Abstract
Multidrug resistance (MDR) significantly restricts the clinical efficacy of gastric cancer (GC) chemotherapy, and it is critical to search novel targets to predict and overcome MDR. Leucine‐rich repeats and immunoglobulin‐like domains 1 (LRIG1) has been proved to be correlated with drug resistance in several cancers. The present study revealed that LRIG1 was overexpressed in chemosensitive GC tissues and decreased expression of LRIG1 predicted poor survival in GC patients. We observed that upregulation of LRIG1 enhanced chemosensitivity in GC cells. Interestingly, miR‐20a, which was overexpressed in GC MDR cell lines and tissues, was identified to regulate LRIG1 expression by directly targeting its 3′ untranslated region. We also found that inhibition of miR‐20a suppressed GC MDR, and upregulation showed opposite effects. Moreover, we demonstrated that the miR‐20a/LRIG1 axis regulated GC cell MDR through epidermal growth factor receptor (EGFR)‐mediated PI3K/AKT and MAPK/ERK signaling pathways. Finally, LRIG1 expression in human GC tissues is inversely correlated with miR‐20a and EGFR. Taken together, the newly identified miR‐20a/LRIG1/EGFR link provides insight into the MDR process of GC, and targeting this axis represents a novel potential therapeutic strategy to block GC chemoresistance.
Keywords: epidermal growth factor receptor, gastric cancer, leucine‐rich repeats and immunoglobulin‐like domains 1, miR‐20a, multidrug resistance
1. INTRODUCTION
Gastric cancer (GC) remains the second leading cause of cancer‐related deaths worldwide in the recent two decade. GC has a low 5‐year survival rate, especially for advanced patients.1, 2 Chemotherapy is the main approach used to manage GC patients of advanced stages.3 However, chemotherapeutic approaches often fail in clinical practice, due to intrinsic or acquired drug resistance, particularly multidrug resistance (MDR).3 Although the mechanisms underlying MDR of GC have been extensively investigated, the key determinants are still largely unknown.
Leucine‐rich repeats and immunoglobulin‐like domains 1 (LRIG1), a transmembrane protein, is a pan‐ERBB negative regulator that inhibits EGFR (epidermal growth factor receptor) signaling by accelerating receptor internalization and degradation in a c‐CBLe‐dependent manner.4, 5, 6 Substantial evidence indicates that LRIG1 is involved in tumorigenesis and functions as a tumor suppressor.6 Low expression of LRIG1 has been associated with poor prognosis in breast,7, 8 oropharyngeal9 and nasopharyngeal cancers.10 Stutz et al (2008) report that overexpression of LRIG1 suppresses malignant glioma cell growth by attenuating EGFR activity and negatively regulates the oncogenic EGF receptor mutant EGFRvIII.11 Moreover, LRIG1 downregulation attenuates tumor necrosis factor α (TNFα)gene expression and confers resistance to Smac mimetics in breast and ovarian cancers.12 Liu et al (2015) demonstrate that LRIG1 could reverse MDR in glioblastoma, by negatively regulating EGFR and suppressing the expression of ATP‐binding cassette, sub‐family B member 1 (ABCB1) and ATP‐binding cassette sub‐family G member 2 (ABCG2).13 However, the function of LRIG1 in GC MDR remains unclear, and needs to be elucidated.
MicroRNA (miRNA) are small, evolutionarily conserved, non‐coding RNA of 18‐25 nucleotides in length, which bind to the 3′‐untranslated regions (3′‐UTR) of target genes, leading to either target mRNA degradation or protein translation reduction.14, 15 It was estimated that approximately 30% of human genes and most genetic pathways are regulated through miRNA.14, 15 As a negative regulator at the posttranscriptional level, miRNA is an important mechanism regulating gene expression.14, 15 In addition, mounting evidence has shown that miRNA are involved in GC development and progression, including MDR.16, 17, 18 We thus speculate that dysregulation of LRIG1's regulatory miRNA might be a main cause of its downregulation in MDR of GC.
In the present study, we showed that LRIG1 was overexpressed in chemosensitive GC tissues compared with chemoresistant ones, and decreased expression of LRIG1 predicted poor survival. We further observed that upregulation of LRIG1 enhanced chemosensitivity to various drugs in GC cells. In addition, we demonstrated that miR‐20a, which was overexpressed in GC MDR cell lines and tissues, regulated LRIG1 expression by directly targeting its 3′‐UTR. We also found that inhibition of miR‐20a suppressed GC MDR, and upregulation of miR‐20a showed opposite effects. Moreover, the miR‐20a/LRIG1 axis regulated GC cell MDR through EGFR‐mediated phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) and MAPK/ERK signaling.
2. MATERIALS AND METHODS
2.1. Cell culture and tissue collection
The human GC cell line SGC7901 and its multidrug‐resistant variants SGC7901/VCR and SGC7901/ADR were obtained from the State Key Laboratory of Cancer Biology and Xijing Hospital of Digestive Diseases, and maintained in RPMI‐1640 medium (Hyclone, Logan, UT, USA) supplemented with 10% FBS (Gibco, Carlsbad, CA, USA) at 37°C under a humidified air atmosphere containing 5% CO2. Paired samples of primary GC and adjacent normal tissues were obtained from patients who had undergone surgery for GC resection at Xijing Hospital, Xi'an, China. This study was approved by the Xijing Hospital Protection of Human Subjects Committee and the Ethics Committee of Nanjing Drum Tower Hospital, and informed consent was obtained from each patient.
2.2. Immunohistochemistry
Immunohistochemistry was performed as in Zhou et al (2014) with an anti‐LRIG1 (Abcam, Cambridge, UK) and anti‐EGFR (Cell Signaling, Danvers, MA, USA) antibodies.19 The protein expression was visualized and classified according to the percentage of positive cells and the intensity of staining, and the histological score (H score) was calculated and graded as described in our previous study.19
2.3. RNA extraction and real‐time quantitative RT‐PCR
Total RNA was extracted with Trizol Reagent (Invitrogen, Carlsbad, CA, USA). The RT and PCR primers for miR‐20a and U6 were purchased from RiBoBio (Guangzhou, China). The PCR primers for LRIG1 and EGFR are shown in Table S1. The first‐strand cDNA was synthesized with the PrimeScript RT Reagent Kit (Takara, Dalian, China). RT‐PCR was performed with SYBR Premix Ex TaqII (Takara) and measured in the LightCycler 480 (Roche, Basel, Switzerland). GAPDH or U6 snRNA was used as an endogenous control, and an internal control was used to verify that sample loading was equal. The fold change was calculated by 2−ΔΔCt. All reactions were performed in triplicate.
2.4. Western blotting
Whole‐cell lysates were prepared in RIPA buffer (Byotime, Haimen, China), and western blot analysis was performed.19 The primary antibodies used were anti‐LRIG1 (Abcam), anti‐EGFR (Cell Signaling), anti‐ERK1/2 (Abcam), anti‐p‐ERK1/2 (Abcam), anti‐AKT (Cell Signaling), anti‐p‐AKT (Cell Signaling), anti‐P‐gp (Sigma, St. Louis, MO, USA), anti‐Bcl‐2 (Abcam) and β‐actin (Sigma).
