Abstract
Transforming growth factor‐β‐induced (TGFΒI) is considered to be a vital gene in several carcinomas. In this study we determined the effect of TGFBI on the proliferative and metastatic potential of human urothelial carcinoma (UC) cells as well as its mRNA and protein expression, which were detected by RT‐PCR and western blot, respectively. UC cell proliferation was analyzed by WST‐1 assay and Hoechst 33258 staining. The effect of TGFBI on UC cell metastasis was analyzed using adhesion, migration and invasion assays. We found that TGFBI increased the proliferation of UC cells. Moreover, TGFBI enhanced the adhesion, migration and invasion of UC cells by upregulating MMP‐2, MMP‐9 and calpain‐2 expression. We evaluated the effect of Epirubicin (EPI) on the regulation of TGFBI expression and found that TGFBI acts as a downstream target of EPI and is suppressed by EPI in UC cells. EPI is more effective in inhibiting the proliferation and metastasis of UC cells with high TGFBI expression. This study demonstrates that TGFBI might lead to tumorigenesis and progression of UC and those cells with high TGFBI expression may be vulnerable to relapse. EPI could prove to be a therapeutic option in patients with high TGFBI expressing UC cells.
Keywords: Epirubicin, metastasis, proliferation, transforming growth factor‐β‐induced, urothelial carcinoma
1. INTRODUCTION
Bladder cancer is second only to prostate cancer as the most common urologic malignancy in men, and urothelial carcinoma (UC) accounts for approximately 90% of all bladder cancers.1 Among UC, 70%‐80% are non‐muscle‐invasive and the remainder are muscle‐invasive. Between 50% and 70% of non‐muscle‐invasive UC have a high rate of recurrence and 10%‐30% will progress to muscle‐invasive UC.2 Transurethral resection (TUR) is the primary regimen for patients with non‐muscle‐invasive UC3 and the 5‐year overall survival rate for non‐muscle‐invasive UC has been reported to be 90%, 60%, 35% and 25% for non‐muscle‐invasive, pT2, pT3 and pT4 UC, respectively.4, 5 Although regular cystoscopic surveillance is essential for early tumor detection, the mortality rate of UC patients is increasing due to tumor metastasis. The molecular mechanisms responsible for the metastasis of UC is still unclear, thus further study should be elucidated to overcome the proliferation and metastasis of UC, it is useful to the development of novel approaches and seeking improved therapeutic strategies.6, 7
Transforming growth factor‐β‐induced (TGFΒI), also known as BIGH3, is a target of transforming growth factor‐β1 (TGF‐β1).8 TGFBI plays an important role in the activation of cell differentiation, proliferation, adhesion and migration; however, conflicting roles have been reported with respect to tumorigenesis and progression.9, 10 Correlative studies have suggested opposite functions of TGFBI as a tumor suppressor or promoter. The expression of TGFΒI is upregulated in some human cancers, such as renal clear cell carcinoma,11, 12 colon carcinoma adenoma,13 osteosarcoma14 and pancreatic carcinoma.15 TGFBI has been reported to be associated with tumorigenesis in these tumors. In contrast, downregulation of TGFBI is also noted in a variety of tumors, such as lung carcinoma,16 neuroblastoma,17 breast carcinoma18 and mixed lineage leukemia.19 Together, these findings suggest that TGFBI functions as a tumor suppressor. TGFBI is an extracellular matrix (ECM) protein consisting of a cell attachment RGD domain at the C‐terminus and 4 fasciclin‐1 (FAS1) homologous internal domains.20, 21 TGFBI serves as a linker protein which mediates integrin binding to extracellular proteins, such as fibronectin, collagen and laminins.22 Another study demonstrated that TGFBI activates matrix metalloproteinase (MMP) secretion and promotes invasion by interacting with integrin via calpain‐2 in astrocytoma cells.23
Transforming growth factor‐β1 belongs to the TGF‐β superfamily and the role of TGF‐β1 in human tumors is complex. A previous study revealed a significant association between the expression of TGF‐β1 and the rate of positive urine cytologies. Moreover, the combined examination of urine cytology with TGF‐β1 and VEGF has achieved higher sensitivity in the detection of UC.24 Another study suggested that TGF‐β1 promotes cell migration and invasion of UC cells by increasing fascin1 expression.25 As a substrate for TGF‐β1, the concentration of TGFBI was also elevated in the serum and urine of patients with UC, and mRNA expression was increased in muscle‐invasive UC.26 These findings suggest that TGFBI may play an important role in tumorigenesis and progression of UC; however, to date, only limited information is available on TGFBI functionality in UC.
In this study we determined the effect of TGFBI on the proliferative and metastatic potential in human UC. Because TGFBI is involved in the proliferation, adhesion, migration and invasion of UC cells, we speculate that TGFBI may enhance metastasis of UC and that TGFBI serves as a candidate marker for the prediction of UC recurrence and a therapeutic target in treating UC.
2. MATERIALS AND METHODS
2.1. Cell culture
Two UC cell lines, RT112 and 253J, were purchased from the ATCC and cultured in 10% heat‐inactivated FBS, supplemented with 1% non‐essential amino acids, RPMI‐1640 medium, 25 mmol/L HEPES, 2 mmol/L l‐glutamine, and 100 units/mL penicillin or 100 μg/mL streptomycin (Invitrogen). All UC cell lines were maintained as monolayers in 10‐cm plastic dish and cultured at 37°C in a humidified atmosphere of 5% CO2.
