Abstract
Neoadjuvant therapy for locally advanced rectal cancer is becoming increasingly common. However, biomarkers predicting the response to neoadjuvant therapy have not been established. Tumor‐infiltrating lymphocytes (TILs) have a crucial effect on tumor progression and survival outcome as the primary host immune response, and an antitumor immune effect has been reported to contribute to the response to radiotherapy and chemotherapy. We investigated the significance of TILs before and after neoadjuvant treatment and the change in the density of those TILs. Sixty‐four patients who underwent radical resection after neoadjuvant treatment for locally advanced rectal cancer were enrolled. The number of TIL subsets was examined using immunohistochemical staining of pretreatment biopsy samples and post‐treatment resected specimens. In both the neoadjuvant chemotherapy cohort and the neoadjuvant chemoradiotherapy cohort, a low density of CD8+ TILs in pretreatment biopsy samples was associated with a poor response, and a low density of CD8+ TILs in post‐treatment resected specimens was similarly associated with a poor response. In the neoadjuvant chemoradiotherapy cohort, the density of CD8+ TILs in post‐treatment resected specimens was significantly increased compared with that in pretreatment biopsy samples. We concluded that T lymphocyte‐mediated immune reactions play an important role in tumor response to neoadjuvant treatment for rectal cancer, and the evaluation of TILs in pretreatment biopsy samples might be a predictor of the clinical effectiveness of neoadjuvant treatment. Furthermore, neoadjuvant therapy, especially chemoradiotherapy, could induce the activation of the local immune status.
Keywords: neoadjuvant therapy, predictive marker, rectal cancer, tumor‐infiltrating lymphocyte, tumor‐infiltrating lymphocyte
Abbreviations
- Bmab
bevacizumab
- CapeOX
capecitabine + oxaliplatin
- Cmab
cetuximab
- CRT
chemoradiotherapy
- CT
chemotherapy
- FOLFOX
5‐fluorouracil + leucovorin + oxaliplatin
- Foxp3
forkhead box P3
- GATA3
GATA‐binding protein 3
- MMR
mismatch repair
- MSI
microsatellite instability
- RORγT
RAR‐related orphan receptor‐γT
- T‐bet
t‐box expressed in T cells
- Th
T helper (cell)
- TIL
tumor‐infiltrating lymphocyte
- TMA
tissue microarray
- Treg
regulatory T (cell)
1. INTRODUCTION
Rectal cancer is one of the most common causes of cancer death worldwide.1 Neoadjuvant CRT combined with surgery has been the standard treatment strategy for locally advanced rectal cancer in order to reduce the local recurrence rate.2 In addition, neoadjuvant CT was proposed as a new treatment strategy in order to reduce not only the local recurrence rate but also the distant metastasis rate.3 However, in unresponsive cases, neoadjuvant therapy could have disadvantages, such as allowing tumor progression or delaying surgery, thereby making it impossible to undertake curative surgery in such patients. Therefore, it is necessary to carry out such surgery as early as possible in unresponsive cases, which requires being able to predict a given patient's clinical response to neoadjuvant treatment. However, the biomarkers predicting the clinical response to neoadjuvant treatment have not been established.
As part of the primary host immune response to malignant tumors, TILs critically influence tumor progression and survival outcomes.4, 5, 6 Galon et al7 established the immunoscore as a prognostic indicator by evaluating the density of TILs and reported that the immunoscore is a more valuable prognostic indicator than traditional histopathological scores, such as the TNM classification.7, 8, 9, 10 Furthermore, the antitumor immune response has also been reported to contribute to the response to radiotherapy and CT.11, 12, 13, 14 However, the mechanisms underlying the correlation between antitumor immunity and the response to radiotherapy and CT have yet to be fully clarified.
In the present study, we investigated the significance of TILs in pretreatment biopsy specimens in advanced rectal cancer as a marker for predicting the response to neoadjuvant treatment. We also investigated the correlation between the antitumor immune status after neoadjuvant treatment and the response to neoadjuvant treatment. In addition, we investigated the change in the local immune status before and after neoadjuvant treatment.
2. MATERIALS AND METHODS
2.1. Patients
Sixty‐four patients with locally advanced rectal cancer were enrolled in this study. All patients underwent resection of rectal cancer after neoadjuvant treatment (neoadjuvant CT or CRT) at the Department of Surgical Oncology, Osaka City University (Osaka, Japan) between January 2010 and February 2017. The indication for neoadjuvant treatment was the depth clinically diagnosed as T3‐T4, or a positive clinical diagnosis of lymph node metastasis. There were no patients who underwent emergency surgery for perforation/obstruction in this study. The resected specimens were pathologically classified according to the UICC TNM classification of malignant tumors (7th edition).7 Chemotherapy was given alone or concomitantly with radiation as one or two cytotoxic agents based on fluoropyrimidine with or without a molecular‐targeting drug. For CRT, patients received 50.4 Gy in 28 fractions to the pelvis using 3‐D conformal irradiation. The tumor response was evaluated by pathologists according to the definitions in the Japanese Classification of Colorectal Carcinoma (8th edition):15 grade 0, no remarkable changes; grade 1a, swelling of cells, enlarged vesicles, pyknosis of nuclei and vacuolated cytoplasm in 1%‐33.3% of tumor cells; grade 1b, grade 1a changes in 33.4%‐66.6% of tumor cells; grade 2, cell nests consisting of markedly damaged cells, often showing a moth‐eaten appearance and simplified granular structures in >66.7% tumor cells; and grade 3, extensive degenerative changes and the replacement of tumor cells with granulomatous or fibrous tissue. We defined patients with grade 0‐1a responses as non‐responders, and those with grade 1b‐3 responses as responders.
2.2. Tissue microarray construction
A TMA with one 3.0‐mm‐diameter punch core per tumor was constructed from formalin‐fixed paraffin‐embedded tissue blocks of pretreatment biopsy specimens and post‐treatment resected specimens, as previously reported.16 We ensured that the specific histological type of cancer was representatively included in the TMA using H&E‐stained TMA sections.
2.3. Immunohistochemistry for TILs, MMR status, and HLA class I expression
Full sections of pretreatment biopsy specimens and post‐treatment surgically resected specimens were used for the evaluation of TILs. Specimen sections, 4‐μm thick, were deparaffined and rehydrated and then subjected to endogenous peroxidase blocking in 1% H2O2 solution in methanol for 15 minutes. Antigen retrieval was carried out by autoclaving the sections at 105°C for 10 minutes each in Dako Target Retrieval Solution (Dako, Glostrup, Denmark). Serum blocking was carried out with antibody in 10% normal rabbit or goat serum for 10 minutes. After H2O2 and serum blocking, the slides were incubated with primary mouse monoclonal anti‐CD4 antibody (1:80 dilution; Dako) at room temperature for 20 minutes, primary mouse monoclonal anti‐CD8 antibody (1:100 dilution; Dako) at room temperature for 30 minutes, primary mouse monoclonal anti‐T‐bet (a Th1‐specific marker) antibody (1:50 dilution; Abcam, Cambridge, UK) at room temperature for 15 minutes, primary rabbit monoclonal anti‐GATA3 (a Th2‐specific marker) antibody (1:50 dilution; Cell Signaling Technology, Danvers, MA, USA) at 4°C for 12 hours, primary mouse monoclonal anti‐RORγT (a Th17‐specific marker) antibody (1:400 dilution; Merck Millipore, Darmstadt, Germany) at 4°C for 12 hours, and primary mouse monoclonal anti‐Foxp3 antibody (1:100 dilution; Abcam) at room temperature for 15 minutes. The secondary antibody was biotin‐labeled rabbit anti‐mouse IgG, IgA, IgM, or biotin‐labeled goat anti‐rabbit IgG (1:500; Nichirei, Tokyo, Japan). Detection was undertaken with a 3,3′‐diaminobenzidine‐tetrachloride kit (Histofine Simple Stain kit; Nichirei). The sections were counterstained with hematoxylin.