2.5. Oligonucleotide construction and transfection
Mimics and inhibitors for has‐miR‐20a and negative control oligonucleotides were purchased from RiboBio. siRNA and plasmids for LRIG1 and corresponding negative control were obtained from Genechem (Shanghai, China). Target cells were transfected with oligonucleotides using Lipofectamine 2000 Reagent (Invitrogen) according to the manufacturer's instructions.
2.6. In vitro drug sensitivity assay
Drug sensitivity was assessed as described previously.20 Briefly, 5 × 103 cells were seeded in 96‐well plates, and medium containing chemotherapeutic drugs was added to each well. After incubation for 48 hours, an MTT (Sigma) assay was performed. Inhibition rates and IC50 values were then calculated.
2.7. Apoptosis assay
Cell apoptosis was evaluated using an Annexin‐V‐FITC apoptosis detection kit (BD, Franklin Lakes, NJ, USA) as previously described.20
2.8. Analysis of intracellular Adriamycin concentrations
Fluorescence intensity of intracellular Adriamycin (ADR) was determined by flow cytometry as described previously.21 Briefly, cells were seeded into 6‐well plates (1 × 106 cells/well) and cultured for 1 hour after ADR addition. Cells were then either harvested to detect ADR accumulation or cultures were continued in a drug‐free medium for another 2 hours, followed by detection of ADR retention. The releasing index of ADR in the GC cells was calculated using the following formula: releasing index = (accumulation value − retention value)/accumulation value.
2.9. Luciferase assay
Plasmids carrying wild‐type Luc‐LRIG1 or mutant Luc‐LRIG1‐ß3′‐UTR were synthesized (GeneCopoeia, Rockville, MD, USA). The luciferase assay was performed as previously described.22
2.10. Immunoprecipitation
An immunoprecipitation assay was performed as previously described using an anti‐LRIG1 antibody.23 The total protein was prepared using M‐PERTM Mammalian Protein Extraction Reagent (Pierce, Appleton, WI, USA); 10% of chromatin was used as an input control, and a non‐specific antibody (anti‐IgG, Abcam) served as a negative control. The obtained proteins were subjected to western blotting in an attempt to amplify the LRIG1‐binding sites.
2.11. Statistical analysis
SPSS software (version 21.0, SPSS, Chicago, IL, USA) was used for the statistical analyses. The continuous data were presented as the means ± SEM, and compared using Student's t test (2‐tailed) or one‐way analysis of variance (ANOVA). Spearman's correlation test was performed to examine the relationship of LRIG1 and miR‐20a or EGFR expression in GC tissues. P < .05 was considered statistically significant (*P < .05, **P < .01 and ***P < .001).
3. RESULTS
3.1. Decreased leucine‐rich repeats and immunoglobulin‐like domains 1 expression is associated with poor prognosis and chemoresistance in gastric cancer
To clarify the expression and clinical significance of LRIG1 in GC, we analyzed the data from Oncomine (https://www.oncomine.org/resource/login.html) and Kaplan Meier plotter (http://www.kmplot.com/analysis/index.php?p=background). It was found that LRIG1 was significantly downregulated in GC compared to normal gastric tissues in 4 independent cohorts (Figure 1A). Furthermore, individuals with lower LRIG1 expression exhibited reduced overall survival in a cohort containing 876 GC cases, and decreased progression free survival in a cohort of 641 GC patients (Figure 1B). These findings indicated that LRIG1 might serve as a biomarker in GC and lower expression of LRIG1 is associated with poor prognosis.
Figure 1.
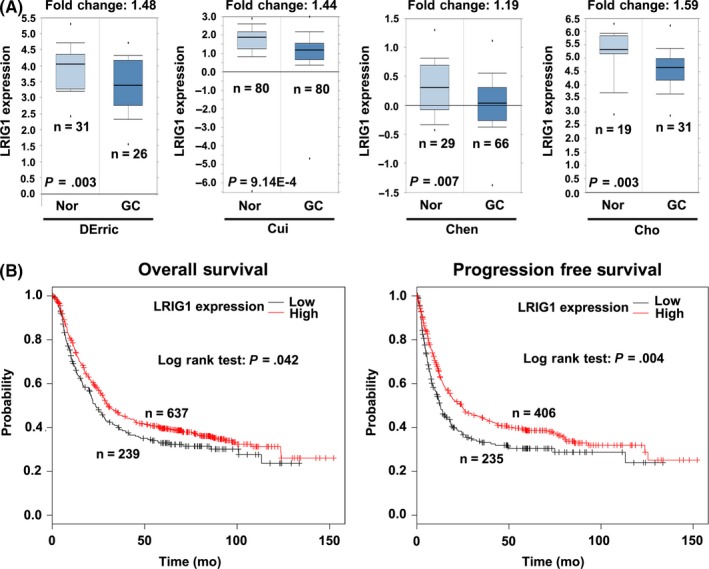
Leucine‐rich repeats and immunoglobulin‐like domains 1 (LRIG1) is downregulated in gastric cancer (GC) tissues and is associated with poor prognosis. A, Comparison of LRIG1 expression between cancerous and normal gastric tissues from Oncomine. GC, gastric cancer; Nor, normal gastric tissues. B, Association of LRIG1 with overall survival and progression free survival in GC from Kaplan Meier plotter
To explore the role of LRIG1 in GC drug resistance, the expression of LRIG1 in GC tissues displaying different chemosensitivities was examined. The response to chemotherapy of 47 GC individuals was estimated according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria, and 13 patients who were evaluated as complete response (CR) or partial response (PR) were regarded as sensitive to chemotherapy. Immunohistochemistry (IHC) and quantitative RT‐PCR (qRT‐PCR) analyses showed that LRIG1 expression was more frequently present in the chemosensitive GC cases than in the chemoresistant ones (Figure 2A,B and Table 1). Moreover, IHC results further confirmed that LRIG1 expression was downregulated in GC tissues compared with adjacent nontumor tissues (Table S2). To further characterize the function of LRIG1 in GC chemoresistance, we measured the expression of LRIG1 in GC chemoresistant cell lines, SGC7901/ADR and SGC7901/VCR, and their parental cell line SGC7901. Western blotting and qRT‐PCR analyses demonstrated that LRIG1 expression was markedly lower in SGC7901/ADR and SGC7901/VCR, compared with SGC7901 (Figure 2C,D). Thus, these findings confirmed that the low expression of LRIG1 correlates with enhanced GC drug resistance.
Figure 2.
