Abstract
Lung cancer remains the leading cause of cancer‐related death worldwide. Previous studies have shown that the novel KIAA0247 gene potentially targeted by the tumor suppressor p53 may inhibit the development of several cancers. However, the exact function of KIAA0247 in non‐small‐cell lung cancer (NSCLC) is unknown. The purpose of the present study was to clarify the role of KIAA0247 in NSCLC. KIAA0247 expression was evaluated in tumors and adjacent normal tissues of 197 NSCLC patients by immunohistochemistry and real‐time PCR and analyzed for association with clinicopathological parameters. Results indicated that KIAA0247 levels positively correlated with cell differentiation (P < .001) and patient survival (P < .0001) and negatively correlated with lymph node metastasis (P < .001) and advanced p‐TNM stage (P < .001). In cultured NSCLC cell lines, KIAA0247 overexpression inhibited cell migration, invasion, and proliferation and downregulated the expression of Jagged1, Notch1 intracellular domain (NICD), Snail, cyclin D1, RhoA, RhoC, and MMP9, while upregulating that of E‐cadherin and p21. The Notch inhibitor DAPT reduced the biological effects of KIAA0247 knockdown, suggesting that KIAA0247 decreased the carcinogenic activity of NSCLC cells through downregulation of Notch signaling. Our results indicate that KIAA0247 inhibits NSCLC progression by reducing the metastatic potential of cancer cells through downregulation of the Notch pathway, which may underlie the association of KIAA0247 expression with favorable clinicopathological characteristics of NSCLC patients. These findings suggest that KIAA0247 is a candidate prognostic biomarker and potential therapeutic target in NSCLC.
Keywords: cell migration, metastasis inhibition, metastasis‐associated gene, p53‐related gene, respiratory organ
1. INTRODUCTION
Lung cancer is the second most frequently diagnosed cancer and the leading cause of all cancer‐related deaths worldwide, with a steadily increasing incidence. Approximately 85%‐90% of lung cancer cases are diagnosed as non‐small‐cell lung cancer (NSCLC).1, 2, 3 When possible, surgery is the best treatment strategy for NSCLC; however, even patients treated with curative intent may have local or systemic recurrence and ultimately succumb to the disease.4, 5 The prognosis of patients with NSCLC principally correlates with tumor metastasis, which is associated with transcriptional regulation of certain critical genes; therefore, it is necessary to identify these pro‐metastatic genes and investigate their effects in detail.
Notch signaling has been shown to participate in development and cell proliferation, differentiation, and apoptosis, and several studies have shown that deregulation of the Notch pathway is involved in such malignancies as breast cancer and T‐cell leukemia.6 The role of Notch in lung cancer was experimentally shown in a transgenic mouse model, where activated Notch overexpressed in the alveolar epithelium cooperated with the Myc pathway in promoting NSCLC development.7 Another study indicated that Notch1 suppressed p53‐mediated apoptosis through the regulation of p53 stability and was required for the growth of lung adenocarcinoma.8 Notch signaling is activated by ligand‐receptor binding involving five ligands (Delta‐like 1, 3, and 4, and Jagged‐1 and ‐2) and four receptors (Notch1‐4). Ligand binding induces a conformational change in Notch, leading to the exposure of the S2 site for the sequential cleavage by a protease of the disintegrin and metalloproteinase (ADAM) family and the gamma‐secretase complex. As a result, the Notch intracellular domain (NICD) is liberated and translocated to the nucleus9 where it induces the expression of target oncogenes coding for cyclin D1, c‐Myc, p21, p27, nuclear factor‐kappa B (NF‐κB), survivin, slug, and Nanog,10 which promote carcinogenesis.
KIAA0247 is a novel gene encoding a 303‐amino acid single‐pass type I membrane protein, which is highly conserved among species. Although KIAA0247 is not mutated in cancers, it has been suggested to play a role in oncogenesis because of a p53‐responsive element found in its promoter region, indicating that KIAA0247 is a prospective target for the tumor suppressor p53.11 KIAA0247 has also been named DRAGO (drug‐activated gene overexpressed) because treatment with certain drugs (cisplatin, alkylating agents, antimetabolites, topoisomerase II inhibitors, taxanes, and nutlin‐3) induces its expression in HCT116 p53+/+ cells but not in HCT116 p53−/− cells.11 KIAA0247 overexpression is associated with the therapeutic benefits of 5‐fluorouracil, and the presence of KIAA0247 mRNA in fecal samples of colon cancer patients correlates with a more favorable prognosis.12 In ovarian cancer, advanced‐stage tumors express approximately 30% less KIAA0247 mRNA compared to levels in early‐stage tumors.11 Another study showed that KIAA0247 mRNA was downregulated in glioma compared to normal brain tissue, whereas KIAA0247 overexpression suppressed the proliferation and angiogenesis of glioma cell lines and promoted apoptosis through inactivation of the AKT and Stat3 signaling pathways.13 The KIAA0247 gene is located on human chromosome 14q24.1, which also contains the SERPINA1 gene responsible for α1‐antitrypsin deficiency that leads to lung tissue damage, pulmonary emphysema, and lung cancer.14
However, the biological function of KIAA0247 in lung cancer is currently unclear, and there are no data regarding KIAA0247 expression pattern or its clinical significance in NSCLC. In the present study, we investigated the role of KIAA0247 in NSCLC by examining KIAA0247 mRNA and protein expression in cancer tissues by real‐time PCR and immunohistochemistry. We also analyzed the effects of KIAA0247 levels on the proliferation, migration, and invasion of lung cancer cell lines and explored the underlying molecular mechanisms.