2.2. Reverse transcription‐PCR
Total RNA from UC cells was extracted using Trizol Reagent (Invitrogen) according to the manufacturer's instructions; first‐strand complementary DNA (cDNA) was reverse‐transcribed using a cDNA Synthesis Kit (Amersham Biosciences). The cDNA was used as the template and was amplified by PCR according to the manufacturer's instructions. The PCR products were visible by agarose gel electrophoresis. GAPDH was used as the internal control to normalize variances, and the primer sets used in this study are shown in Table 1.
Table 1.
Primer sequences and siRNA oligonucleotides used in this study
| Primers | Forward primer (5′‐3′) | Reverse primer (5′‐3′) | Length of PCR products (bp) |
|---|---|---|---|
| TGFBI | GTGTGTGCTGTGCAGAAGGT | CATATCCAGGACAGCACTCG | 124 |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC | 226 |
| siRNA | Sense oligonucleotide (5′‐3′) | Antisense oligonucleotide (5′‐3′) | Target gene sequence (5′‐3′) |
| TGFBI | CCCGCUCGCAGCUUACUUAAC | UAAGUAAGCUGCGAGCGGGAG | CTCCCGCTCGCAGCTTACTTAAC (48‐70 bp) |
| Negative control | GUACCGCACGUCAUUCGUAUC | UACGAAUGACGUGCGGUACGU |
2.3. Western blot
Total protein was isolated and an equal quantity of protein lysates (50 g/well) were performed by SDS‐polyacrylamide gel electrophoresis, then protein was electrotransferred onto polyvinylidene difluoride membranes. The membrane was incubated with a blocking solution (TBS, 0.1% Tween 20 and 5% nonfat dried milk powder) to block nonspecific protein for 2 hour at room temperature. Primary antibody of TGFBI (D31B8) XP Rabbit mAb, MMP‐2 (D8N9Y) Rabbit mAb and MMP‐9 (D6O3H) XP Rabbit mAb were purchased from Cell Signaling Technology. Calpain 2 antibody (ab39165) and β‐actin monoclonal antibody (ab6276) were purchased from Abcam. The blots were incubated with antibodies overnight at room temperature, and the immune complex was detected using a peroxidase‐conjugated secondary antibody and ECL system (Amersham) according to the manufacturer's instructions.
2.4. siRNA and transfection
All siRNA oligonucleotide sequences were designed using siDirect software and the oligonucleotide sets used in this study are shown in Table 1. UC cells were incubated with complete medium until confluence reached 50%‐60%, then UC cells were transfected with siRNA oligonucleotides by lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The cDNA coding sequence of TGFBI was cloned using RT‐PCR and then were subcloned into pcDEF3 vector. Two UC cell lines were transfected with pcDEF3 vector containing full‐length cDNA for TGFBI using lipofectamine 2000 and UC monoclonal cell lines were selected with G418. The expression of TGFBI was confirmed by RT‐PCR and western blot analysis.
2.5. Proliferation analysis
The effect of TGFBI on the proliferative ability of UC cells was analyzed using the WST‐1 assay. Briefly, 3 × 103 exponentially‐growing UC cells (100 μL) were seeded in 96‐well microtiter plates. After 24‐, 48‐ and 72‐hour incubation, 20 μL of WST‐1 Reagent (Roche, Penzberg, Germany) was added to each well according to the protocol, and the incubation was continued for an additional 2 hour at 37°C. The absorbance of each well, representing the cell count, was detected with a microplate reader (Immunoreader NJ‐2000; Japan Intermed, Tokyo, Japan) at 450 nm.
2.6. Hoechst 33258 staining
Urothelial carcinoma cells were harvested and fixed with 5 mL of 10% formalin. Then the fixed cells were seeded onto a slide and dried at room temperature. Fifty microliters of 10 mmol/L Hoechst 33258 solution (Wako, Tokyo, Japan) was added to each slide for 30 minute, then examined under ultraviolet illumination with a Nikon E1000 fluorescence microscope (Tokyo, Japan).
2.7. Cell adhesion assay
A 24‐well culture plate was first coated with 25 μg of Matrigel per well and air dried for 40 minute. Then, a total of 4 × 104 UC cells were harvested with RPMI‐1640 medium containing 0.5% BSA and seeded into each well, and continuously incubated in 5% CO2 at 37°C for 2 hour. The unattached UC cells on the 24‐well culture plate were removed after gentle washing thrice with PBS. The UC cells that remained attached to the culture plate were harvested and stained with Hoechst 33258. All experiments were replicated 3 times.
2.8. Cell migration assay
Chemotaxis was analyzed using the transwell system (Poretics, Livermore, CA, USA) containing 8 μm‐pore polycarbonate membrane filters. UC cells were harvested and re‐suspended in medium with 0.05% FBS, then 1 × 104 cells were seeded into the upper wells. After continuous incubation at 37°C for 24 hour, the membrane was removed and fixed with 70% ethanol at −20°C for 30 minute, and the membrane was harvested and stained with Hoechst 33258. All experiments were performed thrice.