Tissue microarray sections of pretreatment biopsy specimens and post‐treatment resected specimens were used for the evaluation of the MMR status. Staining was carried out as described above except for the following point: antigen retrieval was undertaken at 121°C for 15 minutes. The sections were incubated in primary antibody for 20 minutes for MLH1 (prediluted product), 20 minutes for MSH2 and MSH6 (1:50 dilution), and 30 minutes for PMS2 (1:40 dilution) at room temperature (product codes: IS079, M3639, M3646, and M3647; all Dako).
Tissue microarray sections of pretreatment biopsy specimens and post‐treatment resected specimens were used for evaluation of HLA class I expression. Staining was carried out as described above for immunohistochemistry for TILs except for the following point: antigen retrieval was carried out at 121°C for 15 minutes. The sections were incubated in primary antibody for 12 hours for HLA class I heavy chain (1:200 dilution, MUB2037P; Nordic‐MUbio, Susteren, The Netherlands) at room temperature.
2.4. Immunohistochemical evaluation for TILs
Immunohistochemical evaluations were carried out by a pathologist who was blinded to the clinical information. The numbers of CD4+, CD8+, T‐bet+, GATA3+, RORγT+, and Foxp3+ TILs (Figure 1) in pretreatment biopsy specimens and at the invasive margin and surface of the tumor in post‐treatment resected specimens (Figure 2) were counted with a light microscope in a randomly selected field at a magnification of 400‐fold. In post‐treatment resected specimens with a pathological complete response, we evaluated the density of TILs in the area replaced by granulomatous or fibrous tissue. The mean of values obtained in five different fields was used for the data analysis. We set the median value as the cut‐off value for the density of each type of TIL and then classified patients into high‐ and low‐TIL groups based on this cut‐off value.
Figure 1.
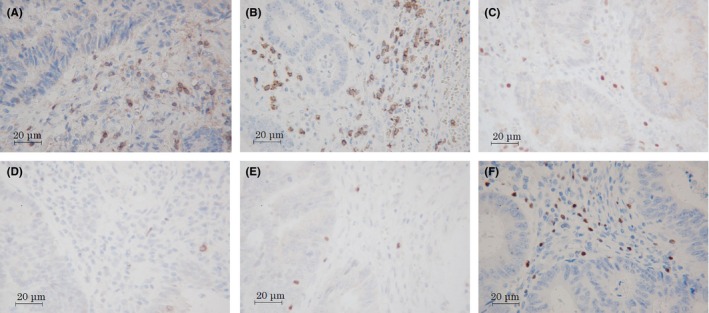
Immune marker expression of CD4 (A), CD8 (B), t‐box expressed in T cells (C), GATA‐binding protein 3 (D), RAR‐related orphan receptor‐γT (E), and forkhead box P3 (F) in locally advanced rectal cancer specimens. Magnification, ×400
Figure 2.
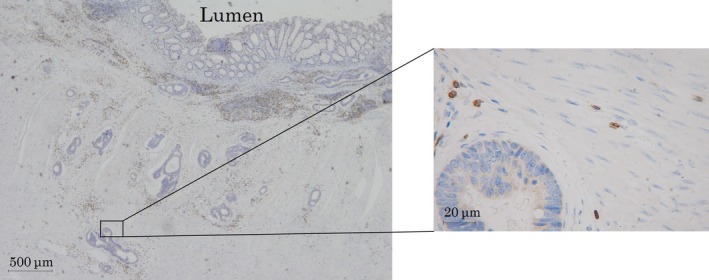
Immunoreactive tumor‐infiltrating lymphocytes determined at the invasive margin in resected specimens of locally advanced rectal cancer after neoadjuvant treatment. Magnification, ×400
2.5. Immunohistochemical evaluation for MMR status
It was reported that the effectiveness of immunohistochemical evaluation of the MMR status is similar to that of genotyping for the MSI status.17 The MSI status therefore was estimated according to the immunohistochemical evaluation of the MMR status, as previously reported.18
Normal colon tissue was used as a positive control, and positive staining within intratumoral immune cells was used as an internal positive control. The expression was evaluated as MMR‐proficient (tumor cell nuclear expression with positive immune cell expression; Figure 3A,B,D,F) or MMR‐deficient (absent tumor cell nuclear expression with positive immune cell expression; Figure 3C,E,G). The tumor was defined as MMR‐deficient when one or more MMR proteins was negatively expressed.
Figure 3.
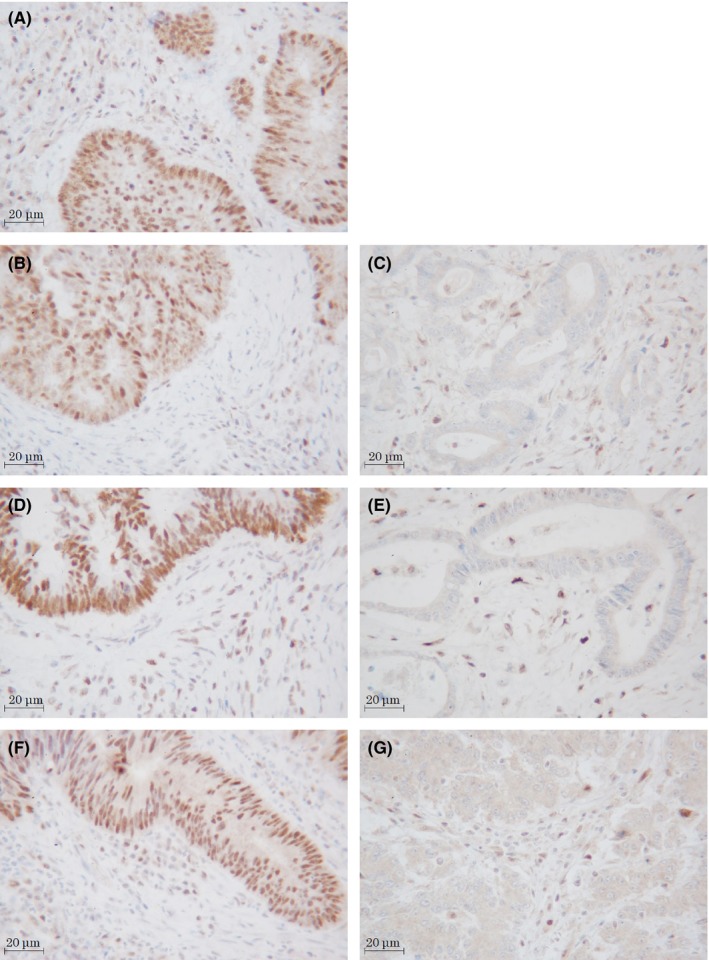
Expression of mismatch repair proteins in resected specimens of locally advanced rectal cancer after neoadjuvant treatment. (A) MLH1: positive. All of the tumor showed MLH1‐positive expression. (B,C) MSH2: positive (B), negative (C). (D,E) MSH6: positive (D), negative (E). (F,G) PMS2: positive (F), negative (G). Magnification, ×400
2.6. Immunohistochemical evaluation for HLA class I expression
We evaluated the HLA class I expression using the criteria established by the HLA and Cancer Component of the 12th International Histocompatibility Workshop.19 The tumor was defined as negative (Figure 4A) when the positive expression rate of the tumor cell membrane was <25%, positive (Figure 4B) when it was >75%, and heterogeneous when intermediate. Normal colon tissue was used as a positive control, and positive staining within intratumoral immune cells was used as an internal positive control.