2. MATERIALS AND METHODS
2.1. Patients and specimens
Data on a total of 197 NSCLC cases documented from 2013 to 2015 were retrieved from the Pathology Archive of the First Affiliated Hospital of China Medical University. All enrolled patients underwent curative surgical resection without having prior chemotherapy or radiation therapy. Clinicopathological information was obtained from the patients’ records. This study was approved by the Medical Research Ethics Committee of China Medical University and informed consent was obtained from all patients.
2.2. Cell culture and treatment
Lung cancer cell lines A549, H292, H1299, H460, H661, and SK‐MES‐1 were purchased from the Cell Bank of the China Academy of Sciences (Shanghai, China), and normal bronchial epithelial HBE cells were obtained from ATCC (Manassas, VA, USA). A549, H292, H1299, H460, and H661 cells were cultured in RPMI 1640 medium (Gibco, Waltham, MA, USA), SK‐MES‐1 cells were cultured in minimal essential medium (Gibco) containing 1.5 g/L NaHCO3 and 0.11 g/L sodium pyruvate, and HBE cells were cultured in DMEM (Gibco) containing 1.5 g/L NaHCO3; all media were supplemented with 10% FBS. The cells were maintained in a 5% CO2 incubator at 37°C.
Cell transfection was carried out using Lipofectamine 3000 reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer's instructions. In KIAA0247 knockdown experiments, cells were transfected with KIAA0247‐specific siRNA and scrambled control siRNA (GenePharma, Shanghai, China) for 48 hours. For KIAA0247 overexpression, cells were transfected with a KIAA0247 expression plasmid and the corresponding empty pCNA3.0 vector, which were kindly donated by Massimo Broggini (Istituto di Ricerche Farmacologiche, Ranica, Italy).11
To inhibit Notch signaling, cells were treated with 2 μmol/L DAPT (Selleck, Houston, TX, USA), a γ‐secretase inhibitor that blocks the Notch pathway. DAPT was dissolved in DMSO and added 6 hours after transfection for 36 hours, whereas the same volume of DMSO was added to control cells.
2.3. Immunohistochemistry
Surgically excised tumor specimens were fixed in 10% neutral formalin, embedded in paraffin, and cut into 4‐μm‐thick sections. The sections were deparaffinized in xylene, rehydrated in a graded alcohol series, and treated with 0.01 mol/L citrate buffer (Maixin‐Bio, Shenzhen, China) under high pressure for 3 minutes. Endogenous peroxidase activity was blocked by hydrogen peroxide (0.3%), and the sections were incubated with normal goat serum (5%) at 20°C for 30 minutes to reduce non‐specific binding. Immunostaining with KIAA0247 rabbit polyclonal antibodies (1:100 dilution; Sigma, St Louis, MO, USA) was carried out at 4°C for 18 hours, and the reaction was visualized using the Elivision super HRP (Mouse/Rabbit) IHC Kit (Maixin‐Bio) and 3,3′‐diaminobenzidine (DAB); nuclei were counterstained with hematoxylin. The sections were dehydrated in ethanol before mounting.
The intensity of KIAA0247 staining was scored as follows: 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (high staining). Percentage scores were assigned as follows: 0 (0%), 1 (1%‐30%), 2 (31%‐70%), and 3 (71%‐100%). The scores of each tumor sample were multiplied to give a final score ranging from 0 to 9; tumor samples with scores >3 were considered to show high KIAA0247 expression, and those with scores ≤3 were considered to show low KIAA0247 expression.
2.4. Immunocytochemistry
Lung cancer cells cultured in 24‐well plates for 24 hours were fixed in paraformaldehyde (2%) for 15 minutes, blocked in BSA (5%) for 2 hours, and incubated with the anti‐KIAA0247 antibodies (1:100) for 18 hours and then with a tetramethylrhodamine (TRITC)‐conjugated secondary antibody for 2 hours; nuclei were counterstained with DAPI. Cell images were captured using an Olympus FV1000 laser‐scanning confocal microscope (Olympus, Tokyo, Japan).
2.5. Quantitative real‐time PCR
Quantitative real‐time PCR was carried out in a 7900HT Fast Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA) using SYBR Green PCR master mix in a total volume of 20 μL under the following cycling conditions: 95°C for 30 seconds, and 45 cycles of 95°C for 5 seconds and 60°C for 30 seconds. The dissociation step was used to generate a melting curve and confirm the specificity of amplification. Relative gene expression was calculated by the 2−ΔΔCt method; the β‐actin‐encoding gene was used as reference. Primer sequences were as follows: KIAA0247 forward, 5′‐GGGATTGAGCACCCTGTACT‐3′; KIAA0247 reverse, 5′‐CTAGCTGTCCAACAGGCAGA‐3′; β‐actin forward, 5′‐ATAGCACAGCCTGGATAGCAACGTAC‐3′; β‐actin reverse, 5′‐CACCTTCTACAATGAGCTGCGTGTG ‐3′. All experiments were carried out in triplicate.
2.6. Western blotting
Expression of proteins directly influencing cell migration and invasion15, 16, 17, 18, 19 and those involved in cell proliferation and cell cycle progression20, 21, 22, 23, 24 was analyzed by western blotting. Total proteins from cells and tumor tissues were extracted in lysis buffer and quantified using the Bradford method. Proteins (80 μg/lane) were separated by SDS‐PAGE (10% gels), transferred to PVDF membranes (Millipore, Billerica, MA, USA), and incubated overnight at 4°C with appropriate primary antibodies (Table 1) and then with HRP‐conjugated anti‐mouse/rabbit IgG at 37°C for 2 hours. Immune reactivity was detected by ECL (Thermo Fisher Scientific, Waltham, MA, USA) using a BioImaging System (UVP Inc., Upland, CA, USA). Relative protein expression was calculated after normalization to GAPDH used as a loading control.
Table 1.