2.9. Cell invasion assay
Cell invasion was analyzed using a transwell system incorporating a polycarbonate filter membrane (Corning). A thin gel layer was formed by adding 12.5 μg Matrigel and 50 μL PBS on the filter and dried overnight. A total of 1 × 105 UC cells were harvested in 100 μL serum‐free medium and then seeded to the upper chamber of the transwell system. After 24‐hour incubation at 37°C, the UC cells that penetrated to the lower surface of the filter were harvested and stained with Hoechst 33258. All experiments were replicated 3 times.
2.10. Statistical analysis
All results are expressed as the mean ± SD. Statistical significance was determined by Student's t test, and a P‐value ≤ .05 was considered significant.
3. RESULTS
3.1. Transforming growth factor‐β‐induced enhances the proliferative ability of urothelial carcinoma cells
The expression of TGFBI was decreased using RNAi, and the expression of TGFBI was also increased by transfection with a vector containing the full‐length cDNA for TGFBI. All transfections were confirmed by RT‐PCR (Figure 1A) and western blot (Figure 1B). TGFBI expression was significantly increased by the TGFBI vector insert and decreased by RNAi. After a 24‐, 48‐ and 72‐hour incubation, the effect of TGFBI on the proliferation of UC cells was analyzed using the WST‐1 assay. Our results indicated that UC cells with a high level of TGFBI expression exhibit significantly higher proliferative ability. In contrast, UC cells with a low level of TGFBI expression exhibited lower proliferative ability (Figure 2). These findings suggest that TGFBI is involved in proliferation of human UC.
Figure 1.
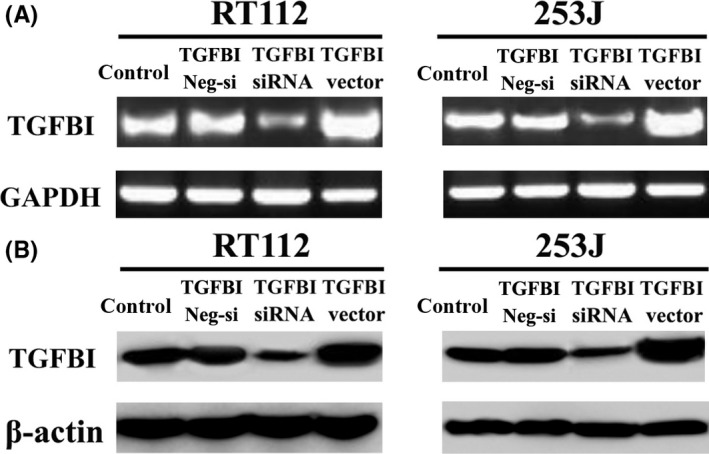
TGFBI expression is confirmed by RT‐PCR (A) and western blot (B) after transfection with TGFBI siRNA oligonucleotide, negative control oligonucleotide and pcDEF3 vector containing the full‐length cDNA for TGFBI
Figure 2.
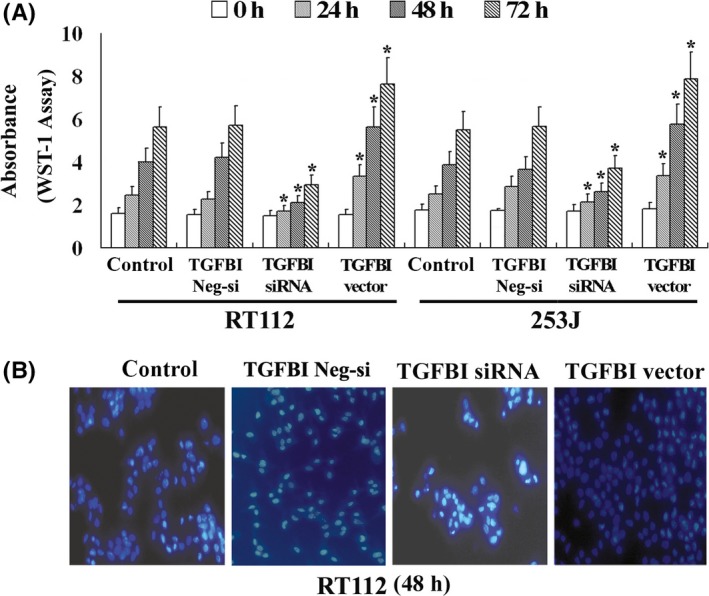
TGFBI enhances the proliferation of UC cells. The proliferative ability of UC cells was evaluated by WST‐1 assay (A) and Hoechst 33258 staining (B). All experiments were performed in triplicate and the error bar represents the SD. *P < .05 vs control
3.2. Transforming growth factor‐β‐induced enhances the metastatic ability of urothelial carcinoma cells
The high rate of relapse in patients with UC is related to spread by intraluminal seeding. Metastasis and recurrence is a major cause of mortality and a complex multistep process in UC patients, which includes UC cell adhesion, migration and invasion. The effects of TGFBI on the adhesion, migration and invasion of UC cells were also evaluated in this study. UC cells with high TGFBI expression had increased adhesion (Figure 3A), migration (Figure 3B) and invasion properties (Figure 3C) compared to control cells. In contrast, those cells with low TGFBI expression due to siRNA transfection had significantly reduced adhesion, migration and invasion properties. These findings suggest that TGFBI is involved in the adhesion, migration and invasion of UC cells, and that TGFBI may play an important role in the metastasis and recurrence of UC.