Figure 4.
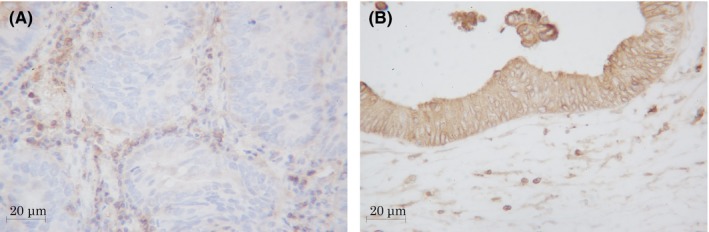
(A) Negative expression (<25%) of HLA class I in tissue microarray sections of pretreatment biopsy specimens. (B) Positive expression (>75%) HLA class I in resected specimens of locally advanced rectal cancer after neoadjuvant treatment. Magnification, ×400
2.7. Statistical analyses
The significance of the correlations between the density of TILs and the response and those between the density of TILs and the clinicopathological characteristics were analyzed using the χ2‐test and Fisher's exact test. The difference between the density of TILs in pretreatment biopsy specimens and that of TILs at the surface of the tumor in post‐treatment resected specimens was evaluated by Wilcoxon's signed rank test. All of the statistical analyses were carried out using the JMP 13.0.0 software program (2016; SAS Institute, Cary, NC, USA). P‐values <.05 were considered to indicate statistical significance.
2.8. Ethical considerations
This study conformed to the provisions of the Declaration of Helsinki. All patients were informed of the investigational nature of this study and provided their written informed consent. This retrospective study was approved by the ethics committee of Osaka City University (approved no. 3853).
3. RESULTS
3.1. Patient analysis
The patient characteristics are listed in Table 1. The distribution of pretreatment clinical depth and lymph node metastasis of cancer was as follows: cT1‐2 in 1 patient, cT3‐4 in 63, cN0 in 18, and cN1‐3 in 46 patients. Neoadjuvant treatment was given as CT in 33 patients: CapeOX, n = 22 patients; CapeOX + Bmab, n = 1; CapeOX + Cmab, n = 1; FOLFOX, n = 5; FOLFOX + Bmab, n = 2; FOLFOX + panitumumab, n = 1; and 5‐fluorouracil + leucovorin + irinotecan + Cmab, n = 1. Neoadjuvant treatment as CRT was given to 31 patients: capecitabine, n = 9; and capecitabine + Bmab, n = 22. The pathological responses of the patients were: grade 0, n = 4 patients; grade 1a, n = 25; grade 1b, n = 17; grade 2, n = 12; and grade 3, n = 6.
Table 1.
Characteristics of 64 patients with locally advanced rectal cancer treated with neoadjuvant chemotherapy (CT) or chemoradiotherapy (CRT)
| Total patients (n = 64) | Neoadjuvant CT (n = 33) | Neoadjuvant CRT (n = 31) | |||
|---|---|---|---|---|---|
| Gender | |||||
| Male | 44 | 23 | 21 | ||
| Female | 20 | 10 | 10 | ||
| Age, y | |||||
| Median (range) | 65 (27‐88) | 62 (27‐78) | 66 (44‐88) | ||
| Histological type | |||||
| Well, moderate | 58 | 29 | 29 | ||
| Poor, mucinous | 5 | 3 | 2 | ||
| cT1, 2a | 1 | 1 | 0 | ||
| cT3, 4a | 63 | 32 | 31 | ||
| cN0a | 18 | 3 | 15 | ||
| cN1‐3a | 46 | 30 | 16 | ||
| Chemotherapy regimen | |||||
| CapeOX | 22 | Cape | 9 | ||
| CapeOX + Bmab | 1 | Cape + Bmab | 22 | ||
| CapeOX + Cmab | 1 | ||||
| FOLFOX | 5 | ||||
| FOLFOX + Bmab | 2 | ||||
| FOLFOX + Pmab | 1 | ||||
| FOLFIRI + Cmab | 1 | ||||
| Pathological responseb | |||||
| Grade 0 | 4 | 4 | 0 | ||
| Grade 1a | 25 | 17 | 8 | ||
| Grade 1b | 17 | 8 | 9 | ||
| Grade 2 | 12 | 3 | 9 | ||
| Grade 3 | 6 | 1 | 5 | ||
Bmab, bevacizumab; Cape, capecitabine; CapeOX, capecitabine + oxaliplatin; Cmab, cetuximab; FOLFIRI, 5‐fluorouracil + leucovorin + irinotecan; FOLFOX, 5‐fluorouracil + leucovorin + oxaliplatin; Pmab, panitumumab.
According to UICC TNM Classification of Malignant Tumors (7th edition).
According to the definitions in the Japanese Classification of Colorectal Carcinoma (8th edition).
A low density of CD8+ and GATA3+ TILs in pretreatment biopsy samples was significantly associated with a poor pathological response (CD8, P < .001; GATA3, P = .011; Table 2). However, the density of all other TILs in pretreatment biopsy samples showed no significant relationship with the pathological response or downstaging of the primary tumor (Table 2). The density of CD8+ TILs in pretreatment biopsy samples showed no significant relationship with any clinicopathological factors or the MMR status or pretreatment HLA class I expression (Table S1).
Table 2.