Antibodies used for western blotting in the present study
| Antibody name | Source | Catalog number | Host | Dilution |
|---|---|---|---|---|
| KIAA0247 | Sigma‐Aldrich | HPA030464 | Rabbit | 1:500 |
| GAPDH | Beyotime | AF0006 | Mouse | 1:1000 |
| RhoA | Cell Signaling Technology Inc. | 2117 | Rabbit | 1:500 |
| RhoB | Cell Signaling Technology Inc. | 2098 | Rabbit | 1:500 |
| RhoC | Cell Signaling Technology Inc. | 3430 | Rabbit | 1:500 |
| MMP9 | Cell Signaling Technology Inc. | 13667 | Rabbit | 1:500 |
| CyclinD1 | Cell Signaling Technology Inc. | 2978 | Rabbit | 1:500 |
| CDK4 | Cell Signaling Technology Inc. | 12790 | Rabbit | 1:500 |
| p21 | Cell Signaling Technology Inc. | 2947 | Rabbit | 1:1000 |
| c‐myc | Cell Signaling Technology Inc. | 13987 | Rabbit | 1:500 |
| E‐Cadherin | Cell Signaling Technology Inc. | 3195 | Rabbit | 1:500 |
| N‐Cadherin | Cell Signaling Technology Inc. | 13116 | Rabbit | 1:500 |
| Vimentin | Cell Signaling Technology Inc. | 5741 | Rabbit | 1:1000 |
| Snail | Cell Signaling Technology Inc. | 3879 | Rabbit | 1:500 |
| Slug | Cell Signaling Technology Inc. | 9585 | Rabbit | 1:500 |
| Jagged1 | Cell Signaling Technology Inc. | 2620 | Rabbit | 1:500 |
| NICD | Wanlei Bio | wl03097a | Rabbit | 1:1000 |
| Anti‐mouse IgG | Cell Signaling Technology Inc. | 7076 | Horse | 1:1000 |
| Anti‐rabbit IgG | Cell Signaling Technology Inc. | 7074 | Goat | 1:1000 |
2.7. Cell proliferation and colony formation assays
Cell proliferation was evaluated daily for 5 days using the colorimetric MTT assay. In brief, cells were seeded at the concentration of 3000 cells/100 μL per well in 96‐well culture plates, incubated for appropriate times, and MTT solution (10 μL/well) was added for 4 hours. MTT crystals in living cells were solubilized with 150 μL DMSO and optical density was measured at 490 nm using a microplate reader.
For colony formation assay, cells were seeded on 6‐cm2 culture dishes at a density of 500 cells/dish and incubated for 10‐15 days. The cells were then washed with PBS, stained with Giemsa solution, and colonies with more than 50 cells were counted. At least 3 independent experiments were carried out under identical conditions.
2.8. Cell cycle analysis
Cells were fixed in 75% ethanol 48 hours after transfection, washed with PBS, stained with 5 mg/mL propidium iodide in PBS supplemented with RNase A for 30 minutes at 37°C, and analyzed by flow cytometry using a FACSCalibur flow cytometer with CellQuest 3.0 software (BD Biosciences, Billerica, MA, USA).
2.9. Cell migration and invasion analysis
Cell migration and invasion assays were carried out in 24‐well Transwell chambers containing inserts with a pore size of 8 μm (Costar, Washington, DC, USA). For the invasion assay, the inserts were coated with 100 μL Matrigel (1:9 dilution; BD Biosciences). Cells were trypsinized 24 hours after transfection, and 5 × 104 cells in 100 μL medium supplemented with 2% FBS were transferred to the upper Transwell chamber, whereas 600 μL medium supplemented with 10% FBS was added to the lower chamber. After incubation for 24 hours, cells on the upper membrane surface were removed with a cotton tip, and those that passed through the membrane were fixed with polyformaldehyde and stained with hematoxylin. Number of migrated/invaded cells was counted in 10 randomly selected fields under a microscope at high magnification. All experiments were repeated independently at least three times under identical conditions.
2.10. Statistical analysis
SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for data processing. Survival of NSCLC patients was analyzed using the Kaplan‐Meier estimator.25, 26 Correlations between KIAA0247 expression and clinicopathological features were examined by chi‐squared test, and differences between cell groups were compared by paired t test. Two‐sided P‐values <.05 were considered statistically significant.
3. RESULTS
3.1. Downregulation of KIAA0247 expression has clinical significance
Analysis of KIAA0247 protein expression by immunocytochemistry indicated that KIAA0247 was localized both in the cytoplasm and in the nuclei of NSCLC A549, H292, H1299, H460, H661, and SK‐MES‐1 cells, as well as in normal bronchial epithelial HBE cells (Figure 1A). We next examined KIAA0247 protein levels in the same lung cancer cell lines (Figure 1B) and quantified relative expression levels. A549 and H1299 cells showed the highest KIAA0247 expression levels, whereas H292 and H661 cells showed the lowest. Because of the relationship between KIAA0247 and p53, and based on these basal expression levels, A549 and H1299 cells were used for subsequent experiments.
Figure 1.