Figure 3.
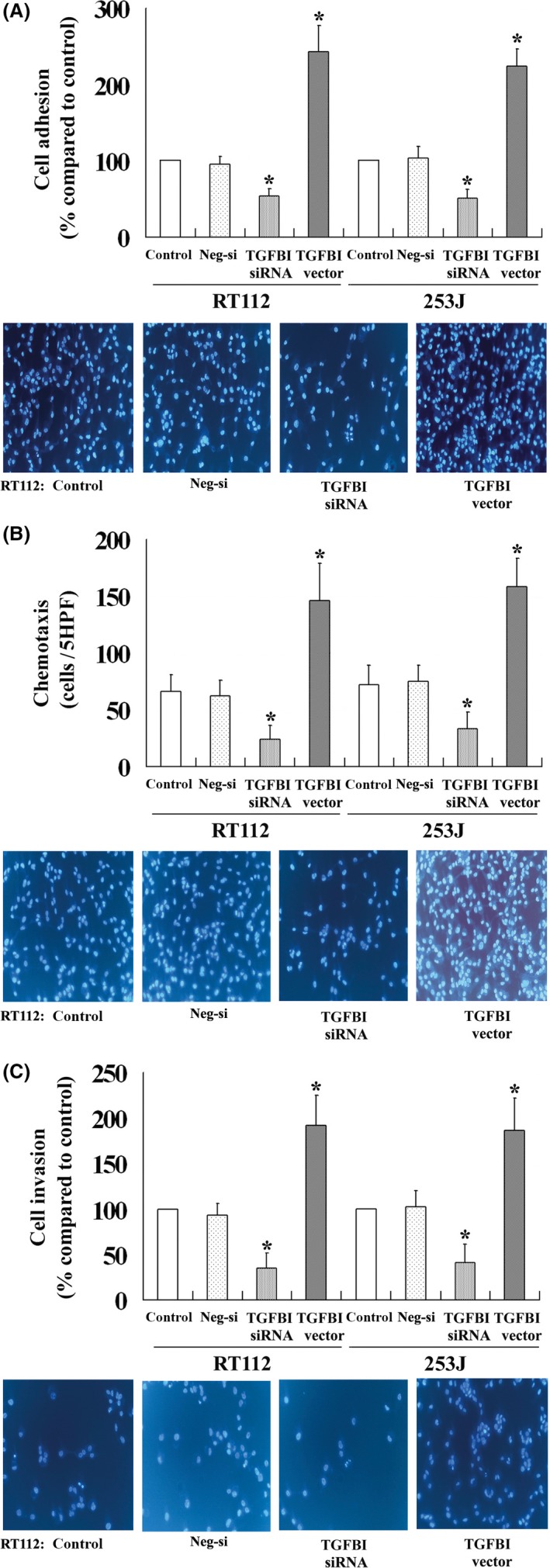
TGFBI enhances the adhesion (A), migration (B) and invasion (C) of UC cells. All experiments were performed in triplicate and the error bar represents the SD. *P < .05 vs control
3.3. Transforming growth factor‐β‐induced upregulates the expression of matrix metalloproteinase and calpain‐2
Calpain‐2 promotes the invasion of tumor cells and matrix metalloproteinase (MMP) secretion. To further understand the molecular mechanism underlying TGFBI activity in UC cells, we evaluated the effect of TGFBI on the regulation of MMP and calpain‐2 expression by western blot analysis. Our results indicated that UC cells with a high level of TGFBI expression exhibit increased expression of MMP‐2, MMP‐9 and calpain‐2. In contrast, UC cells with a low level of TGFBI expression exhibited decreased expression of these proteins (Figure 4). These findings suggest that TGFBI is involved in the regulation of MMP and calpain‐2 secretion, and that TGFBI enhances the metastatic potential of human UC.
Figure 4.
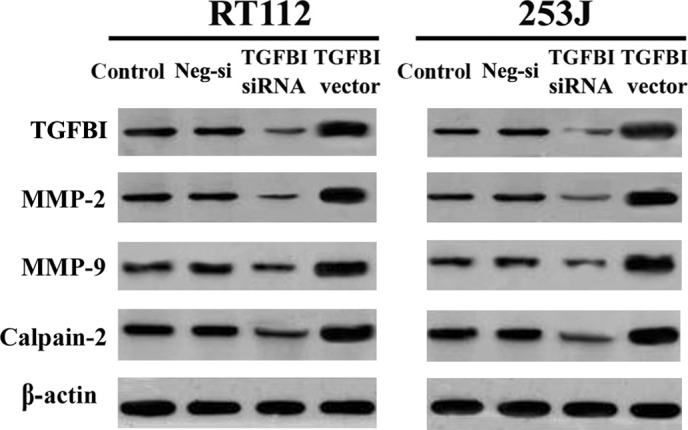
TGFBI increases the expression of MMP2, MMP9, and calpain‐2 confirmed by Western blot
3.4. Epirubicin decreases the expression of transforming growth factor‐β‐induced
Epirubicin is the first‐line treatment for patients with bladder UC after TUR.27 Although EPI can effectively reduce the recurrence rate of UC, the mechanism of EPI is still unclear. We found that EPI caused time‐dependent cell growth suppression in 2 UC cell lines (Figure 5A). We also evaluated the effect of EPI (4 μmol/L for 48 hour) on the regulation of TGFBI expression by RT‐PCR (Figure 5B) and western blot analysis (Figure 5C). Our results suggest that EPI can significantly downregulate the expression of TGFBI in 2 UC cells. These findings suggest that TGFBI acts as a downstream molecule of EPI, and that EPI exerts its biological functions by decreasing the expression of TGFBI in human UC.