Correlations between the density of tumor‐infiltrating lymphocytes (TILs) in pretreatment biopsy specimens and response to neoadjuvant treatment in 64 patients with locally advanced rectal cancer
| Pathological responsea | Downstaging of primary tumor | |||||
|---|---|---|---|---|---|---|
| Non‐respondersb | Respondersc | P‐value | Negative | Positive | P‐value | |
| Density of CD8+ TILs | ||||||
| <8 | 13 | 2 | <.001 | 13 | 2 | .21 |
| ≥8 | 3 | 12 | 9 | 6 | ||
| Density of CD4+ TILs | ||||||
| <4.6 | 8 | 5 | .480 | 10 | 3 | 1.00 |
| ≥4.6 | 8 | 9 | 12 | 5 | ||
| Density of T‐bet+ TILs | ||||||
| <2.3 | 9 | 6 | .720 | 11 | 4 | 1.00 |
| ≥2.3 | 7 | 8 | 11 | 4 | ||
| Density of GATA3+ TILs | ||||||
| <0.75 | 10 | 2 | .011 | 10 | 2 | .42 |
| ≥0.75 | 6 | 12 | 12 | 6 | ||
| Density of RORγT+TILs | ||||||
| <1.2 | 7 | 6 | 1.000 | 10 | 3 | 1.00 |
| ≥1.2 | 9 | 8 | 12 | 5 | ||
| Density of Foxp3+ TILs | ||||||
| <13.6 | 10 | 5 | .270 | 10 | 5 | .68 |
| ≥13.6 | 6 | 9 | 12 | 3 | ||
Foxp3, forkhead box P3; GATA3, GATA‐binding protein 3; RORγT, RAR‐related orphan receptor‐γT; T‐bet, t‐box expressed in T cells.
According to definitions in the Japanese Classification of Colorectal Carcinoma (8th edition).
Grade 0‐1a.
Grade 1b‐3.
A low density of CD8+ TILs at the invasive margin in post‐treatment resected specimens was significantly associated with a poor pathological response (P < .001) and a low rate of downstaging of the primary tumor (P = .007; Table 3). A low density of T‐bet+ and RORγT+ TILs in post‐treatment resected specimens was significantly associated with a low rate of downstaging of the primary tumor (T‐bet, P < .001; RORγT, P = .032) (Table 3). However, the density of all other TILs in post‐treatment resected specimens showed no significant relationship with the pathological response or the downstaging of the primary tumor (Table 3).
Table 3.
Correlations between the density of tumor‐infiltrating lymphocytes (TILs) in post‐treatment resected specimens and response to neoadjuvant treatment in 64 patients with locally advanced rectal cancer
| Pathological responsea | Downstaging of primary tumor | |||||
|---|---|---|---|---|---|---|
| Non‐respondersb | Respondersc | P‐value | Negative | Positive | P‐value | |
| Density of CD8+ TILs | ||||||
| <13.4 | 23 | 9 | <.001 | 27 | 5 | .007 |
| ≥13.4 | 6 | 26 | 16 | 15 | ||
| Density of CD4+ TILs | ||||||
| <8.2 | 15 | 16 | .800 | 21 | 10 | 1.000 |
| ≥8.2 | 14 | 19 | 22 | 10 | ||
| Density of T‐bet+ TILs | ||||||
| <8 | 17 | 13 | .130 | 27 | 3 | <.001 |
| ≥8 | 12 | 22 | 16 | 17 | ||
| Density of GATA3+ TILs | ||||||
| <0.5 | 10 | 14 | .800 | 16 | 7 | 1.000 |
| ≥0.5 | 19 | 21 | 27 | 13 | ||
| Density of RORγT+ TILs | ||||||
| <1.3 | 17 | 15 | .320 | 26 | 6 | .032 |
| ≥1.3 | 12 | 20 | 17 | 14 | ||
| Density of Foxp3+ TILs | ||||||
| <5.7 | 13 | 19 | .620 | 25 | 7 | .110 |
| ≥5.7 | 16 | 16 | 18 | 13 | ||
Foxp3, forkhead box P3; GATA3, GATA‐binding protein 3; RORγT, RAR‐related orphan receptor‐γT; T‐bet, t‐box expressed in T cells.
According to definitions in the Japanese Classification of Colorectal Carcinoma (8th edition).
Grade 0‐1a.
Grade 1b‐3.
A high density of CD8+ TILs at the invasive margin in post‐treatment resected specimens was significantly associated with a decrease in tumor depth (P = .005; Table S2). Furthermore, the density of CD8+ TILs in the post‐treatment resected specimens was significantly associated with pathological lymph node metastases (P < .001), lymphatic involvement (P = .004), and the post‐treatment serum carcinoembryonic antigen concentration (P = .002; Table S2). A one‐to‐one correspondence of CD8+ TILs was observed between pretreatment biopsy and post‐treatment resected specimens (Figure 5). In total patients and in responders, the density of CD8+ TILs at the surface of the tumor in post‐treatment resected specimens was significantly higher than in the pretreatment biopsy specimens (all patients, P = .013; responders, P = .034) (Figure 5A,B). However, in non‐responders, the density of CD8+ TILs in post‐treatment resected specimens was not higher than in the pretreatment biopsy specimens (Figure 5C).
Figure 5.
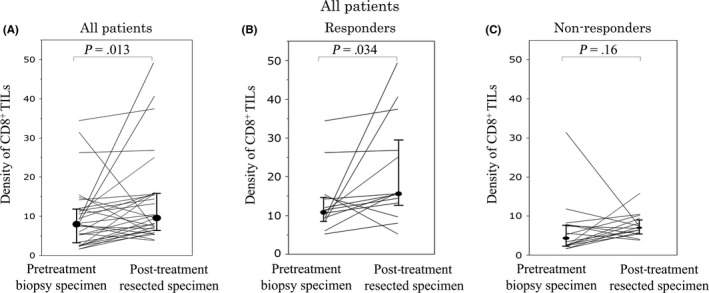
One‐to‐one correspondence of the density of CD8+ tumor‐infiltrating lymphocytes (TILs) at pretreatment biopsy and at the surface of the tumor in post‐treatment resected specimens from patients with locally advanced rectal cancer treated with neoadjuvant therapy. Changes in the density of CD8+ TILs in all patients (A) responders (i.e. pathological response grade 1b‐3) (B), and non‐responders (i.e. pathological response grade 0‐1a) (C)
3.2. Analysis of neoadjuvant CT cohort
Patient characteristics are listed in Table 1. The distribution of pretreatment clinical depth and lymph node metastasis of cancer was as follows: cT1‐2 in 1 patient, cT3‐4 in 32, cN0 in 3, and cN1‐3 in 30. The pathological response of the patients was as follows: grade 0, n = 4; grade 1a, n = 17; grade 1b, n = 8; grade 2, n = 3; and grade 3, n = 1. A low density of CD8+ TILs in pretreatment biopsy samples was significantly associated with a poor pathological response (CD8, P = .022; Table 4). However, the density of all other TILs in pretreatment biopsy samples showed no significant relationship with the pathological response or downstaging of the primary tumor (Table 4). The density of CD8+ TILs in pretreatment biopsy samples showed no significant relationship with any clinicopathological factors or the MMR status or pretreatment HLA class I expression (Table S3).
Table 4.