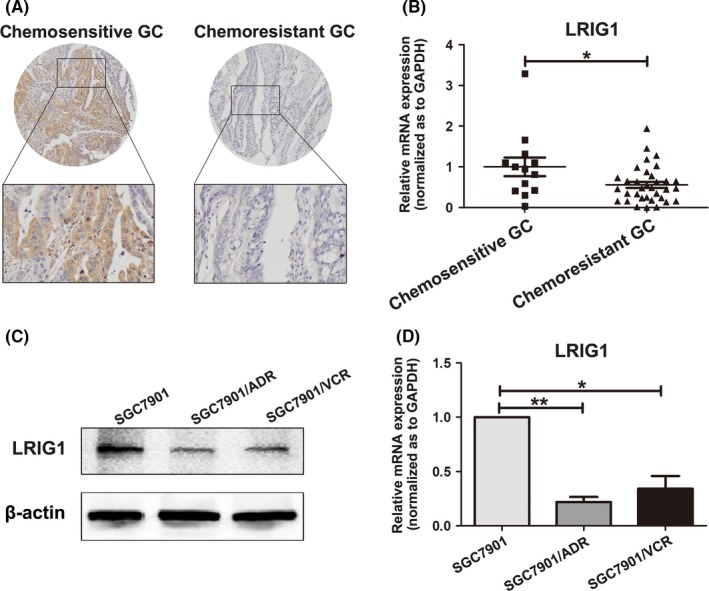
Decreased leucine‐rich repeats and immunoglobulin‐like domains 1 (LRIG1) expression is associated with chemoresistance in gastric cancer (GC). A, Immunohistochemistry analysis of LRIG1 expression in chemosensitive and chemoresistant GC tissues. B, Expression level of LRIG1 mRNA in chemosensitive and chemoresistant GC tissues was measured using quantitative RT‐PCR. GAPDH was used as an internal control and the fold change was calculated by 2−ΔΔCt. *P < .05. C, Expression of LRIG1 in GC cell line SGC7901 and its multidrug resistance variants (MDR) SGC7901/VCR and SGC7901/ADR were examined through western blot analysis. β‐actin was used as an internal control. D, Expression level of LRIG mRNA in GC cell line SGC7901 and its MDR variants SGC7901/VCR and SGC7901/ADR were measured using quantitative RT‐PCR. GAPDH was used as an internal control. *P < .05, **P < .01
Table 1.
LRIG1 expression in chemosensitive and chemoresistant gastric cancer tissues
| n | Expression level of LRIG1 | P | ||||
|---|---|---|---|---|---|---|
| − | + | ++ | +++ | |||
| Chemosensitive GC | 13 | 4 | 2 | 3 | 4 | <.05 |
| Chemoresistant GC | 34 | 19 | 9 | 5 | 1 | |
χ2‐tests were used to evaluate the significance of differences in 2 groups.
GC, gastric cancer; LRIG1, leucine‐rich repeats and immunoglobulin‐like domains 1.
3.2. Upregulation of leucine‐rich repeats and immunoglobulin‐like domains 1 enhances chemosensitivity of gastric cancer cells
Next, we investigated whether upregulation of LRIG1 increased sensitivity to chemotherapeutic agents in GC cells. To this end, SGC7901 cells were transfected with siRNA against LRIG1 (siLRIG1) or NC siRNA (siNC), and SGC7901/ADR and SGC7901/VCR cells were transfected with LRIG1 vector or negative control (NC). Western blotting assay confirmed that siLRIG1 dramatically suppressed LRIG1 expression in SGC7901, while LRIG1 vector markedly upregulated LRIG1 expression in SGC7901/ADR and SGC7901/VCR (Figure 3A). We found that silencing of LRIG1 significantly inhibited sensitivity to chemotherapy, as indicated by an increase in the IC50 values of vincristine (VCR), ADR, 5‐fluorouracil (5‐FU) and cisplatin (CDDP) in SGC7901 cells (Figure 3B). Meanwhile, deceased IC50 values for these 4 chemotherapeutic agents were observed after LRIG1 expression was upregulated by LRIG1 vector in SGC7901/ADR and SGC7901/VCR cells (Figure 3C,D). Because one major mechanism through which tumor cells escape from drug‐induced damage is alterations in drug influx and efflux, we analyzed intracellular accumulation and release of ADR. As shown in Table 2, downregulation of LRIG1 in SGC7901 cells manifested an increased releasing index for ADR compared with SGC7901‐siNC cells. Conversely, restoration of LRIG1 in SGC7901/ADR and SGC7901/VCR cells showed a diminished releasing index for ADR. Moreover, the function of LRIG1 in GC cell MDR was also verified by the increase of apoptosis induced by 5‐FU in SGC7901/ADR and SGC7901/VCR cells after upregulation of LRIG1. In contrast, reduced LRIG1 expression in SGC7901 cells led to a decrease in the apoptosis (Figure 3E). Taken together, these results suggested that LRIG1 correlates with GC MDR and that upregulation of LRIG1 could increase sensitivity to chemotherapeutic drugs.
Figure 3.
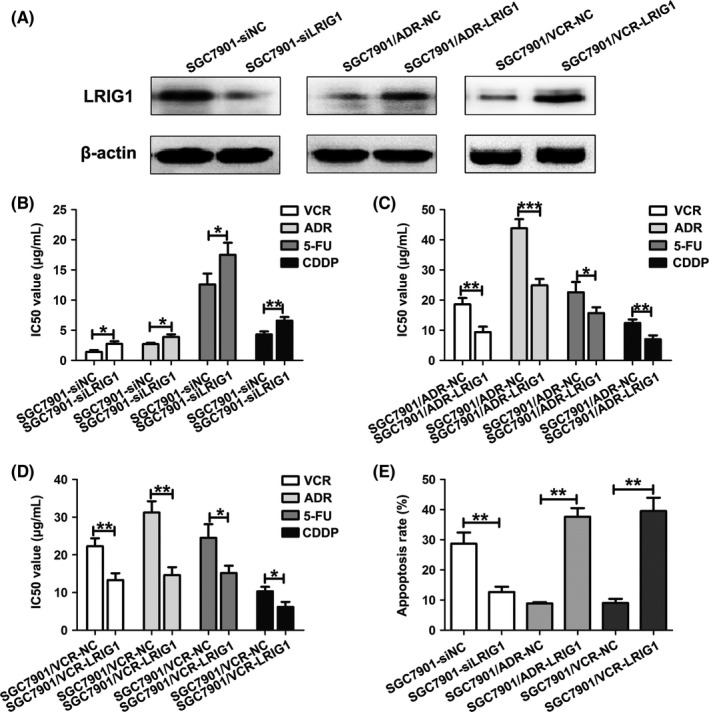
Upregulation of leucine‐rich repeats and immunoglobulin‐like domains 1 (LRIG1) enhances chemosensitivity of gastric cancer (GC) cells. A, Western blot analysis of LRIG1 expression in SGC7901 cells transfected with LRIG1 siRNA (siLRIG1) or negative control (siNC), and SGC7901/ADR and SGC7901/VCR cells transfected with LRIG1 plasmid or vector control (NC). β‐actin was used as an internal control. B, IC50 values of SGC7901 cells to vincristine (VCR), Adriamycin (ADR), 5‐fluorouracil (5‐FU) and cisplatin (CDDP) calculated from MTT assays after transfection with siLRIG1 or siNC. *P < .05, **P < .01. C, IC50 values of SGC7901/ADR cells to VCR, ADR, 5‐FU and CDDP calculated from MTT assays after transfection with LRIG1 or NC. *P < .05, **P < .01, ***P < .001. D, IC50 values of SGC7901/VCR cells to VCR, ADR, 5‐FU and CDDP calculated from MTT assays after transfection with LRIG1 or NC. *P < .05, **P < .01. E, Apoptotic rates of cells treated with 5‐FU (50 mg/mL for SGC7901/ADR and SGC7901/VCR; 20 mg/mL for SGC7901) for 24 h were measured by flow cytometry. **P < .01
Table 2.