Figure 5.
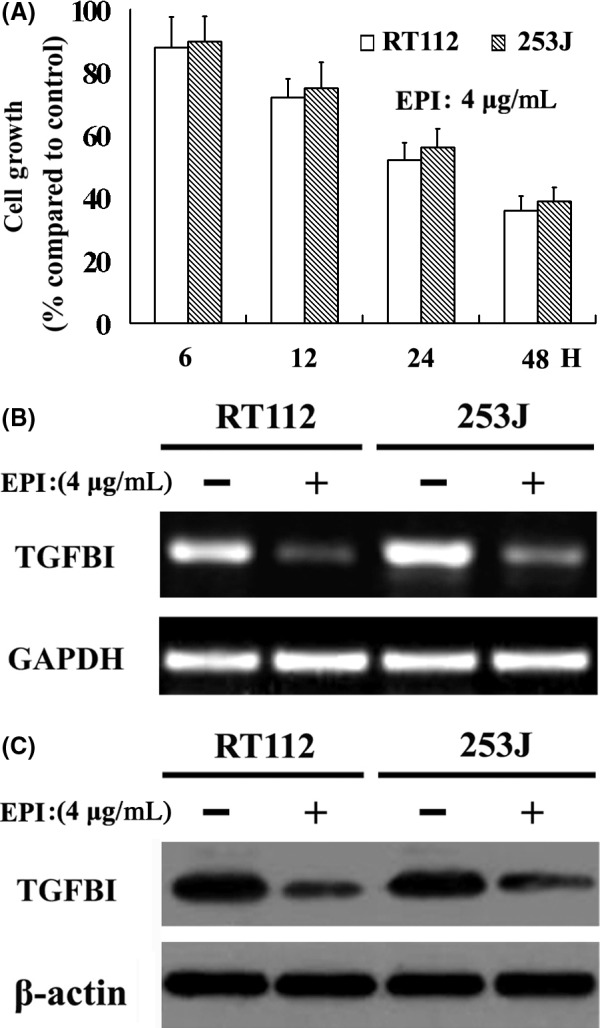
EPI caused time‐dependent cell growth suppression in 2 UC cell lines (A), and EPI decreases TGFBI expression confirmed by RT‐PCR (B) and western blot (C)
3.5. Transforming growth factor‐β‐induced affects Epirubicin‐induced tumor‐suppressive functions
As a downstream target gene of EPI, whether or not TGFBI affects the EPI tumor inhibitory effect is unknown. In this study we evaluated the effect of TGFBI on EPI‐induced tumor‐suppressive functions in 2 UC cell lines. Despite TGFBI expression, EPI caused dose‐dependent cell growth suppression in all UC cells; however, increased susceptibility to EPI was observed in UC cells with a high level of TGFBI expression. In contrast, decreased susceptibility was observed in UC cells with a low level of TGFBI expression compared to control cells (Figure 6A; only the results for 24 hour are shown). Similar results were also found with respect to EPI‐induced anti‐metastatic function and higher suppression of adhesion, migration and invasion in high TGFBI‐expressing UC cells after EPI treatment (4 μg/mL for 48 hour), and lower suppression in low TGFBI‐expressing UC cells compared to control cells (Figure 6B‐D). These findings suggest that TGFBI enhances EPI‐induced tumor‐suppressive functions in UC cells and that TGFBI may be a potential marker of EPI treatment for human UC.
Figure 6.