Correlations between the density of tumor‐infiltrating lymphocytes (TILs) in pretreatment biopsy specimens and response to treatment in the neoadjuvant chemotherapy cohort of patients with locally advanced rectal cancer (n = 33)
| Pathological responsea | Downstaging of primary tumor | |||||
|---|---|---|---|---|---|---|
| Non‐respondersb | Respondersc | P‐value | Negative | Positive | P‐value | |
| Density of CD8+ TILs | ||||||
| <8 | 10 | 0 | .022 | 9 | 1 | 1.00 |
| ≥8 | 2 | 3 | 4 | 1 | ||
| Density of CD4+ TILs | ||||||
| <4.6 | 6 | 0 | .230 | 6 | 0 | .49 |
| ≥4.6 | 6 | 3 | 7 | 2 | ||
| Density of T‐bet+ TILs | ||||||
| <2.3 | 7 | 2 | 1.000 | 8 | 1 | 1.00 |
| ≥2.3 | 5 | 1 | 5 | 1 | ||
| Density of GATA3+ TILs | ||||||
| <0.75 | 9 | 1 | .240 | 9 | 1 | 1.00 |
| ≥0.75 | 3 | 2 | 4 | 1 | ||
| Density of RORγT+ TILs | ||||||
| <1.2 | 5 | 1 | 1.000 | 6 | 0 | .49 |
| ≥1.2 | 7 | 2 | 7 | 2 | ||
| Density of Foxp3+ TILs | ||||||
| <13.6 | 8 | 1 | .530 | 8 | 1 | 1.00 |
| ≥13.6 | 4 | 2 | 5 | 1 | ||
GATA3, GATA‐binding protein 3; RORγT, RAR‐related orphan receptor gamma T; T‐bet, t‐box expressed in T cells; Foxp3, forkhead box P3.
According to definitions in the Japanese Classification of Colorectal Carcinoma (8th edition).
Grade 0‐1a.
Grade 1b‐3.
A low density of CD8+ TILs at the invasive margin in post‐treatment resected specimens was significantly associated with a poor pathological response (P < .001) (Table 5). A low density of Foxp3+ TILs in post‐treatment resected specimens was significantly associated with a low rate of downstaging of the primary tumor (P = .024), and a low density of T‐bet+ TILs in post‐treatment resected specimens tended to be associated with a low rate of downstaging of the primary tumor (P = .060; Table 5). However, the density of all other TILs in post‐treatment resected specimens showed no significant relationship with the pathological response or downstaging of the primary tumor (Table 5).
Table 5.
Correlations between the density of tumor‐infiltrating lymphocytes (TILs) in post‐treatment resected specimens and response to treatment in the neoadjuvant chemotherapy cohort of patients with locally advanced rectal cancer (n = 33)
| Pathological responsea | Downstaging of primary tumor | |||||
|---|---|---|---|---|---|---|
| Non‐respondersb | Respondersc | P‐value | Negative | Positive | P‐value | |
| Density of CD8+ TILs | ||||||
| <13.4 | 18 | 3 | <.001 | 18 | 3 | .390 |
| ≥13.4 | 3 | 9 | 8 | 3 | ||
| Density of CD4+ TILs | ||||||
| <8.2 | 10 | 4 | .490 | 12 | 2 | .670 |
| ≥8.2 | 11 | 8 | 14 | 4 | ||
| Density of T‐bet+ TILs | ||||||
| <8 | 14 | 8 | 1.000 | 20 | 2 | .060 |
| ≥8 | 7 | 4 | 6 | 4 | ||
| Density of GATA3+ TILs | ||||||
| <0.5 | 7 | 4 | 1.000 | 8 | 2 | 1.000 |
| ≥0.5 | 14 | 8 | 18 | 4 | ||
| Density of RORγT+ TILs | ||||||
| <1.3 | 12 | 7 | 1.000 | 17 | 2 | .190 |
| ≥1.3 | 9 | 5 | 9 | 4 | ||
| Density of Foxp3+ TILs | ||||||
| <5.7 | 10 | 4 | .490 | 14 | 0 | .024 |
| ≥5.7 | 11 | 8 | 12 | 6 | ||
Foxp3, forkhead box P3; GATA3, GATA‐binding protein 3; RORγT, RAR‐related orphan receptor‐γT; T‐bet, t‐box expressed in T cells.
According to definitions in the Japanese Classification of Colorectal Carcinoma (8th edition).
Grade 0‐1a.
Grade 1b‐3.
The density of CD8+ TILs at the invasive margin in post‐treatment resected specimens showed no significant relationship with any clinicopathological factors or MMR status or pretreatment HLA class I expression (Table S4). In the total patient cohort, as well as in the responders and non‐responders, the density of CD8+ TILs was not markedly changed after neoadjuvant chemotherapy (Figure 6).
Figure 6.
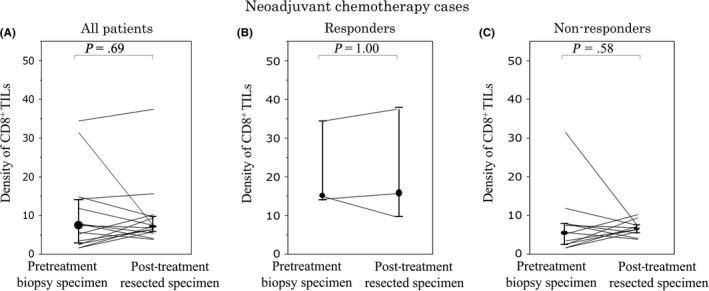
One‐to‐one correspondence of the density of CD8+ tumor‐infiltrating lymphocytes (TILs) at the pretreatment biopsy and at the surface of the tumor in post‐treatment resected specimens from patients with locally advanced rectal cancer treated with neoadjuvant chemotherapy. Changes in the density of CD8+ TILs in all patients (A) responders (i.e. pathological response grade 1b‐3) (B), and non‐responders (i.e. pathological response grade 0‐1a) (C)
3.3. Analysis of neoadjuvant CRT cohort
Patient characteristics are listed in Table 1. The distribution of pretreatment clinical depth and lymph node metastasis of cancer was as follows: cT1‐2 in no patients, cT3‐4 in 31, cN0 in 15, and cN1‐3 in 16. The pathological responses of the patients were: grade 0, n = 0; grade 1a, n = 8; grade 1b, n = 9; grade 2, n = 9; and grade 3, n = 5. A low density of CD8+ TILs in pretreatment biopsy samples tended to be associated with a poor pathological response (P = .077; Table 5). However, the density of all other TILs in pretreatment biopsy samples showed no significant relationship with the pathological response or downstaging of the primary tumor (Table 6). The density of CD8+ TILs in pretreatment biopsy samples showed no significant relationship with any clinicopathological factors or the MMR status or pretreatment HLA class I expression (Table S5).
Table 6.
No statistically significant correlation between the density of tumor‐infiltrating lymphocytes (TILs) in pretreatment biopsy specimens and response to treatment in the neoadjuvant chemoradiotherapy cohort of patients with locally advanced rectal cancer (n = 31)
| Pathological responsea | Downstaging of primary tumor | |||||
|---|---|---|---|---|---|---|
| Non‐respondersb | Respondersc | P‐value | Negative | Positive | P‐value | |
| Density of CD8+ TILs | ||||||
| <8 | 3 | 2 | .077 | 4 | 1 | .58 |
| ≥8 | 1 | 9 | 5 | 5 | ||
| Density of CD4+ TILs | ||||||
| <4.6 | 2 | 5 | 1.000 | 4 | 3 | 1.00 |
| ≥4.6 | 2 | 6 | 5 | 3 | ||
| Density of T‐bet+ TILs | ||||||
| <2.3 | 2 | 4 | 1.000 | 3 | 3 | .62 |
| ≥2.3 | 2 | 7 | 6 | 3 | ||
| Density of GATA3+ TILs | ||||||
| <0.75 | 1 | 1 | .480 | 1 | 1 | 1.00 |
| ≥0.75 | 3 | 10 | 8 | 5 | ||
| Density of RORγT+ TILs | ||||||
| <1.2 | 2 | 5 | 1.000 | 4 | 3 | 1.00 |
| ≥1.2 | 2 | 6 | 5 | 3 | ||
| Density of Foxp3+ TILs | ||||||
| <13.6 | 2 | 4 | 1.000 | 2 | 4 | .14 |
| ≥13.6 | 2 | 7 | 7 | 2 | ||
Foxp3, forkhead box P3; GATA3, GATA‐binding protein 3; RORγT, RAR‐related orphan receptor‐γT; T‐bet, t‐box expressed in T cells.