Intracellular ADR accumulation and retention in gastric cancer cells
| Cell lines | Fluorescence intensity | Releasing index | P | |
|---|---|---|---|---|
| Accumulation | Retention | |||
| SGC7901‐siNC | 2.82 ± 0.20 | 2.08 ± 0.18 | 0.26 ± 0.06 | <.05 |
| SGC7901‐siLRIG1 | 1.41 ± 0.16 | 0.87 ± 0.09 | 0.38 ± 0.04 | |
| SGC7901/VCR‐NC | 2.01 ± 0.18 | 1.09 ± 0.13 | 0.46 ± 0.06 | <.05 |
| SGC7901/VCR‐LRIG1 | 3.08 ± 0.31 | 2.05 ± 0.24 | 0.33 ± 0.03 | |
| SGC7901/ADR‐NC | 1.94 ± 0.13 | 1.09 ± 0.13 | 0.44 ± 0.05 | <.01 |
| SGC7901/ADR‐LRIG1 | 3.63 ± 0.29 | 2.85 ± 0.17 | 0.21 ± 0.03 | |
| SGC7901‐mimic NC | 2.75 ± 0.14 | 1.99 ± 0.15 | 0.28 ± 0.07 | <.05 |
| SGC7901‐miR‐20a mimic | 1.29 ± 0.08 | 0.72 ± 0.10 | 0.44 ± 0.06 | |
| SGC7901/VCR‐inhibitor NC | 2.04 ± 0.20 | 1.11 ± 0.11 | 0.46 ± 0.08 | <.05 |
| SGC7901/VCR‐miR‐20a inhibitor | 3.16 ± 0.29 | 2.25 ± 0.18 | 0.29 ± 0.03 | |
| SGC7901/ADR‐ inhibitor NC | 2.01 ± 0.23 | 1.14 ± 0.14 | 0.43 ± 0.10 | <.05 |
| SGC7901/ADR‐ miR‐20a inhibitor | 3.41 ± 0.31 | 2.82 ± 0.20 | 0.17 ± 0.06 | |
ADR, Adriamycin; GC, gastric cancer; LRIG1, leucine‐rich repeats and immunoglobulin‐like domains 1; NC, negative control; VCR, vincristine.
3.3. miR‐20a downregulates leucine‐rich repeats and immunoglobulin‐like domains 1 expression by binding its 3′‐untranslated regions
To explore the regulatory mechanism of LRIG1 at miRNA level, we employed bioinformatic methods to identify the potential miRNA regulating LRIG1. Based on the bioinformatics analysis of 3 different programs (miRanda, Pictar and targetscan), a highly conserved miR‐20a targeting sequence was predicted in the 3′‐UTR of the LRIG1 mRNA. We then examined the relationship between the expression levels of LRIG1 mRNA and miR‐20a in the GC drug resistant cell lines. Compared with the parent cell line SGC7901, the expression of miR‐20a was markedly higher in the resistant cell lines SGC7901/ADR and SGC7901/VCR (Figure 4A), inverse with the expression of LRIG1 in the GC cell lines. To further address whether miR‐20a directly targeted LRIG1 3′‐UTR to inhibit its expression in GC, PCR products containing wildtype or mutant LRIG1 3′‐UTR binding sites were inserted into reporter vectors (Figure 4B). Luciferase reporter assays showed that miR‐20a brought a significant reduction in relative luciferase activity when the LRIG1 plasmid containing wild‐type 3′‐UTR was present, but the luciferase activity was not significantly changed in the 3′‐UTR with mutant binding sites (Figure 4C). This result indicated that miR‐20a might repress LRIG1 expression by targeting the binding sites of the LRIG1 3′‐UTR. Moreover, both qRT‐PCR and western blotting analysis showed that upregulation of miR‐20a markedly decreased LRIG1 expression in SGC7901 cells and silencing of miR‐20a dramatically raised LRIG1 expression in SGC7901/ADR and SGC7901/VCR cells (Figure 4D,E). Collectively, these results indicated that miR‐20a suppresses LRIG1 expression by directly targeting its 3′‐UTR.
Figure 4.
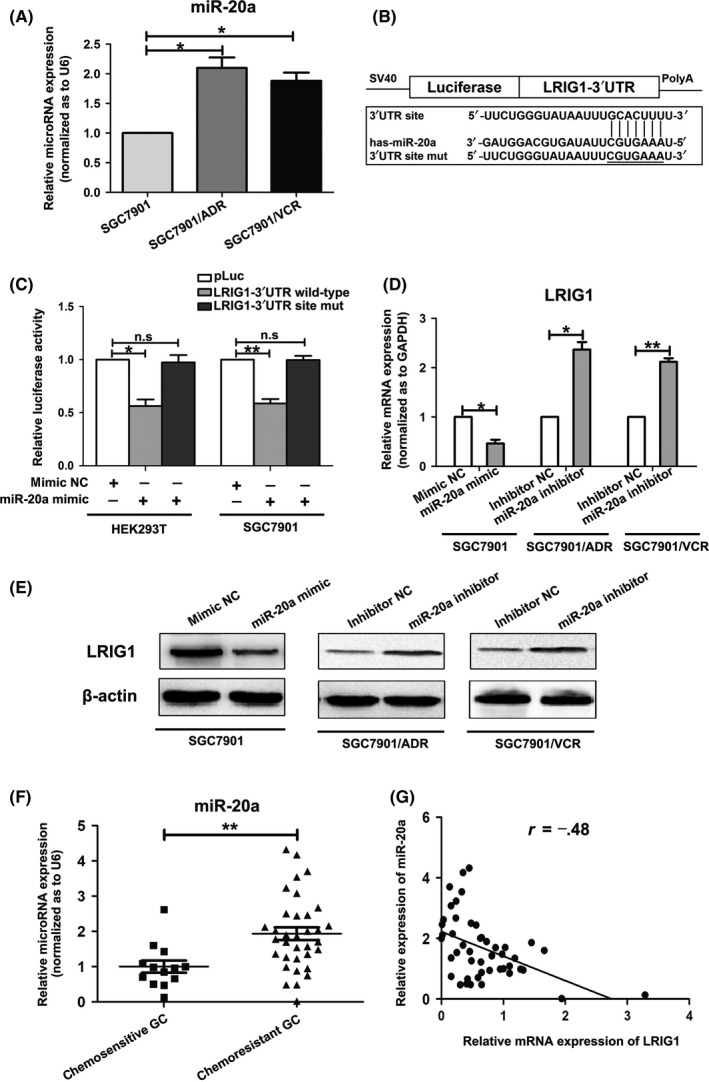
miR‐20a downregulates leucine‐rich repeats and immunoglobulin‐like domains 1 (LRIG1) expression by binding its 3′‐UTR. A, Expression level of miR‐20a in GC cell line SGC7901 and its multidrug resistance (MDR) variants SGC7901/VCR and SGC7901/ADR. The U6 small nuclear RNA was used as an internal control and the fold change was calculated using 2−ΔΔCt. *P < .05. B, Diagram of LRIG1 3′‐UTR‐containing reporter construct. Mutations were generated at the predicted miR‐20a binding site located in the LRIG1 3′‐UTR. C, Luciferase activity after the wild‐type or mutant reporter plasmids were co‐transfected with miR‐20a mimic or negative control (NC) in HEK293T and SGC7901 cells. *P < .05, **P < .01, n.s. no significance. D, Expression of LRIG1 mRNA in SGC7901 cells after transfection with miR‐20a mimic or mimic control (mimic NC), and SGC7901/ADR and SGC7901/VCR cells transfected