity by targeting the JAK2‐STAT3 signaling pathway. Oncotarget. 2016;7:75539‐75550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu X, Han K, Tang X, et al. The ring finger protein RNF6 induces leukemia cell proliferation as a direct target of pre‐B‐cell leukemia homeobox 1. J Biol Chem. 2016;291:9617‐9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han K, Xu X, Xu Z, et al. SC06, a novel small molecule compound, displays preclinical activity against multiple myeloma by disrupting the mTOR signaling pathway. Sci Rep. 2015;5:12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han K, Xu X, Chen G, et al. Identification of a promising PI3K inhibitor for the treatment of multiple myeloma through the structural optimization. J Hematol Oncol. 2014;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sato A, Kudo C, Yamakoshi H, et al. Curcumin analog GO‐Y030 is a novel inhibitor of IKKbeta that suppresses NF‐kappaB signaling and induces apoptosis. Cancer Sci. 2011;102:1045‐1051. [DOI] [PubMed] [Google Scholar]
- 21. Karin M, Ben‐Neriah Y. Phosphorylation meets ubiquitination: the control of NF‐[kappa]B activity. Annu Rev Immunol. 2000;18:621‐663. [DOI] [PubMed] [Google Scholar]
- 22. Chen Z, Hagler J, Palombella VJ, et al. Signal‐induced site‐specific phosphorylation targets I kappa B alpha to the ubiquitin‐proteasome pathway. Genes Dev. 1995;9:1586‐1597. [DOI] [PubMed] [Google Scholar]
- 23. Cox J, Weinman S. Mechanisms of doxorubicin resistance in hepatocellular carcinoma. Hepat Oncol. 2016;3:57‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pimentel‐Gutierrez HJ, Bobadilla‐Morales L, Barba‐Barba CC, et al. Curcumin potentiates the effect of chemotherapy against acute lymphoblastic leukemia cells via downregulation of NF‐kappaB. Oncol Lett. 2016;12:4117‐4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Z, Duan ZJ, Chang JY, et al. Sinomenine sensitizes multidrug‐resistant colon cancer cells (Caco‐2) to doxorubicin by downregulation of MDR‐1 expression. PLoS ONE. 2014;9:e98560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Huang Y, Yang X, Xu T, et al. Overcoming resistance to TRAIL‐induced apoptosis in solid tumor cells by simultaneously targeting death receptors, c‐FLIP and IAPs. Int J Oncol. 2016;49:153‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iacopetta D, Carocci A, Sinicropi MS, et al. Old drug scaffold, new activity: thalidomide‐correlated compounds exert different effects on breast cancer cell growth and progression. ChemMedChem. 2017;12:381‐389. [DOI] [PubMed] [Google Scholar]
- 28. Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778‐790. [DOI] [PubMed] [Google Scholar]
- 29. Yan KH, Lin YW, Hsiao CH, et al. Mefloquine induces cell death in prostate cancer cells and provides a potential novel treatment strategy in vivo. Oncol Lett. 2013;5:1567‐1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan KH, Yao CJ, Hsiao CH, et al. Mefloquine exerts anticancer activity in prostate cancer cells via ROS‐mediated modulation of Akt, ERK. JNK and AMPK signaling. Oncol Lett. 2013;5:1541‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma N, Thomas S, Golden EB, et al. Inhibition of autophagy and induction of breast cancer cell death by mefloquine, an antimalarial agent. Cancer Lett. 2012;326:143‐154. [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Zhang R, Li J, et al. Comprehensive resistome analysis reveals the prevalence of NDM and MCR‐1 in Chinese poultry production. Nat Microbiol. 2017;2:16260. [DOI] [PubMed] [Google Scholar]
- 33. Klug DM, Gelb MH, Pollastri MP. Repurposing strategies for tropical disease drug discovery. Bioorg Med Chem Lett. 2016;26:2569‐2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hou X, Yang C, Zhang L, et al. Killing colon cancer cells through PCD pathways by a novel hyaluronic acid‐modified shell‐core nanoparticle loaded with RIP3 in combination with chloroquine. Biomaterials. 2017;124:195‐210. [DOI] [PubMed] [Google Scholar]
- 35. Cao B, Li J, Zhou X, et al. Clioquinol induces pro‐death autophagy in leukemia and myeloma cells by disrupting the mTOR signaling pathway. Sci Rep. 2014;4:5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen G, Han K, Xu X, et al. An anti‐leishmanial thiadiazine agent induces multiple myeloma cell apoptosis by suppressing the nuclear factor kappaB signalling pathway. Br J Cancer. 2014;110:63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim NK, Park JK, Shin E, Kim YW. The combination of nuclear factor kappa B, cyclo‐oxygenase‐2 and vascular endothelial growth factor expression predicts poor prognosis in stage II and III colorectal cancer. Anticancer Res. 2014;34:6451‐6457. [PubMed] [Google Scholar]
- 38. Wu D, Wu P, Zhao L, et al. NF‐kappaB expression and outcomes in solid tumors: a systematic review and meta‐analysis. Medicine (Baltimore). 2015;94:e1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bremner P, Heinrich M. Natural products as targeted modulators of the nuclear factor‐kappaB pathway. J Pharm Pharmacol. 2002;54:453‐472. [DOI] [PubMed] [Google Scholar]
- 40. Kwak JH, Jung JK, Lee H. Nuclear factor‐kappa B inhibitors; a patent review (2006–2010). Expert Opin Ther Pat. 2011;21:1897‐1910. [DOI] [PubMed] [Google Scholar]
- 41. Liu F, Xia Y, Parker AS, Verma IM. IKK biology. Immunol Rev. 2012;246:239‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grabias B, Kumar S. Adverse neuropsychiatric effects of antimalarial drugs. Expert Opin Drug Saf. 2016;15:903‐910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