According to definitions in the Japanese Classification of Colorectal Carcinoma (8th edition).
Grade 0‐1a.
Grade 1b‐3.
A low density of CD8+ TILs at the invasive margin in post‐treatment resected specimens tended to be associated with a poor pathological response (P = .095) and a low rate of downstaging of the primary tumor (P = .057; Table 7). Furthermore, a low density of T‐bet+ TILs in post‐treatment resected specimens was significantly associated with a low rate of downstaging of the primary tumor (P = .045; Table 7). However, the density of all other TILs in post‐treatment resected specimens showed no significant relationship with the pathological response or downstaging of the primary tumor (Table 7).
Table 7.
Correlations between the density of tumor‐infiltrating lymphocytes (TILs) in post‐treatment resected specimens and response to treatment in the neoadjuvant chemoradiotherapy cohort of patients with locally advanced rectal cancer (n = 31)
| Pathological responsea | Downstaging of the primary tumor | |||||
|---|---|---|---|---|---|---|
| Non‐respondersb | Respondersc | P‐value | Negative | Positive | P‐value | |
| Density of CD8+ TILs | ||||||
| <13.4 | 5 | 6 | .095 | 9 | 2 | .057 |
| ≥13.4 | 3 | 17 | 8 | 12 | ||
| Density of CD4+ TILs | ||||||
| <8.2 | 5 | 12 | .700 | 9 | 8 | 1.000 |
| ≥8.2 | 3 | 11 | 8 | 6 | ||
| Density of T‐bet+ TILs | ||||||
| <8 | 3 | 5 | .390 | 7 | 1 | .045 |
| ≥8 | 5 | 18 | 10 | 13 | ||
| Density of GATA3+ TILs | ||||||
| <0.5 | 3 | 10 | 1.000 | 8 | 5 | .720 |
| ≥0.5 | 5 | 13 | 9 | 9 | ||
| Density of RORγT+ TILs | ||||||
| <1.3 | 5 | 8 | .230 | 9 | 4 | .270 |
| ≥1.3 | 3 | 15 | 8 | 10 | ||
| Density of Foxp3+ TILs | ||||||
| <5.7 | 3 | 15 | .230 | 11 | 7 | .480 |
| ≥5.7 | 5 | 8 | 6 | 7 | ||
Foxp3, forkhead box P3; GATA3, GATA‐binding protein 3; RORγT, RAR‐related orphan receptor‐γT; T‐bet, t‐box expressed in T cells.
According to definitions in the Japanese Classification of Colorectal Carcinoma (8th edition).
Grade 0‐1a.
Grade 1b‐3.
The density of CD8+ TILs at the invasive margin in post‐treatment resected specimens was significantly associated with lymph node metastases (P = .004) and the post‐treatment serum carcinoembryonic antigen concentration (P = .026) (Table S6). Furthermore, the high density of CD8+TILs at the invasive margin in post‐treatment resected specimens tended to be associated with a decrease in tumor depth (P = .057; Table S6).
In the total patient cohort as well as in the responders, the density of CD8+ TILs at the surface of the tumor in post‐treatment resected specimens was significantly higher than in the pretreatment biopsy specimens (all patients, P = .003; responders, P = .024) (Figure 7A,B). However, in the non‐responders, the density of CD8+ TILs in post‐treatment resected specimens was not higher than in the pretreatment biopsy specimens (Figure 7C).
Figure 7.
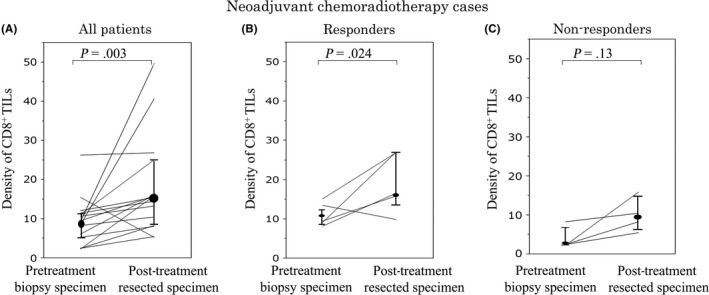
One‐to‐one correspondence of the density of CD8+ tumor‐infiltrating lymphocytes (TILs) at the pretreatment biopsy and at the surface of the tumor in post‐treatment resected specimens from patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Changes in the density of CD8+ TILs in all patients (A), responders (i.e. pathological response grade 1b‐3) (B), and non‐responders (i.e. pathological response grade 0‐1a) (C)
4. DISCUSSION
The current study showed that the local antitumor immune status before and after neoadjuvant therapy was significantly associated with the response to neoadjuvant therapy. Furthermore, we showed that neoadjuvant therapy, especially CRT, might activate local antitumor immunity.
The current study suggests that tumors attracting abundant CD8+ TILs before neoadjuvant CT or CRT could be more sensitive to CT or radiotherapy than others. Therefore, the quantitative measurement of CD8+ TILs in pretreatment biopsy samples might be a predictor of the clinical effectiveness of neoadjuvant CT and neoadjuvant CRT. Our results agreed with those of previous studies.11, 12, 13, 20 Our results were also supported by the basic research studies that have shown that CT or radiotherapy is more effective in immunocompetent mice than in immunodeficient mice and that immunosuppression counteracts the effect of CRT.21, 22 The attraction of increased numbers of TILs before neoadjuvant treatment was reported to imply that a tumor was originally immunogenic.20 For tumors attracting large numbers of TILs before neoadjuvant treatment, it is considered that not only the direct cytotoxic effects of CT or radiotherapy but also the antitumor immune response of the host will improve the effects of the treatment.
The current study showed that the density of CD8+ TILs after neoadjuvant CT or CRT positively correlated with the efficacy of the neoadjuvant treatment, such as the pathological response and downstaging of the primary tumor. Cytotoxic agents and radiation have been reported to be extremely effective as treatments, not only because they exert direct cytotoxic effects on tumor cells, but also because they activate the antitumor immune response.23, 24 Cytotoxic agents and radiation reportedly release the tumor antigens through necrotic tumor cell death, relieve the immunosuppression mechanism, and facilitate the recruitment of CD8+ TILs by stimulating the immune cells. We concluded that a better effect of the treatment was produced as a result of not only the CT‐ or radiotherapy‐mediated antitumor effect but also the antitumor effect of the antitumor immunity, as CT or radiotherapy facilitated the antitumor immune response.