with miR‐20a inhibitors or inhibitor control (inhibitor NC) were analyzed using quantitative RT‐PCR. GAPDH was used as an internal control. *P < .05, **P < .01. E, Expression of LRIG1 protein was analyzed in SGC7901 cells after transfection with miR‐20a mimic or mimic NC, and SGC7901/ADR and SGC7901/VCR cells transfected with miR‐20a inhibitors or inhibitor NC through western blotting. β‐actin served as an internal control. F, Expression of miR‐20a in chemosensitive and chemoresistant GC tissues was examined by quantitative RT‐PCR. U6 was used as an internal control. **P < .01. G, Statistically significant inverse correlation between miR‐20a and LRIG1 mRNA levels in GC specimens (Spearman's correlation analysis, r = −.48; P < .01)
We next measured whether miR‐20a expression was associated with LRIG1 expression in GC samples to evaluate the clinical relevance of the miR‐20a/LRIG1 axis. The expression of miR‐20a in GC tissues from 47 GC patients was detected by qRT‐PCR. We found that miR‐20a was significantly overexpressed in chemoresistant GC tissues, compared with chemosensitive cases (Figure 4F). Statistical analysis revealed that LRIG1 expression was inversely correlated with miR‐20a expression (Figure 4G). These observations suggested that miR‐20a and LRIG1 are inversely expressed in GC specimens.
3.4. miR‐20a is involved in chemoresistance of gastric cancer cells
To verify the effect of miR‐20a on GC drug resistance, we transfected miR‐20a mimic into SGC7901 cells, and miR‐20a inhibitor into SGC7901/ADR and SGC7901/VCR cells, to conduct gain‐of‐function and loss‐of‐function experiments. Remarkably, the restoration of miR‐20a in SGC7901 cells increased the IC50 values to VCR, ADR, 5FU and CDDP (Figure 5A), and exhibited an increased releasing index for ADR (Table 2). Meanwhile, upregulation of miR‐20a led to reduced apoptosis (Figure 5D). In contrast, inhibition of miR‐20a in SGC7901/ADR and SGC7901/VCR cells sensitized drug‐resistant cells to chemotherapeutics by increasing the intracellular concentration of ADR (Table 2). Silencing of miR‐20a decreased the IC50 values and also markedly raised apoptosis rates (Figure 5B‐D). Therefore, these results indicated that miR‐20a has a negative effect on GC chemosensitivity.
Figure 5.
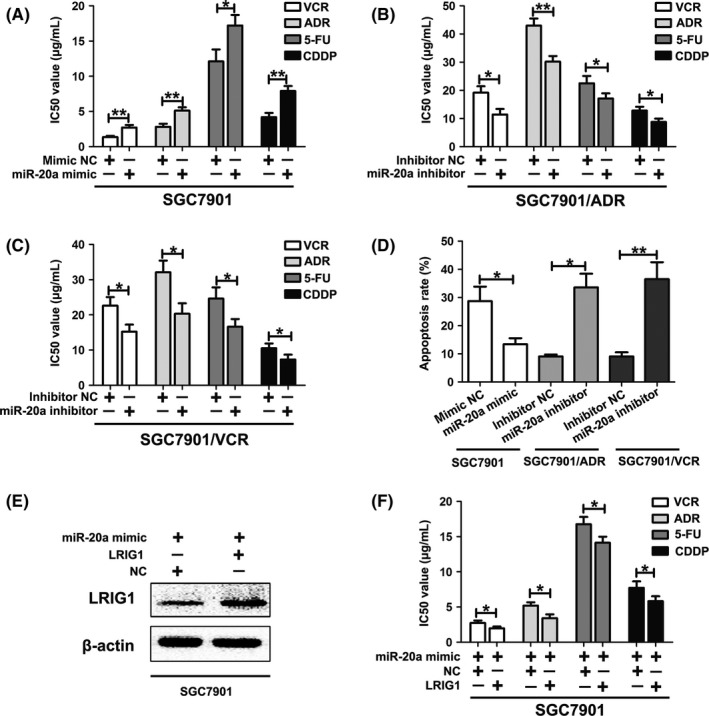
miR‐20a is involved in chemoresistance of gastric cancer (GC) cells. A, IC50 values of SGC7901 cells to vincristine (VCR), Adriamycin (ADR), 5‐fluorouracil (5‐FU) and cisplatin (CDDP) calculated from MTT assays after transfection with miR‐20a mimic or control. *P < .05, **P < .01. B, IC50 values of SGC7901/ADR cells to VCR, ADR, 5‐FU and CDDP calculated from MTT assays after transfection with miR‐20a inhibitor or control. *P < .05, **P < .01. C, IC50 values of SGC7901/VCR cells to VCR, ADR, 5‐FU, and CDDP calculated from MTT assays after transfection with miR‐20a inhibitor or control. *P < .05. D, Apoptotic rates of cells treated with 5‐FU for 24 h were measured by flow cytometry. *P < .05, **P < .01. E, Western blotting of LRIG1 in SGC7901 cells co‐transfected with miR‐20a mimic and LRIG1 plasmid. β‐actin was used as an internal control. F, IC50 values of SGC7901 cells to VCR, ADR, 5‐FU and CDDP calculated from MTT assays after co‐transfection with miR‐20a mimic and leucine‐rich repeats and immunoglobulin‐like domains 1 (LRIG1) plasmid. *P < .05
Because LRIG1 can inhibit GC cell chemoresistance and miR‐20a can post‐transcriptionally regulate the expression of LRIG1, we hypothesized that miR‐20a‐mediated downregulation of LRIG1 directly promotes GC chemoresistance. To address this hypothesis, we first transfected SGC7901 cells with LRIG1 plasmids, and then miR‐20a mimic was co‐transfected into SGC7901 cells (Figure 5E). In vitro drug sensitivity assay demonstrated that the restoration of miR‐20a expression significantly reduced LRIG1‐induced GC cell chemosensitivity (Figure 5F).