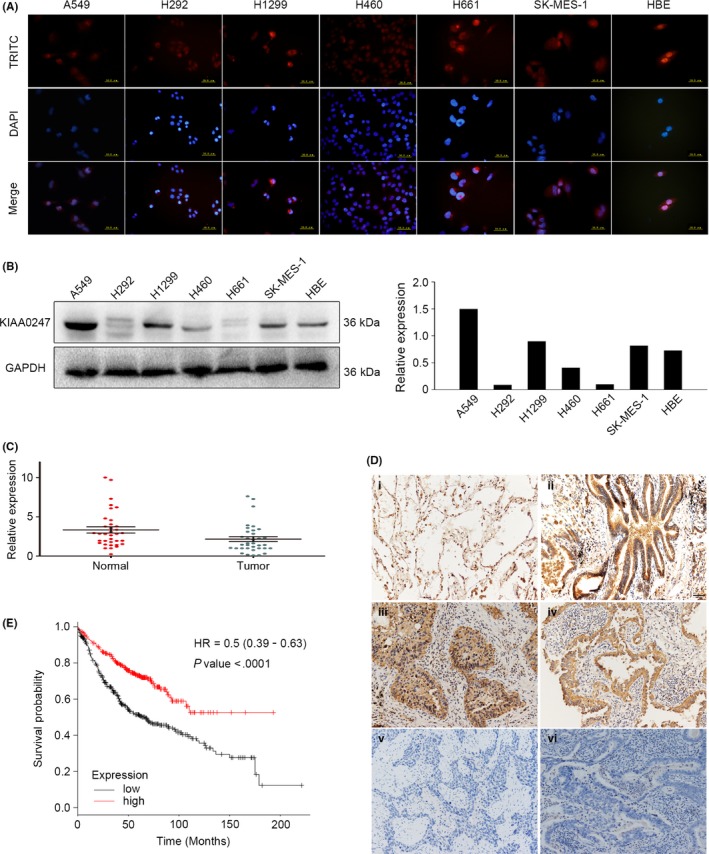
Expression pattern of KIAA0247. A, KIAA0247 expression in non‐small‐cell lung cancer (NSCLC) cell lines analyzed by immunocytochemistry using tetramethylrhodamine (TRITC)‐labeled antibodies; nuclei were stained with DAPI. B, KIAA0247 protein levels in six lung cancer cell lines and a normal bronchial cell line (HBE) assessed by immunoblotting and analyzed with ImageJ software. C, Relative expression of KIAA0247 mRNA in 35 paired NSCLC samples (green points) and adjacent normal lung tissue (red points). D, KIAA0247 protein expression analyzed by immunohistochemistry in (i) alveolar and (ii) normal bronchial epithelial cells; (iii) well‐differentiated squamous cell carcinoma, and (iv) adenocarcinoma; and (v) poorly differentiated squamous cell carcinoma and (vi) adenocarcinoma. Magnification, ×200. E, Survival of NSCLC patients with high and low KIAA0247 expression analyzed based on Kaplan‐Meier curves; hazard ratio (HR) and P‐value are indicated
Furthermore, real‐time PCR analysis of 35 NSCLC specimens and the corresponding normal tissues indicated that the expression of KIAA0247 mRNA was lower in tumors than in the matched surrounding tissue (Figure 1C). This observation was supported by the downregulated expression of KIAA0247 protein in tumors compared to that in adjacent normal lung tissue which showed strong staining, as indicated by immunohistochemistry analysis of 197 paired NSCLC/normal tissue specimens (Figure 1D).
We also investigated the relationship between KIAA0247 protein expression and clinical parameters. As shown in Table 2, KIAA0247 levels negatively correlated with poor differentiation (P < .001), lymph node metastasis (P < .001), and advanced p‐TNM stage (P < .001), but did not significantly vary depending on age (P = .865), gender (P = .119), tumor histological type (P = .311), and size (P = .134). Regarding the correlation of KIAA0247 expression with NSCLC prognosis, Kaplan‐Meier analysis in adenocarcinoma patients of all grades, stages, genders, smoking history, surgery success, chemotherapy, and radiotherapy showed that high KIAA0247 levels were associated with survival (Figure 1E).
Table 2.
Association of KIAA0247 expression with clinical and pathological characteristics of NSCLC patients
| Clinicopathological characteristics | Total N | KIAA0247‐positive | KIAA0247‐negative | P‐value |
|---|---|---|---|---|
| Age (years) | ||||
| ≤60 | 109 | 31 | 78 | .865 |
| >60 | 88 | 26 | 62 | |
| Gender | ||||
| Male | 127 | 32 | 95 | .119 |
| Female | 70 | 25 | 45 | |
| Histological type | ||||
| Squamous cell carcinoma | 101 | 26 | 75 | .311 |
| Adenocarcinoma | 96 | 31 | 65 | |
| Differentiation | ||||
| Well‐Moderate | 126 | 49 | 77 | <.001 |
| Poor | 71 | 8 | 63 | |
| Tumor size (cm) | ||||
| ≤3 | 108 | 36 | 72 | .134 |
| >3 | 89 | 21 | 68 | |
| Lymph node metastasis | ||||
| Negative | 113 | 46 | 67 | <.001 |
| Positive | 84 | 11 | 73 | |
| TNM stage | ||||
| I | 83 | 35 | 48 | <.001 |
| II‐III | 114 | 22 | 92 | |
NSCLC, non‐small‐cell lung cancer.
3.2. KIAA0247 inhibits migration and invasion of lung cancer cells
As KIAA0247 levels correlated with NSCLC progression, we next explored the biological function of KIAA0247 in lung cancer cells by genetically manipulating KIAA0247 expression. KIAA0247 knockdown with siRNA promoted, whereas KIAA0247 overexpression inhibited, migration and invasion of A549 and H1299 cells compared with control cells, and the difference was statistically significant (Figure 2A). Consistent with these results, variations in KIAA0247 protein levels affected the expression of proteins involved in cell migration and invasion. Thus, RhoA, RhoC, and MMP9 were upregulated and RhoB was downregulated by KIAA0247 knockdown, whereas KIAA0247 overexpression caused the opposite effects (Figure 2B,C). Taken together, these findings show that KIAA0247 suppresses the migration and invasion of lung cancer cells.
Figure 2.