Each TIL subset reportedly exerts a different effect on antitumor immunity. The current study showed that the density of CD8+ TILs before and after neoadjuvant treatment and the density of T‐bet+ TILs after neoadjuvant treatment were significantly associated with the response to treatment. Activated CD8+ T cells (i.e. cytotoxic T lymphocytes) are capable of directly killing tumor cells and play a crucial role in antitumor immunity.4, 6 However, we found that the density of CD4+ TILs before and after neoadjuvant treatment had no significant relationship with the response to treatment. Some authors have reported that the density of CD4+ TILs is positively correlated with the antitumor immune status and the response to CRT.11, 20, 25 However, a metastatic review reported that the significance of the density of CD4+ TILs in antitumor immunity is still unclear.4 The difference in the results between the previous and present studies was thought to be due to the fact that each subset of CD4+ T lymphocytes (i.e. Th1, Th2, and Th17 cells, Treg cells, and T follicular helper cells) exerts a different effect on antitumor immunity. T helper 1 cells are reportedly able to activate CD8+ T cells and enhance antitumor immunity.4, 26, 27 This fact supports our finding that a high density of T‐bet+ TILs after neoadjuvant therapy was positively correlated with a high rate of downstaging of the primary tumor. In contrast, Th2 cells, through the activation of B cells or the production of the immunosuppressive cytokine interleukin‐10, seem to suppress the antitumor immunity.4 Regarding Th17 cells, some authors have reported that these cells facilitate the antitumor immunity, whereas others have reported that they accelerate tumor growth through neoangiogenesis of the tumor.4, 26 In colorectal cancer, the significance of Foxp3+ TILs seems to be controversial. We found in the present study that the density of Foxp3+ TILs after neoadjuvant chemotherapy was positively associated with downstaging of the primary tumor, although the density of Foxp3+ TILs before and after neoadjuvant chemotherapy was not associated with the pathological response to treatment. Regulatory T cells, which express the transcription factor Foxp3, suppress the activation of T cells. A high density of Foxp3+ TILs was reported to be associated with poor prognosis in several types of cancer.28, 29 In contrast, in colorectal cancer, a previous study with a large cohort showed that a high density of Foxp3+ TILs was associated with a good prognosis.30 Furthermore, another recent study suggested that two Foxp3+ T‐cell subsets (immunosuppressive Foxp3+ Treg cells and non‐suppressive Foxp3+ T cells) distinctly control the prognosis of colorectal cancer.31 The association between the density of Foxp3+ TILs and the response to neoadjuvant therapy also remains unclear, with conflicting findings reported. Indeed, some reports have found that a low density of Foxp3+ TILs after neoadjuvant CRT for rectal cancer was associated with a complete response,32 whereas others have found that the density of Foxp3+ TILs before and after neoadjuvant therapy for rectal cancer was not associated with the response to treatment.11, 33
The findings obtained in the current study suggest that local antitumor immunity could be enhanced by increased numbers of CD8+ TILs after neoadjuvant therapy, especially in responders. Cytotoxic agents and radiation have been previously reported to be immunosuppressants because lymphocytes are very sensitive to cytotoxic agents and radiation.34 However, another aspect to the effects of cytotoxic agents and radiation was recently reported, involving their effects on enhancing the antitumor immunity and thereby contributing to their direct antitumor effects.23, 24 Both CT‐ and radiotherapy‐mediated necrotic tumor cell death were reported to induce the release of tumor antigens and facilitate the recruitment of immune cells to the tumor by mediators such as pro‐inflammatory cytokine interferon‐γ.35 In this way, CT and radiotherapy have been reported to enhance antitumor immunity.35 In addition, cytotoxic agents and radiation also enhance antitumor immunity through other mechanisms. For example, 5‐fluorouracil was reported to prevent immunosuppression by reducing the numbers of myeloid‐derived suppressor cells and promoting the production of interferon‐γ.36 Oxaliplatin was reported to induce immunogenic cancer cell death and result in the activation of the immune response of T cells.37 Radiation was further reported to activate the antitumor immune response through cancer cell death and inducing the abscopal effect.38 Furthermore, recent reports have shown that anti‐epidermal growth factor receptor antibody might increase the density of CD3+ and CD8+ TILs and enhance the antitumor immune response by inducing antibody‐dependent cell‐mediated cytotoxicity through immune cells and immunogenic cell death.39, 40 As described above, many previous reports have shown that CT and radiotherapy can induce the activation of the antitumor immune response. The subgroup analysis in the current study showed that the density of CD8+ TILs after neoadjuvant CT was not increased, whereas that after neoadjuvant CRT was notably increased. Radiation, therefore, might induce the activation of the antitumor immune response more strongly than chemotherapy.
The antitumor immune status in colorectal cancer patients reportedly depends on the MSI status of the tumor and the immunological phenotypes of cancer cells, such as HLA class I expression. High‐grade MSI colorectal cancer is reportedly more immunogenic than microsatellite stable colorectal cancer and has a high density of TILs because of the large number of tumor antigens produced by frameshift mutations.41, 42 The HLA class I expression on tumor cells reportedly plays a key role in the presentation of tumor antigens for the recognition of tumor cells by cytotoxic T cells,43 and transporters associated with antigen processing (TAP) and tapasin are involved in the processing of tumor antigens.44 The downregulation of the HLA class I expression on tumor cells might induce the immune escape of tumor cells. Indeed, the downregulation of HLA class I expression was reportedly associated with low infiltration of CD3+ and CD8+ T cells, a poor prognosis and a poor response to chemotherapy.44, 45 However, the current study showed that the density of CD8+ TILs was not associated with the MMR status of the tumor or HLA class I expression on cancer cells. We therefore believe that the density of TILs may depend on not only the immunological phenotype of cancer cells and the MSI status of the tumor, but also other immune cells and stromal cells.46
Several limitations associated with the present study warrant mention. First, the current study was retrospective in nature with included relatively few patients. Second, the current study did not analyze the molecular mechanism underlying the antitumor immunity response induced by cytotoxic agents or radiation. Third, the heterogeneity of TILs in the tumor remains an unresolved issue, although we evaluated the average number of TILs in five different fields in order to reduce the effect of this issue. Fourth, in cases of a pathological complete response after neoadjuvant treatment, we evaluated the density of lymphocytes in scar tissue in order to evaluate the TILs. However, no adequate method for evaluating the density of TILs in cases of complete response has been established. Fifth, whether the density of TILs at the surface of the tumor reflects the antitumor immune status of the whole tumor, as well as those at the invasive margin and in the center of the tumor, remains to be clarified. Finally, we did not analyze the association between the density of TILs and relapse‐free survival because the follow‐up was too short and the number of relapse cases was too few.
We concluded that T lymphocyte‐mediated immune reactions play an important role in tumor response to neoadjuvant treatment for locally advanced rectal cancer, and the quantitative measurement of CD8+ TILs in pretreatment biopsy samples could be a predictor of the clinical effectiveness of neoadjuvant treatment. Furthermore, CRT may notably activate the local immune status.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We thank Brian Quinn for providing medical writing services on behalf of JMC.