3.5. miR‐20a/leucine‐rich repeats and immunoglobulin‐like domains 1 axis regulates gastric cancer drug resistance through epidermal growth factor receptor‐mediated phosphatidylinositol 3 kinase/protein kinase B and MAPK/ERK signaling
LRIG1 is capable of interacting with EGFR and enhancing both its basal and ligand‐stimulated ubiquitination and degradation.4, 5, 6 Thus, we speculated that LRIG1 modulated GC drug resistance through EGFR signaling. qRT‐PCR and western blotting assays were performed to investigate whether EGFR activity was changed after LRIG1 expression altered. Downregulation of LRIG1 in SGC7901 cells did not result in an impact on the mRNA level of EGFR but led to a substantial increase in the protein level of EGFR (Figure 6A,B). It can be inferred that LRIG1 might directly interact with EGFR protein, but not through transcription regulation. Moreover, we next examined whether siLRIG1 treatment led to a decrease in proteasomal‐mediated degradation of EGFR protein. SGC7901 cells were treated with the proteasome inhibitor MG132 (10 μM; Torcris Biosciences, Bristol, UK). MG132 pre‐treatment resulted in a relatively smaller increase in EGFR level (Figure 6B), suggesting that LRIG1‐induced EGFR depletion may be, at least partially, attributed to an increase in protein degradation by the proteasome. Subsequently, a co‐immunoprecipitation method was carried out to determine whether there was a physical interaction between LRIG1 and EGFR molecules. As shown in Figure 6C, EGFR could be specifically co‐immunoprecipitated with LRIG1, but not with control IgG, indicating that these 2 proteins were specifically associated with each other in complex. In addition, EGFR expression was detected through IHC in the 47 GC tissues, and statistical analysis revealed that LRIG1 expression was significantly negatively correlated with EGFR levels (Table 3).
Figure 6.
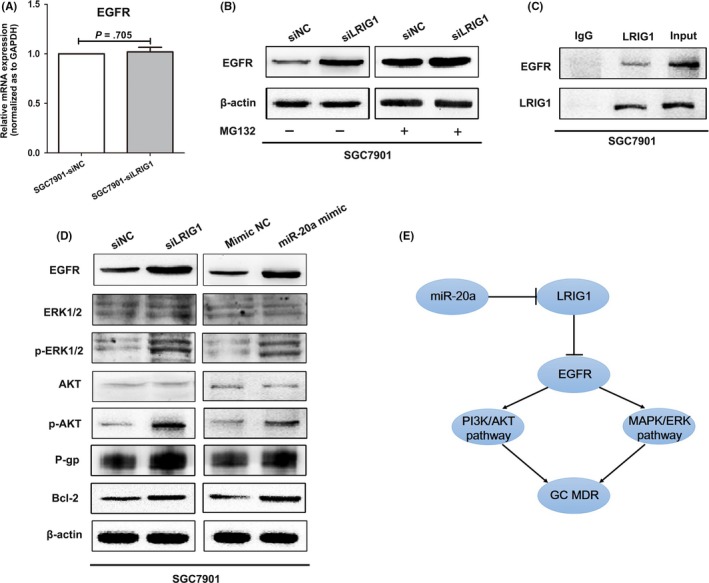
miR‐20a/leucine‐rich repeats and immunoglobulin‐like domains 1 (LRIG1) axis regulates gastric cancer (GC) chemoresistance through epidermal growth factor receptor (EGFR)‐mediated phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) and MAPK/ERK signaling. A, mRNA expression of EGFR in SGC7901 was examined by quantitative RT‐PCR after transfection with siLRIG1 or siNC. GAPDH was used as an internal control. B, Western blotting of EGFR in SGC7901 cells transfected with siLRIG1 or siNC, incubated without or with the proteasome inhibitor MG132. β‐actin was used as an internal control. C, Lysates of SGC7901 cells were immunoprecipitated with anti‐LRIG1 or control IgG and blotted with antibodies to EGFR or LRIG1. D, Western blotting of ERK1/2, AKT, their phosphorylated forms, P‐gp and Bcl‐2 in SGC7901 cells after transfection with siLRIG1 or miR‐20a mimic. β‐actin was used as an internal control. E, Schematic representation of the miR‐20a/LRIG1 axis as an multidrug resistance (MDR) regulator in GC
Table 3.
The correlation between LRIG1 and EGFR in gastric cancer tissues
| Expression level of LRIG1 | Spearman's test | ||||||
|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | r | P | ||
| Expression level of EGFR | − | 4 | 2 | 3 | 3 | −.299 | <.05 |
| + | 5 | 3 | 2 | 1 | |||
| ++ | 7 | 3 | 2 | 1 | |||
| +++ | 7 | 3 | 1 | 0 | |||
EGFR, epidermal growth factor receptor; LRIG1, leucine‐rich repeats and immunoglobulin‐like domains 1.
It is well known that EGFR mediated PI3K/AKT and MAPK/ERK pathways play very important roles in GC drug resistance.21, 24, 25, 26 Therefore, we next measured the expression alteration of key molecules in PI3K/AKT and MAPK/ERK pathways to further clarify the miR‐20a/LRIG1 downstream mechanism. Western blotting demonstrated that downregulation of LRIG1 or upregulation of miR‐20a significantly enhanced EGFR activity, and increased p‐AKT (phospho‐AKT) and p‐ERK1/2 (phospho‐ERK1/2) protein expression (Figure 6D). In addition, P‐gp and Bcl‐2, as important downstream molecules of EGFR signal, were remarkably increased after LRIG1 knockdown or miR‐20a upregulation.27, 28, 29 Taken together, these results indicated that the miR‐20a/LRIG1 axis might regulate GC cell drug resistance by increasing EGFR activity and through secondary accelerating PI3K/AKT and MAPK/ERK signaling pathways (Figure 6E).
4. DISCUSSION
Multidrug resistance accounts for most cases of treatment failure among GC patients.3 Although a wide range of MDR‐associated molecules have been discovered, the mechanisms underlying their functions and their interactions are not fully understood. The human LRIG1 gene at chromosome 3p14.1 encodes LRIG1 protein, which negatively regulates the ERRB2 gene product, human epidermal growth factor receptor 2 (HER2), and other oncogenic receptor tyrosine kinases, including EGFR, HER3 and HER4.4, 5, 6, 30, 31, 32 Powell et al (2012) demonstrate that LRIG1 functions as a tumor suppressor in the mouse intestine,5 and increasing evidence indicates that LRIG1 may act as a tumor suppressor in humans.33 Reduced expression of LRIG1 has been reported in breast, cervical and skin cancers, as reviewed.6, 34 The soluble ectodomain of LRIG1 inhibits in vivo growth of EGFRVIII mutant gliomas, and restoration of LRIG1 expression sensitizes glioma cells to chemotherapy.11 In the present study, we observed that LRIG1 was downregulated in GC tissues, associated with chemosensitivity, and that low expression of LRIG1 predicted poor prognosis in GC individuals. Moreover, LRIG1 was correlated with MDR of GC and upregulation of LRIG1 could increase sensitivity to chemotherapeutic drugs.