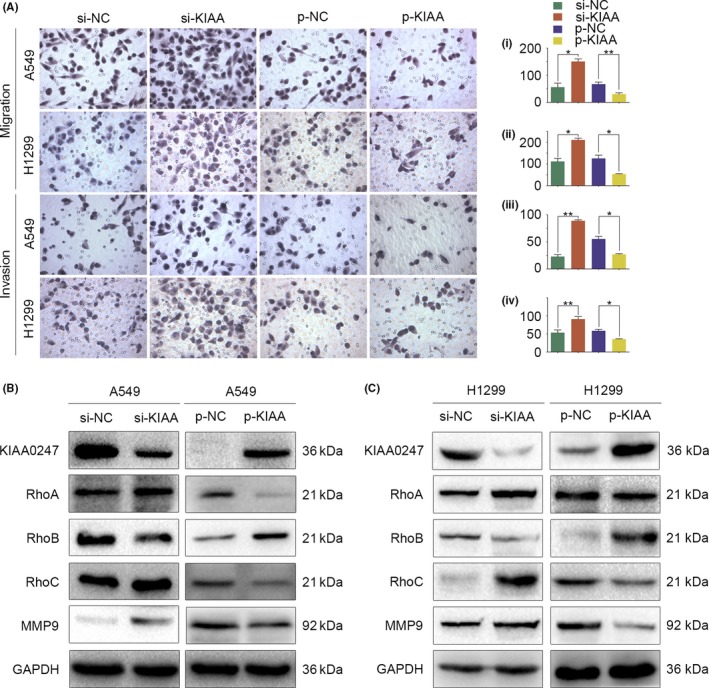
Effect of KIAA0247 expression on the migration and invasion of non‐small‐cell lung cancer (NSCLC) cells. A549 and H1299 cells were transfected with KIAA0247‐specific siRNA (si‐KIAA0247) or control siRNA, or with a KIAA0247 expression plasmid (p‐KIAA0247) or vehicle (control). A, Cell migration analyzed by Transwell migration assay; cells that migrated to the lower chamber were stained with hematoxylin and counted. *P < .05; **P < .01. (i) A549 migration: si‐KIAA0247 vs control siRNA, P = .024; p‐KIAA0247 vs control, P = .005. (ii) H1299 migration: si‐KIAA0247 vs control siRNA, P = .046; p‐KIAA0247 vs control, P = .047. (iii) A549 invasion: si‐KIAA0247 vs control siRNA, P = .005; p‐KIAA0247 vs control P = .035. (iv) H1299 invasion: si‐KIAA0247 vs control siRNA, P = .007; p‐KIAA0247 vs control, P = .023. (B,C) Expression of KIAA0247 and cell migration‐ and invasion‐related proteins in transfected (B) A549 and (C) H1299 cells
3.3. KIAA0247 inhibits growth of lung cancer cells
Next, we analyzed the effect of KIAA0247 expression on the clinically relevant properties of cancer cells, such as colony formation and cell proliferation. In A549 and H1299 cells, KIAA0247 downregulation promoted clone formation, which was inhibited by KIAA0247 upregulation compared with control groups (Figure 3A). Consistent with these observations, KIAA0247 knockdown induced cell proliferation, whereas KIAA0247 overexpression reduced cell proliferation as evidenced by the MTT assay (Figure 3B), and blocked the cell cycle at the G1/S phase as evidenced by flow cytometry (Figure 3C). Analysis of proteins involved in cell proliferation and cell cycle progression showed that expression of cyclin D1, CDK4, and c‐Myc was upregulated and that of p21 was downregulated by KIAA0247 knockdown, whereas KIAA0247 overexpression had the opposite effects (Figure 3D,E).
Figure 3.
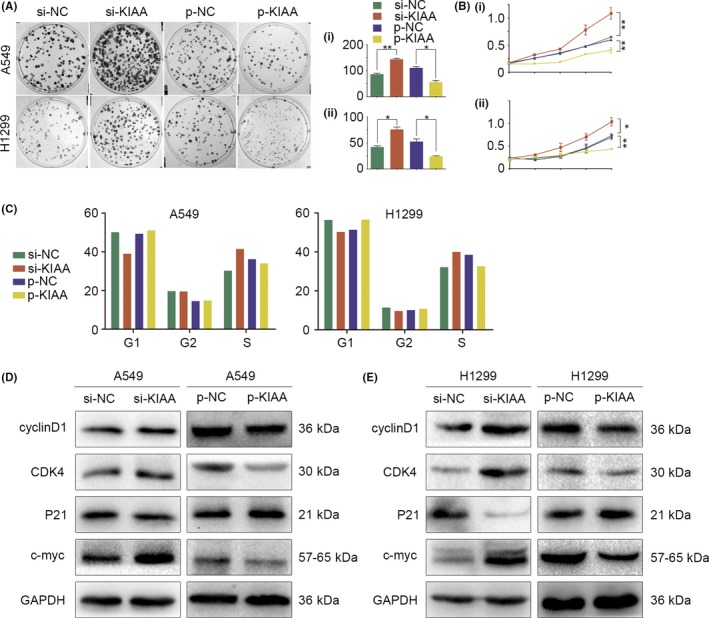
Effect of KIAA0247 expression on colony formation, proliferation, and cell cycle progression of non‐small‐cell lung cancer (NSCLC) cells. A549 and H1299 cells were transfected with KIAA0247‐specific siRNA (si‐KIAA0247) or control siRNA, or with a KIAA0247 expression plasmid (p‐KIAA0247) or vehicle (control). *P < .05 and **P < .01. A, Colony formation assay. (i) A549: si‐KIAA0247 vs control, P = .002; p‐KIAA0247 vs control, P = .04. (ii) H2199: si‐KIAA0247 vs control, P = .039; p‐KIAA0247 vs control, P = .02. B, MTT assay. (i) A549: si‐KIAA0247 vs control, P = .001; p‐KIAA0247 vs P = .002. (ii) H1299: si‐KIAA0247 vs control, P = .05; p‐KIAA0247 vs control, P = .001. C, Cell cycle analysis. KIAA0247 overexpression results in blockage of the cell cycle at the G1/S transition. D,E, Changes in expression of cell proliferation‐related proteins in (D) A549 and (E) H1299 cells
3.4. KIAA0247 negatively regulates the expression of proteins related to epithelial‐mesenchymal transition
To address the mechanism underlying the suppression of lung cancer proliferation and migration/invasion by KIAA0247, we examined the expression of epithelial‐mesenchymal transition (EMT)‐related proteins by western blotting. In A549 cells transfected with KIAA0247‐siRNA, protein levels of E‐cadherin and claudin‐1 were decreased, whereas those of N‐cadherin, vimentin, Snail, and Twist1 were increased. In contrast, cells transfected with the KIAA0247 overexpression plasmid showed the opposite expression profile of EMT proteins (Figure 4A). Similar effects were observed in H1299 cells (Figure 4B).