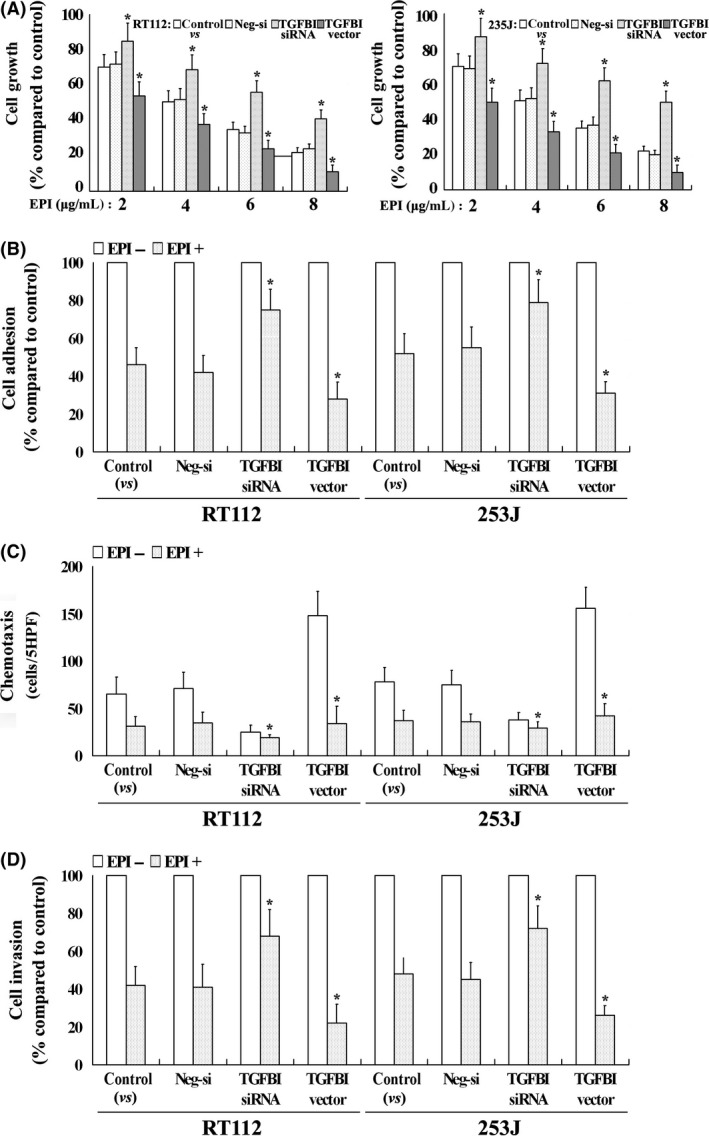
TGFBI affects EPI‐induced tumor‐suppressive functions. EPI caused dose‐dependent cell growth suppression in 2 UC cell lines, and an increased susceptibility to EPI was observed in UC cells with a high level of TGFBI expression (A). EPI had higher suppressive ability of adhesion (B), migration (C) and invasion (D) in high TGFBI‐expressing UC cells. All experiments were performed in triplicate and the error bar represents the SD; only the results for RT112 are shown. *P < .05 vs control
4. DISCUSSION
Bladder UC is well‐known for its high rate of recurrence. UC is a life‐threatening disease; thus, routine cystoscopic checks and intravesical instillation are usually performed to prevent the recurrence of UC following TUR. Moreover, radical cystectomy is the recommended treatment for muscle‐invasive and frequently recurring disease.28 Therefore, novel molecular mechanisms and therapeutic innovations are needed for patients with UC.
Although some attempts have been made to predict the recurrence of UC, the mechanisms underlying recurrence and progression remain obscure.29, 30, 31 TGFBI is a multi‐functional protein that commonly exists in normal as well as tumor cells. Reports on TGFBI's tumor suppressive and potentiating roles in various malignancies have conflicting results. Divya et al.26 indicate that the expression of TGFBI is elevated in the serum and urine of patients with UC; moreover, mRNA expression is also increased in muscle‐invasive UC, which suggests that TGFBI may prove useful as a diagnostic or prognostic marker for UC.26 The roles of TGFBI in UC are still indistinct, and the functions of TGFBI in UC should be further determined.
In the present study we evaluated the effect of TGFBI on the proliferation of UC cells. We found that TGFBI increased the UC cells proliferation and that TGFBI may be involved in tumorigenesis of human urothelial cells. In addition, we analyzed the effect of TGFBI on the adhesion, migration and invasion of UC cells. Our results suggest that TGFBI enhances the adhesion, migration and invasion of UC cells, and that TGFBI may play an important role in the metastasis and recurrence of UC. MMP are ECM‐degrading proteinases with biological functions in cancer metastasis.32, 33, 34 MMP promote metastasis through proteolysis of the ECM and activation of various signals.35 Calpain‐2 is a calcium‐dependent thiol proteinase, which consists of a regulatory subunit and a catalytic subunit.36 Calpain‐2 is activated by millimolar levels of Ca2+.37 It has been reported that calpain‐2 mediates the invasion of glioma cells.38 Our results indicate that TGFBI increases the expression of MMP‐2, MMP‐9 and calpain‐2. TGFBI may promote metastasis, by regulating the secretion of MMP and calpain‐2 in human UC.
Routine intravesical instillation with EPI has been widely performed to reduce the recurrence of UC following TUR. Although EPI chemotherapy has achieved an average 16 months of mean disease‐free survival, and suppressed the progression and recurrence in patients with non‐muscle‐invasive UC,39 acquired resistance to EPI is still a barrier to achieving successful treatment of UC.40 Some studies with combination treatments have shown antitumor drug action and possible synergistic effects against UC. Ciprofloxacin has been shown to synergistically increase EPI‐induced apoptosis in UC cell lines,41 and icaritin acts synergistically with EPI to suppress UC cell growth through inhibition of autophagy.40 However, the molecular mechanisms of EPI in UC remain unclear. In this study we examined the effect of EPI on the regulation of TGFBI expression. TGFBI acts as a downstream target of EPI, and EPI can suppress the expression of TGFBI in UC cells. In addition, we evaluated the effect of TGFBI on EPI‐induced tumor‐suppressive functions. Our results suggest that EPI is more effective in inhibiting the proliferation and metastasis of UC cells with high TGFBI expression. These findings indicated that decreased expression of TGFBI may contribute to the resistance to EPI therapy.