Accumulating evidence indicates that miRNA play important roles in the MDR of various cancers. miRNA, including miR‐27a, miR‐497, miR‐363, miR‐181b, miR‐15b and miR‐16, have been reported to be implicated in the MDR of GC.16, 17, 18, 33, 35, 36 Our previous studies identified 11 miRNA, which regulate MDR in GC, using high‐throughput functional screening, and further confirmed that miR‐27b/CCNG1/P53/miR‐508‐5p axis plays important roles in GC‐associated MDR.20, 37 Here we identified miR‐20a as an upstream miRNA of LRIG1, playing an important role in GC drug resistance. miR‐20a suppressed LRIG1 expression by directly targeting its 3′‐UTR, and was inversely expressed with LRIG1 in GC specimens. In addition, restoration of miR‐20a expression significantly reduced LRIG1‐induced GC cell chemosensitivity.
miR‑20a, a member of the miR‑17‑92 cluster, has already been widely studied in different cancers, acting as an oncogene in tumor development and progression.38, 39, 40, 41 According to Change et al (2013), ectopic expression of miR‐20a could lead to epithelial‐mesenchymal transition and promote metastasis of gallbladder carcinoma, by directly binding the 3′‐UTR of Smad7, and subsequently enhancing nuclear translocation of β‐catenin.38 More recently, Liu et al39 demonstrated that miR‐20a‐mediated autophagy defect might be a new mechanism underlying the oncogenic function of miRNA during breast tumorigenesis. Furthermore, miR‐20a was shown to modulate sensitivity to some anti‐cancer drugs, such as the anti‐leukemia drug etoposide (VP‐16), 5‐FU, oxaliplatin (L‐OHP) and teniposide (VM‐26). For example, suppression of miR‐20a expression by treatment with either miR‐20a inhibitor or oridonin sensitized leukemia cells to VP‐16.40 Chai et al (2011) report that miR‐20a regulated chemotherapeutic sensitivity of colorectal adenocarcinoma cancer cells to the most used cancer chemotherapy agents.42 Furthermore, miR‐20a enhanced cisplatin resistance of GC by targeting cylindromatosis.43 This study further confirmed that miR‐20a is involved in the MDR of GC, and acts as an oncogenic miRNA.
Currently, the tumor suppressive function of LRIG1 is mainly attributed to its inhibitory effect on EGFR signaling.4, 5, 6 Over the last decade, it has been shown that LRIG1 binds to EGFR and attenuates EGFR signaling through both receptor degradation and catalytic inhibition.4, 5, 6, 10 EGFR is a well‐studied, versatile signal transducer that is overexpressed in many types of tumor cells, including lung, colon and prostatic carcinoma, and upregulation of EGFR is associated with poor clinical prognosis.26, 44, 45 EGFR mediates signals that stimulate proliferation, migration and metastasis in many tumor types, and its signal transduction is regulated by stimulatory and inhibitory inputs.26, 46 Yu et al demonstrate that PI3K/AKT pathway could be inactivated by doxorubicin and etoposide, and wortmannin could increase the sensitivity of GC cells to chemotherapy.24 Zhang et al demonstrated that miR‐939 contributed to chemosensitivity by inhibiting the SLC34A2/Raf/MEK/ERK pathway.47 The present study showed that the miR‐20a/LRIG1 axis might regulate GC cell drug resistance by increasing EGFR activity and through secondary promotion of PI3K/AKT and MAPK/ERK signaling pathways.
In conclusion, the results obtained in the present study reveal that low expression of LRIG1 in GC drug resistance might reflect the increased levels of miR‐20a, which could significantly promote MDR in GC. Furthermore, the miR‐20a/LRIG1 axis might regulate GC drug resistance through EGFR‐mediated PI3K/AKT and MAPK/ERK signaling. The newly identified miR‐20a/LRIG1/EGFR link provides a novel potential therapeutic strategy for the future treatment of GC patients with MDR.
CONFLICT OF INTEREST
Authors declare no conflicts of interest for this article.
Supporting information
Zhou L, Li X, Zhou F, et al. Downregulation of leucine‐rich repeats and immunoglobulin‐like domains 1 by microRNA‐20a modulates gastric cancer multidrug resistance. Cancer Sci. 2018;109:1044–1054. https://doi.org/10.1111/cas.13538
Funding information
National Natural Science Foundation of China grant numbers 81402337 and 81402387.
Zhou, Li, Zhou and Jin equally contributed to this study.
Contributor Information
Yulong Shang, Email: shangyul870222@163.com.
Xiaoping Zou, Email: 13770771661@163.com.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 3. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654‐2664. [DOI] [PubMed] [Google Scholar]
- 4. Gur G, Rubin C, Katz M, et al. LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. EMBO J. 2004;23:3270‐3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Powell AE, Wang Y, Li Y, et al. The pan‐ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Poulin EJ, Coffey RJ. LRIG1 is a triple threat: ERBB negative regulator, intestinal stem cell marker and tumour suppressor. Br J Cancer. 2013;108:1765‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krig SR, Frietze S, Simion C, et al. Lrig1 is an estrogen‐regulated growth suppressor and correlates with longer relapse‐free survival in ERalpha‐positive breast cancer. Mol Cancer Res. 2011;9:1406‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson PA, Ljuslinder I, Tsavachidis S, et al. Loss of LRIG1 locus increases risk of early and late relapse of stage I/II breast cancer. Cancer Res. 2014;74:2928‐2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindquist D, Nasman A, Tarjan M, et al. Expression of LRIG1 is associated with good prognosis and human papillomavirus status in oropharyngeal cancer. Br J Cancer. 2014;110:1793‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheu JJ, Lee CC, Hua CH, et al. LRIG1 modulates aggressiveness of head and neck cancers by regulating EGFR‐MAPK‐SPHK1 signaling and extracellular matrix remodeling. Oncogene. 2014;33:1375‐1384. [DOI] [PubMed] [Google Scholar]
- 11. Stutz MA, Shattuck DL, Laederich MB, Carraway KL III, Sweeney C. LRIG1 negatively regulates the oncogenic EGF receptor mutant EGFRvIII. Oncogene. 2008;27:5741‐5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai L, McEachern D, Yang CY, Lu J, Sun H, Wang S. LRIG1 modulates cancer cell sensitivity to Smac mimetics by regulating TNFalpha expression and receptor tyrosine kinase signaling. Cancer Res. 2012;72:1229‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu B, Guo Z, Dong H, et al. LRIG1, human EGFR inhibitor, reverses multidrug resistance through modulation of ABCB1 and ABCG2. Brain Res. 2015;1611:93‐100. [DOI] [PubMed] [Google Scholar]