Figure 4.
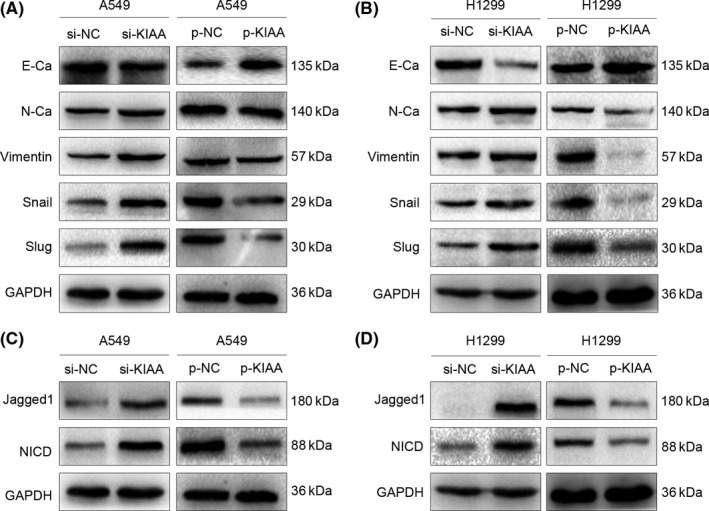
Effect of KIAA0247 on the expression of epithelial‐mesenchymal transition (EMT)‐ and Notch‐related proteins. A549 and H1299 cells were transfected with KIAA0247‐specific siRNA (si‐KIAA0247) or control siRNA, or with a KIAA0247 expression plasmid (p‐KIAA0247) or vehicle (control). A,B, Changes in the expression of EMT‐related proteins in (A) A549 and (B) H1299 cells. C,D, Changes in the expression of Notch‐related proteins in (C) A549 and (D) H1299 cells
3.5. KIAA0247 inhibits tumorigenic behavior and downregulates expression of EMT proteins in NSCLC cells through Notch signaling
The Notch pathway is implicated in the development of several cancers by affecting the expression of EMT‐related factors. As our results showed that KIAA0247 levels negatively correlated with the expression of N‐cadherin, RhoA, MMP9, cyclin D1, c‐Myc, Snail, and Twist1, and positively correlated with that of E‐cadherin and p21, we examined the involvement of KIAA0247 in the regulation of Notch signaling in NSCLC cells. As shown in Figure 4C, KIAA0247 knockdown upregulated, whereas KIAA0247 overexpression, downregulated the levels of Jagged1 and NICD in A549 cells. The same effects were detected in H1299 cells (Figure 4D).
To investigate whether the functional activity of KIAA0247 is mediated through Notch, we treated NSCLC cells with the Notch inhibitor DAPT. In A549 and H1299 cells transfected with KIAA0247‐siRNA, DAPT partially reduced the induction of cell migration, invasion, and proliferation (Figure 5) and reversed the effect of KIAA0247 knockdown on protein expression (Figure 6). These results suggested that KIAA0247 functions through inhibition of the Notch pathway.
Figure 5.
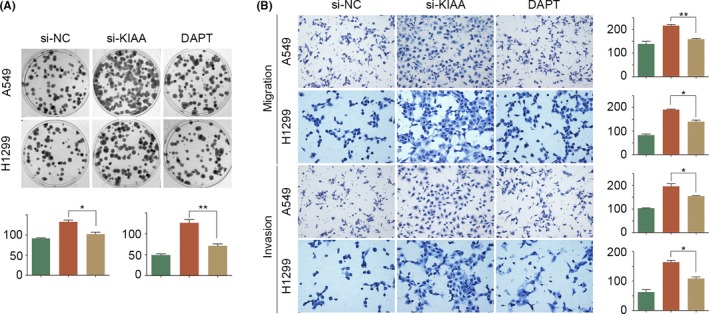
Effect of KIAA0247 with or without Notch inhibition on the behavior of non‐small‐cell lung cancer cells. A549 and H1299 cells transfected with si‐KIAA0247 were treated with or without Notch inhibitor DAPT and analyzed for (A) cell proliferation and (B) migration and invasion
Figure 6.