Matsutani S, Shibutani M, Maeda K, et al. Significance of tumor‐infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci. 2018;109:966–979. https://doi.org/10.1111/cas.13542
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 2. Rodel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO‐04 randomised phase 3 trial. Lancet Oncol. 2012;13:679‐687. [DOI] [PubMed] [Google Scholar]
- 3. Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fridman WH, Pages F, Sautes‐Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298‐306. [DOI] [PubMed] [Google Scholar]
- 5. Galon J, Costes A, Sanchez‐Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960‐1964. [DOI] [PubMed] [Google Scholar]
- 6. Pages F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early‐stage colorectal cancer. J Clin Oncol. 2009;27:5944‐5951. [DOI] [PubMed] [Google Scholar]
- 7. Sobin LH, Gospodarowicz MK, Wittekind C. UICC. TNM classification of malignant tumors (7th ed.). New York, NY: Wiley‐Liss; 2009. [Google Scholar]
- 8. Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261‐267. [DOI] [PubMed] [Google Scholar]
- 10. Galon J, Pages F, Marincola FM, et al. The immune score as a new possible approach for the classification of cancer. J Trans Med. 2012;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Teng F, Meng X, Kong L, et al. Tumor‐infiltrating lymphocytes, forkhead box P3, programmed death ligand‐1, and cytotoxic T lymphocyte‐associated antigen‐4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Trans Res. 2015;166:e1. [DOI] [PubMed] [Google Scholar]
- 12. Teng F, Mu D, Meng X, et al. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am J Cancer Res. 2015;5:2064‐2074. [PMC free article] [PubMed] [Google Scholar]
- 13. Shinto E, Hase K, Hashiguchi Y, et al. CD8 + and FOXP3 + tumor‐infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21(Suppl 3):S414‐S421. [DOI] [PubMed] [Google Scholar]
- 14. Shibutani M, Maeda K, Nagahara H, et al. Tumor‐infiltrating lymphocytes predict the chemotherapeutic outcomes in patients with stage IV colorectal cancer. In vivo. 2018;32:151‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sugihara K, Ajioka Y, Ishiguro S, et al. Japanese classification of colorectal carcinoma (8th ed.). Tokyo, Japan: Kanehara; 2013. [Google Scholar]
- 16. Milanes‐Yearsley M, Hammond ME, Pajak TF, et al. Tissue micro‐array: a cost and time‐effective method for correlative studies by regional and national cancer study groups. Mod Pathol. 2002;15:1366‐1373. [DOI] [PubMed] [Google Scholar]
- 17. Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352:1851‐1860. [DOI] [PubMed] [Google Scholar]
- 18. Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite Instability as a Biomarker for PD‐1 Blockade. Clin Cancer Res. 2016;22:813‐820. [DOI] [PubMed] [Google Scholar]
- 19. 12th International Histocompatibility Conference . Genetic diversity of HLA: functional and medical implications. Paris, France, June 9–12, 1996. Abstract. Hum Immunol. 1996;1996:1‐184. [PubMed] [Google Scholar]
- 20. Yasuda K, Nirei T, Sunami E, Nagawa H, Kitayama J. Density of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol. 2011;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plotnikov A, Niego B, Ophir R, Korenstein R, Keisari Y. Effective treatment of mouse metastatic prostate cancer by low electric field enhanced chemotherapy. Prostate. 2006;66:1620‐1630. [DOI] [PubMed] [Google Scholar]
- 22. Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll‐like receptor 4‐dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050‐1059. [DOI] [PubMed] [Google Scholar]
- 23. Gupta A, Sharma A, von Boehmer L, Surace L, Knuth A, van den Broek M. Radiotherapy supports protective tumor‐specific immunity. Oncoimmunology. 2012;1:1610‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andre F, Dieci MV, Dubsky P, et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res. 2013;19:28‐33. [DOI] [PubMed] [Google Scholar]
- 25. Noble F, Mellows T, McCormick Matthews LH, et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with oesophageal adenocarcinoma. Cancer Immunol Immunother. 2016;65:651‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Can Res. 2011;71:1263‐1271. [DOI] [PubMed] [Google Scholar]
- 27. Xie Y, Akpinarli A, Maris C, et al. Naive tumor‐specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942‐949. [DOI] [PubMed] [Google Scholar]
- 29. deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3 + tumor‐infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022‐3029. [DOI] [PubMed] [Google Scholar]
- 30. Salama P, Phillips M, Grieu F, et al. Tumor‐infiltrating FOXP3 + T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186‐192. [DOI] [PubMed] [Google Scholar]
- 31. Saito T, Nishikawa H, Wada H, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679‐684. [DOI] [PubMed] [Google Scholar]
- 32. McCoy MJ, Hemmings C, Miller TJ, et al. Low stromal Foxp3 + regulatory T‐cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer. Br J Cancer. 2015;113:1677‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCoy MJ, Hemmings C, Anyaegbu CC, et al. Tumour‐infiltrating regulatory T cell density before neoadjuvant chemoradiotherapy for rectal cancer does not predict treatment response. Oncotarget. 2017;8:19803‐19813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Order SE. The effects of therapeutic irradiation on lymphocytes and immunity. Cancer. 1977;39:737‐743. [DOI] [PubMed] [Google Scholar]
- 35. Frey B, Rubner Y, Kulzer L, et al. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2014;63:29‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vincent J, Mignot G, Chalmin F, et al. 5‐Fluorouracil selectively kills tumor‐associated myeloid‐derived suppressor cells resulting in enhanced T cell‐dependent antitumor immunity. Can Res. 2010;70:3052‐3061. [DOI] [PubMed] [Google Scholar]
- 37. Vacchelli E, Senovilla L, Eggermont A, et al. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2013;2:e23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925‐931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weiner GJ. Monoclonal antibody mechanisms of action in cancer. Immunol Res. 2007;39:271‐278. [DOI] [PubMed] [Google Scholar]
- 40. Inoue Y, Hazama S, Suzuki N, et al. Cetuximab strongly enhances immune cell infiltration into liver metastatic sites in colorectal cancer. Cancer Sci. 2017;108:455‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S. Tumour‐infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. 2004;91:469‐475. [DOI] [PubMed] [Google Scholar]
- 42. Ishikawa T, Fujita T, Suzuki Y, et al. Tumor‐specific immunological recognition of frameshift‐mutated peptides in colon cancer with microsatellite instability. Can Res. 2003;63:5564‐5572. [PubMed] [Google Scholar]
- 43. Tanaka K, Tsuchikawa T, Miyamoto M, et al. Down‐regulation of Human Leukocyte Antigen class I heavy chain in tumors is associated with a poor prognosis in advanced esophageal cancer patients. Int J Oncol. 2012;40:965‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han LY, Fletcher MS, Urbauer DL, et al. HLA class I antigen processing machinery component expression and intratumoral T‐Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res. 2008;14:3372‐3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Kruijf EM, van Nes JG, Sajet A, et al. The predictive value of HLA class I tumor cell expression and presence of intratumoral Tregs for chemotherapy in patients with early breast cancer. Clin Cancer Res. 2010;16:1272‐1280. [DOI] [PubMed] [Google Scholar]
- 46. Yaguchi T, Sumimoto H, Kudo‐Saito C, et al. The mechanisms of cancer immunoescape and development of overcoming strategies. Int J Hematol. 2011;93:294‐300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