- 14. Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153:516‐519. [DOI] [PubMed] [Google Scholar]
- 15. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riquelme I, Letelier P, Riffo‐Campos AL, Brebi P, Roa JC. Emerging Role of miRNAs in the Drug Resistance of Gastric Cancer. Int J Mol Sci. 2016;17:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song S, Ajani JA. The role of microRNAs in cancers of the upper gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2013;10:109‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishimoto T, Baba H, Izumi D, et al. Current perspectives toward the identification of key players in gastric cancer microRNA dysregulation. Int J Cancer. 2016;138:1337‐1349. [DOI] [PubMed] [Google Scholar]
- 19. Zhou L, Shang Y, Liu C, et al. Overexpression of PrPc, combined with MGr1‐Ag/37LRP, is predictive of poor prognosis in gastric cancer. Int J Cancer. 2014;135:2329‐2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shang Y, Zhang Z, Liu Z, et al. miR‐508‐5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33:3267‐3276. [DOI] [PubMed] [Google Scholar]
- 21. Liang J, Ge F, Guo C, et al. Inhibition of PI3K/Akt partially leads to the inhibition of PrP(C)‐induced drug resistance in gastric cancer cells. FEBS J. 2009;276:685‐694. [DOI] [PubMed] [Google Scholar]
- 22. Zhou L, Zhao X, Han Y, et al. Regulation of UHRF1 by miR‐146a/b modulates gastric cancer invasion and metastasis. FASEB J. 2013;27:4929‐4939. [DOI] [PubMed] [Google Scholar]
- 23. Dong J, Wang R, Ren G, et al. HMGA2‐FOXL2 axis regulates metastases and epithelial‐to‐mesenchymal transition of chemoresistant gastric cancer. Clin Cancer Res. 2017;23:3461‐3473. [DOI] [PubMed] [Google Scholar]
- 24. Yu HG, Ai YW, Yu LL, et al. Phosphoinositide 3‐kinase/Akt pathway plays an important role in chemoresistance of gastric cancer cells against etoposide and doxorubicin induced cell death. Int J Cancer. 2008;12:433‐443. [DOI] [PubMed] [Google Scholar]
- 25. Sun L, Liu L, Liu X, et al. MGr1‐Ag/37LRP induces cell adhesion‐mediated drug resistance through FAK/PI3K and MAPK pathway in gastric cancer. Cancer Sci. 2014;105:651‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roskoski R Jr. The ErbB/HER family of protein‐tyrosine kinases and cancer. Pharmacol Res. 2014;79:34‐74. [DOI] [PubMed] [Google Scholar]
- 27. Jin W, Liao X, Lv Y, et al. MUC1 induces acquired chemoresistance by upregulating ABCB1 in EGFR‐dependent manner. Cell Death Dis. 2017;8:e2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hour TC, Chung SD, Kang WY, et al. EGFR mediates docetaxel resistance in human castration‐resistant prostate cancer through the Akt‐dependent expression of ABCB1 (MDR1). Arch Toxicol. 2015;89:591‐605. [DOI] [PubMed] [Google Scholar]
- 29. Yan Z, Jiang J, Li F, et al. Adenovirus‐mediated LRIG1 expression enhances the chemosensitivity of bladder cancer cells to cisplatin. Oncol Rep. 2015;33:1791‐1798. [DOI] [PubMed] [Google Scholar]
- 30. Morrison MM, Williams MM, Vaught DB, et al. Decreased LRIG1 in fulvestrant‐treated luminal breast cancer cells permits ErbB3 upregulation and increased growth. Oncogene. 2016;35:1143‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller JK, Shattuck DL, Ingalla EQ, et al. Suppression of the negative regulator LRIG1 contributes to ErbB2 overexpression in breast cancer. Cancer Res. 2008;68:8286‐8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shattuck DL, Miller JK, Laederich M, et al. LRIG1 is a novel negative regulator of the Met receptor and opposes Met and Her2 synergy. Mol Cell Biol. 2007;27:1934‐1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu W, Shan X, Wang T, Shu Y, Liu P. miR‐181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. Int J Cancer. 2010;127:2520‐2529. [DOI] [PubMed] [Google Scholar]
- 34. Hedman H, Henriksson R. LRIG inhibitors of growth factor signalling ‐ double‐edged swords in human cancer? Eur J Cancer. 2007;43:676‐682. [DOI] [PubMed] [Google Scholar]
- 35. Xia L, Zhang D, Du R, et al. miR‐15b and miR‐16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372‐379. [DOI] [PubMed] [Google Scholar]
- 36. Zhang PF, Sheng LL, Wang G, et al. miR‐363 promotes proliferation and chemo‐resistance of human gastric cancer via targeting of FBW7 ubiquitin ligase expression. Oncotarget. 2016;7:35284‐35292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shang Y, Feng B, Zhou L, et al. The miR27b‐CCNG1‐P53‐miR‐508‐5p axis regulates multidrug resistance of gastric cancer. Oncotarget. 2016;7:538‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang Y, Liu C, Yang J, et al. MiR‐20a triggers metastasis of gallbladder carcinoma. J Hepatol. 2013;59:518‐527. [DOI] [PubMed] [Google Scholar]
- 39. Liu L, He J, Wei X, et al. MicroRNA‐20a‐mediated loss of autophagy contributes to breast tumorigenesis by promoting genomic damage and instability. Oncogene. 2017;36:5874‐5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weng H, Huang H, Dong B, Zhao P, Zhou H, Qu L. Inhibition of miR‐17 and miR‐20a by oridonin triggers apoptosis and reverses chemoresistance by derepressing BIM‐S. Cancer Res. 2014;74:4409‐4419. [DOI] [PubMed] [Google Scholar]
- 41. Wang Z, Wang B, Shi Y, et al. Oncogenic miR‐20a and miR‐106a enhance the invasiveness of human glioma stem cells by directly targeting TIMP‐2. Oncogene. 2015;34:1407‐1419. [DOI] [PubMed] [Google Scholar]
- 42. Chai H, Liu M, Tian R, Li X, Tang H. miR‐20a targets BNIP2 and contributes chemotherapeutic resistance in colorectal adenocarcinoma SW480 and SW620 cell lines. Acta Biochim Biophys Sin (Shanghai). 2011;43:217‐225. [DOI] [PubMed] [Google Scholar]
- 43. Zhu M, Zhou X, Du Y, et al. miR‐20a induces cisplatin resistance of a human gastric cancer cell line via targeting CYLD. Mol Med Rep. 2016;14:1742‐1750. [DOI] [PubMed] [Google Scholar]
- 44. Burgess AW, Cho HS, Eigenbrot C, et al. An open‐and‐shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541‐552. [DOI] [PubMed] [Google Scholar]
- 45. Guo G, Gong K, Wohlfeld B, Hatanpaa KJ, Zhao D, Habib AA. Ligand‐Independent EGFR Signaling. Cancer Res. 2015;75:3436‐3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang JX, Xu Y, Gao Y, et al. Decreased expression of miR‐939 contributes to chemoresistance and metastasis of gastric cancer via dysregulation of SLC34A2 and Raf/MEK/ERK pathway. Mol Cancer. 2017;16:18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