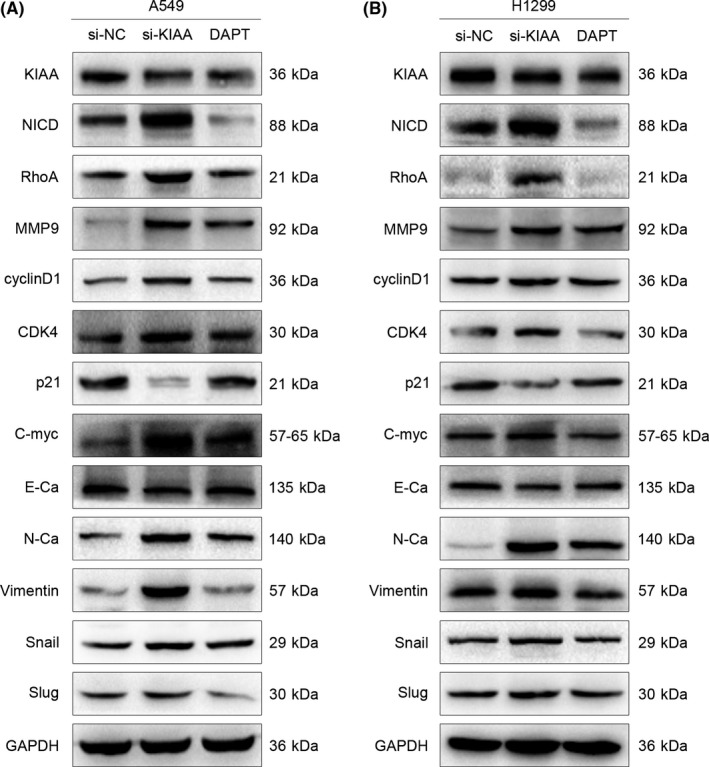
Effect of KIAA0247 with or without Notch inhibition on protein expression in non‐small‐cell lung cancer cells. A, A549 and (B) H1299 cells transfected with si‐KIAA0247 were treated with or without Notch inhibitor DAPT and analyzed by western blotting for expression of proteins involved in cell proliferation, migration, invasion, and epithelial‐mesenchymal transition
4. DISCUSSION
Lung cancer, especially NSCLC, remains the leading cause of cancer‐related death;1, 3 therefore, it is important to explore novel biomarkers that can be used for targeted therapy in NSCLC.
KIAA0247 expression is suggested to be regulated by the tumor suppressor p53 and thus may be involved in carcinogenesis. Previous studies have shown that low KIAA0247 mRNA levels in feces strongly correlate with colorectal tumor size and poor prognosis12 and that KIAA0247 expression inversely correlates with tumor stage in ovarian cancer11 and histological grade in glioma.13 Therefore, we hypothesized that KIAA0247 may also play an important role in the progression of NSCLC. To the best of our knowledge, this is the first demonstration of a correlation between KIAA0247 expression and clinicopathological features of NSCLC. We showed that high KIAA0247 expression positively correlated with cancer cell differentiation and survival of NSCLC patients, while negatively correlating with lymph node metastasis and p‐TNM stage, suggesting that increased KIAA0247 levels can predict a better outcome. Investigation of the underlying mechanisms using cultured NSCLC cells showed that KIAA0247 reduced cancer cell migration, invasion, and proliferation by regulating the expression of functional proteins influencing the malignant behavior of tumor cells.
The Rho family of GTPases comprises RhoA, RhoB, and RhoC, which regulate cell migration and adhesion.15, 16, 17, 27 In our study, KIAA0247 suppressed cell migration and invasion by downregulating RhoA, RhoC, and MMP9, and upregulating RhoB. Cell proliferation depends on progression through the G1 phase of the cell cycle, requiring the activity of cyclin D‐dependent kinase CDK4,23, 24 which is negatively regulated by cyclin‐dependent kinase inhibitors p16, p18, p21, and p27.24, 28 Our results showed that KIAA0247 decreased the expression of cyclin D1 and increased that of p21, suggesting that KIAA0247 inhibits cell proliferation by arresting the cell cycle at the G1/S transition point through regulation of the p21‐cyclin D1‐CDK4 axis.
Furthermore, KIAA0247 influenced the expression of several key EMT‐related proteins: E‐cadherin and claudin‐1 were upregulated and N‐cadherin, vimentin, Snail, and Twist1 were downregulated, suggesting a novel mechanism underlying the involvement of KIAA0247 in cancer. E‐cadherin and N‐cadherin are Ca2+‐dependent adhesion molecules that play a central role in the regulation of cell‐cell interactions.29 Upregulation of N‐cadherin expression is linked to NSCLC metastasis, whereas its downregulation was shown to reduce NSCLC proliferation and invasion.30 Snail and Twist1 are key transcription factors driving EMT and regulating cell adhesion and proliferation through Notch signaling.31
The role of the Notch pathway in cancer, defined by its effects on cell proliferation, migration, and EMT, is well established.31, 32, 33 Activation of Notch signaling occurs through the binding of a ligand such as Jagged1 to Notch receptors, which triggers downstream intracellular signaling events. Jagged1 is associated with multiple forms of cancer through its effects on cancer biology, including tumor cell growth, cancer stem cell maintenance, and metastasis. In our study, the expression of Jagged1 and NICD was downregulated by KIAA0247. NICD translocates to the nucleus, where it binds several transcription factors and modulates the expression of target genes, including those encoding Snail, E‐cadherin, cyclin D1, c‐Myc, and p21.3 The γ‐secretase inhibitor DAPT blocks Notch signaling by reducing NICD formation,10 which, in our case, was induced by KIAA0247 knockdown; consequently, DAPT partially suppressed cell migration, invasion, and proliferation, thus suggesting that KIAA0247, indeed, functions by regulating Notch signaling.
Our results indicate that KIAA0247 plays a critical role in NSCLC by inhibiting cancer cell proliferation and metastatic potential through the Notch signaling pathway, suggesting that KIAA0247 is a candidate prognostic biomarker and possible therapeutic target in NSCLC. Given that the Notch ligand Jagged1 not only participates in the Notch signaling pathway but also has other activities, further experiments should clarify whether Jagged1 mediates KIAA0247 effects through NCID and/or through Notch‐independent mechanisms.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
ACKNOWLEDGMENTS
This work was supported by the Liaoning Province Colleges and Universities Innovation Team (LC2015029), Liaoning Provincial Education Department. We thank Massimo Broggini for providing the HA‐pcDNA‐KIAA0247 plasmid and empty HA‐pcDNA plasmid. We would like to thank Editage (http://www.editage.cn) for English language editing.