In conclusion, we found that TGFBI plays an important role in the proliferation and metastasis of UC, and that TGFBI might lead to tumorigenesis and progression of UC. It is also an intriguing possibility that UC cells with high TGFBI expression may be vulnerable to relapse. TGFBI may be a potential marker for recurrence and EPI could be a therapeutic option for UC patients with high TGFBI expression. The molecular mechanisms underlying TGFBI in UC warrant further investigation.
DISCLOSURE STATEMENT
The authors have no conflicts of interest to declare.
Shang D, Song B, Liu Y. Epirubicin suppresses proliferative and metastatic potential by downregulating transforming growth factor‐β‐induced expression in urothelial carcinoma. Cancer Sci. 2018;109:980–987. https://doi.org/10.1111/cas.13403
Funding information
Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201604).
Shang and Song contributed equally to this study and should be considered as co‐first authors.
REFERENCES
- 1. Sharma S, Ksheersagar P, Sharma P. Diagnosis and treatment of bladder cancer. Am Fam Physician. 2009;80:717‐723. [PubMed] [Google Scholar]
- 2. Jacobs BL, Lee CT, Montie JE. Bladder cancer in 2010: how far have we come? CA Cancer J Clin. 2010;60:244‐272. [DOI] [PubMed] [Google Scholar]
- 3. Parekh DJ, Bochner BH, Dalbagni G. Superficial and muscle invasive bladder cancer: principles of management for outcomes assessments. J Clin Oncol. 2006;24:5519‐5527. [DOI] [PubMed] [Google Scholar]
- 4. Morgan TM, Clark PE. Bladder cancer. Curr Opin Oncol. 2010;22:242‐249. [DOI] [PubMed] [Google Scholar]
- 5. Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol. 2006;176:2414‐2422. [DOI] [PubMed] [Google Scholar]
- 6. Lokeshwar VB, Habuchi T, Grossman HB. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology. 2005;66:35‐63. [DOI] [PubMed] [Google Scholar]
- 7. Bassi P, De Marco V, De Lisa A, et al. Non‐invasive diagnostic tests for bladder cancer: a review of the literature. Urol Int. 2005;75:193‐200. [DOI] [PubMed] [Google Scholar]
- 8. Thapa N, Lee BH, Kim IS. TGFBIp/betaig‐h3 protein. A versatile matrix molecule induced by TGF‐beta. Int J Biochem Cell Biol. 2007;39:2183‐2194. [DOI] [PubMed] [Google Scholar]
- 9. Thapa N, Kang KB, Kim IS. Beta ig‐h3 mediates osteoblast adhesion and inhibits differentiation. Bone. 2005;36:232‐242. [DOI] [PubMed] [Google Scholar]
- 10. Kim JE, Jeong HW, Nam JO, et al. Identification of motifs in the fasciclin domains of the transforming growth factor‐beta‐induced matrix protein betaig‐h3 that interact with the alphaVbeta5 integrin. J Biol Chem. 2002;277:46159‐46165. [DOI] [PubMed] [Google Scholar]
- 11. Ivanov SV, Ivanova AV, Salnikow K, et al. Two novel VHL targets, TGFBI (BIGH3) and its transactivator KLF10, are up‐regulated in renal clear cell carcinoma and other tumors. Biochem Biophys Res Commun. 2008;370:536‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shang DH, Liu YT, Yang PQ, Chen YQ, Tian Y. TGFBI‐promoted adhesion, migration and invasion of human renal cell carcinoma depends on inactivation of von Hippel‐Lindau tumor suppressor. Urology. 2012;79:966e1‐966e7. [DOI] [PubMed] [Google Scholar]
- 13. Ma C, Rong Y, Radiloff DR, Datto MB, et al. Extracellular matrix protein betaig‐h3/TGFΒI promotes metastasis of colon cancer by enhancing cell extravasation. Genes Dev. 2008;22:308‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo YS, Zhao R, Ma J, et al. βig‐h3 promotes human osteosarcoma cells metastasis by interacting with integrin α2β1 and activating PI3K. PLoS ONE. 2014;9:e90220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schneider D, Kleeff J, Berberat PO, et al. Induction and expression of betaig‐h3 in pancreatic cancer cells. Biochim Biophys Acta. 2002;1588:1‐6. [DOI] [PubMed] [Google Scholar]
- 16. Irigoyen M, Pajares MJ, Agorreta J, et al. TGFΒI expression is associated with a better response to chemotherapy in NSCLC. Mol Cancer. 2010;9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Becker J, Erdlenbruch B, Noskova I, et al. Keratoepithelin suppresses the progression of experimental human neuroblastomas. Cancer Res. 2006;66:5314‐5321. [DOI] [PubMed] [Google Scholar]
- 18. Wen G, Partridge MA, Li B, et al. TGFBI expression reduces in vitro and in vivo metastatic potential of lung and breast tumor cells. Cancer Lett. 2011;308:23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Z, Luo RT, Mi S, et al. Consistent deregulation of gene expression between human and murine MLL rearrangement leukemias. Cancer Res. 2009;69:1109‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanssen E, Reinboth B, Gibson MA. Covalent and non‐covalent interactions of betaig‐h3 with collagen VI. Beta ig‐h3 is covalently attached to the amino‐terminal region of collagen VI in tissue microfibrils. J Biol Chem. 2003;278:24334‐24341. [DOI] [PubMed] [Google Scholar]