Xu Y, Ren H, Jiang J, et al. KIAA0247 inhibits growth, migration, invasion of non‐small‐cell lung cancer through regulating the Notch pathway. Cancer Sci. 2018;109:1055–1065. https://doi.org/10.1111/cas.13539
Funding information
Liaoning Province Colleges and Universities Innovation Team (Grant/Award Number: ‘LC2015029’).
REFERENCES
- 1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277‐300. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7‐30. [DOI] [PubMed] [Google Scholar]
- 3. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 4. Khanna P, Blais N, Gaudreau PO, Corrales‐Rodriguez L. Immunotherapy comes of age in lung cancer. Clin Lung Cancer. 2017;18:13‐22. [DOI] [PubMed] [Google Scholar]
- 5. Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. N Engl J Med. 2002;346:92‐98. [DOI] [PubMed] [Google Scholar]
- 6. Yuan X, Wu H, Han N, et al. Notch signaling and EMT in non‐small cell lung cancer: biological significance and therapeutic application. J Hematol Oncol. 2014;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allen TD, Rodriguez EM, Jones KD, Bishop JM. Activated Notch1 induces lung adenomas in mice and cooperates with Myc in the generation of lung adenocarcinoma. Cancer Res. 2011;71:6010‐6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Licciulli S, Avila JL, Hanlon L, et al. Notch1 is required for Kras‐induced lung adenocarcinoma and controls tumor cell survival via p53. Cancer Res. 2013;73:5974‐5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling‐are we there yet? Nat Rev Drug Discov. 2014;13:357‐378. [DOI] [PubMed] [Google Scholar]
- 10. Niessen K, Fu Y, Chang L, Hoodless PA, McFadden D, Karsan A. Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J Cell Biol. 2008;182:315‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polato F, Rusconi P, Zangrossi S, et al. DRAGO (KIAA0247), a new DNA damage‐responsive, p53‐inducible gene that cooperates with p53 as oncosuppressor [Corrected]. J Natl Cancer Inst 2014;106:dju053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang CJ, Yang SH, Huang SM, et al. A predicted protein, KIAA0247, is a cell cycle modulator in colorectal cancer cells under 5‐FU treatment. J Transl Med. 2011;9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan Y, Huang N, Zhang X, et al. KIAA0247 suppresses the proliferation, angiogenesis and promote apoptosis of human glioma through inactivation of the AKT and Stat3 signaling pathway. Oncotarget. 2016;7:87100‐87113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janciauskiene SM, Bals R, Koczulla R, Vogelmeier C, Köhnlein T, Welte T. The discovery of α1‐antitrypsin and its role in health and disease. Respir Med. 2011;105:1129‐1139. [DOI] [PubMed] [Google Scholar]
- 15. Bravo‐Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012;24:277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spiering D, Hodgson L. Dynamics of the Rho‐family small GTPases in actin regulation and motility. Cell Adh Migr. 2011;5:170‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanna S, El‐Sibai M. Signaling networks of Rho GTPases in cell motility. Cell Signal. 2013;25:1955‐1961. [DOI] [PubMed] [Google Scholar]
- 18. Gong L, Wu D, Zou J, et al. Prognostic impact of serum and tissue MMP‐9 in non‐small cell lung cancer: a systematic review and meta‐analysis. Oncotarget. 2016;7:18458‐18468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase‐9 (MMP‐9): the next decade. Crit Rev Biochem Mol Biol. 2013;48:222‐272. [DOI] [PubMed] [Google Scholar]
- 20. Hilakivi‐Clarke L, Wang C, Kalil M, Riggins R, Pestell RG. Nutritional modulation of the cell cycle and breast cancer. Endocr Relat Cancer. 2004;11:603‐622. [DOI] [PubMed] [Google Scholar]
- 21. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 22. Hall M, Peters G. Genetic alterations of cyclins, cyclin‐dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res. 1996;68:67‐108. [DOI] [PubMed] [Google Scholar]
- 23. Sherr CJ. D‐type cyclins. Trends Biochem Sci. 1995;20:187‐190. [DOI] [PubMed] [Google Scholar]
- 24. Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin‐dependent kinase inhibitors (CKIs). Oncogene. 1995;11:211‐219. [PubMed] [Google Scholar]
- 25. Lanczky A, Nagy A, Bottai G, et al. miRpower: a web‐tool to validate survival‐associated miRNAs utilizing expression data from 2,178 breast cancer patients. Breast Cancer Res Treat. 2016; 160(3): 439‐446. [DOI] [PubMed] [Google Scholar]
- 26. Gyorffy B, Surowiak P, Budczies J, Lanczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non‐small‐cell lung cancer. PLoS ONE. 2013;8:e82241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713‐2722. [DOI] [PubMed] [Google Scholar]
- 28. Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14:518128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng H, Kang Y. Multilayer control of the EMT master regulators. Oncogene. 2014;33:1755‐1763. [DOI] [PubMed] [Google Scholar]
- 30. Zhang X, Liu G, Kang Y, Dong Z, Qian Q, Ma X. N‐cadherin expression is associated with acquisition of EMT phenotype and with enhanced invasion in erlotinib‐resistant lung cancer cell lines. PLoS ONE. 2013;8:357692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Z, Li Y, Kong D, Sarkar FH. The role of Notch signaling pathway in epithelial‐mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets. 2010;100:745‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miele L, Miao H, Nickoloff BJ. Notch signaling as a novel cancer therapeutic target. Curr Cancer Drug Targets. 2006;6:313‐323. [DOI] [PubMed] [Google Scholar]
- 33. Miele L. Notch signaling. Clin Cancer Res. 2006;12:1074‐1079. [DOI] [PubMed] [Google Scholar]