- 21. Hashimoto K, Noshiro M, Ohno S, et al. Characterization of a cartilage‐derived 66‐kDa protein (RGD‐CAP/Big‐h3) that binds to collagen. Biochim Biophys Acta. 1997;1355:303‐314. [DOI] [PubMed] [Google Scholar]
- 22. Kim JE, Kim SJ, Lee BH, Park RW, Kim KS, Kim IS. Identification of motifs for cell adhesion within the repeated domains of transforming growth factor‐beta‐induced gene, betaig‐h3. J Biol Chem. 2000;275:30907‐30915. [DOI] [PubMed] [Google Scholar]
- 23. Ma J, Cui W, He SM, et al. Human U87 astrocytoma cell invasion induced by interaction of big‐h3 with integrin a5b1 involves Calpain‐2. PLoS ONE. 2012;7:e37297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eissa S, Salem AM, Zohny SF, Hegazy MG. The diagnostic efficacy of bladder cancer: comparison with voided urine and VEGF in TGF‐beta1 urinary cytology. Cancer Biomark. 2007;3:275‐285. [DOI] [PubMed] [Google Scholar]
- 25. Zhang N, Bi X, Zeng Y, Zhu Y, et al. TGF‐β1 promotes the migration and invasion of bladder carcinoma cells by increasing fascin1 expression. Oncol Rep. 2016;36:977‐983. [DOI] [PubMed] [Google Scholar]
- 26. Divya B, Nitin A, Rehan K, Manoj S, Amlesh S, Alpana S. Expression of CD147, BIGH3 and Stathmin and their potential role as diagnostic marker in patients with urothelial carcinoma of the bladder. Clin Chim Acta. 2012;413:1641‐1646. [DOI] [PubMed] [Google Scholar]
- 27. Onrust SV, Wiseman LR, Goa KL. Epirubicin: a review of its intravesical use in superficial bladder cancer. Drugs Aging. 1999;15:307‐333. [DOI] [PubMed] [Google Scholar]
- 28. Mitsumori K, Tsuchiya N, Habuchi T, et al. Early and large‐dose intravesical instillation of epirubicin to prevent superficial bladder carcinoma recurrence after transurethral resection. BJU Int. 2004;94:317‐321. [DOI] [PubMed] [Google Scholar]
- 29. Xu T, Zhu Z, Zhang X, et al. Predicting recurrence and progression in Chinese patients with nonmuscle‐invasive bladder cancer using EORTC and CUETO scoring models. Urology. 2013;82:387‐393. [DOI] [PubMed] [Google Scholar]
- 30. Ajili F, Darouiche A, Chebil M, Boubaker S. The efficiency of the EORTC scoring system for the prediction of recurrence and progression of non‐muscle‐invasive bladder cancer treated by bacillus calmette‐guerin immunotherapy. Ultrastruct Pathol. 2013;37:249‐253. [DOI] [PubMed] [Google Scholar]
- 31. Mares J, Szakacsova M, Soukup V, Duskova J, Horinek A, Babjuk M. Prediction of recurrence in low and intermediate risk non‐muscle invasive bladder cancer by real‐time quantitative PCR analysis: cDNA microarray results. Neoplasma. 2013;60:295‐301. [DOI] [PubMed] [Google Scholar]
- 32. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases. Structure, function, and biochemistry. Circ Res. 2003;92:827‐839. [DOI] [PubMed] [Google Scholar]
- 33. Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer. 2005;115:849‐860. [DOI] [PubMed] [Google Scholar]
- 34. Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624‐632. [DOI] [PubMed] [Google Scholar]
- 35. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Macqueen DJ, Wilcox AH. Characterization of the definitive of vertebrates using phylogenetic, evolutionary and calpain family classical expression analyses. Open Biol. 2014;4:130219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roumes H, Leloup L, Dargelos E, Brustis JJ, Daury L, Cottin P. Calpains: markers of tumor aggressiveness? Exp Cell Res. 2010;316:1587‐1599. [DOI] [PubMed] [Google Scholar]
- 38. Jang HS, Lal S, Greenwood JA. Calpain 2 is required for glioblastoma cell invasion: regulation of matrix metalloproteinase 2. Neurochem Res. 2010;35:1796‐1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Türkeri L, Tanıdır Y, Çal Ç, Özen H, Şahin H. Comparison of the efficacy of single or double intravesical epirubicininstillation in the early postoperative period to prevent recurrences in non‐muscle‐invasive urothelial carcinoma of the bladder: prospective, randomized multicenter study. Urol Int. 2010;85:261‐265. [DOI] [PubMed] [Google Scholar]
- 40. Pan XW, Li L, Huang Y, et al. Icaritin acts synergistically with epirubicin to suppress bladder cancer growth through inhibition of autophagy. Oncol Rep. 2016;35:334‐342. [DOI] [PubMed] [Google Scholar]
- 41. Engeler DS, Scandella E, Ludewig B, Schmid HP. Ciprofloxacin and epirubicin synergistically induce apoptosis in human urothelial cancer cell lines. Urol Int. 2012;88:343‐349. [DOI] [PubMed] [Google Scholar]


