Abstract
This study aimed to describe the demographic characteristics, hospital utilizations, patterns of inpatient surgical management, and the overall state/regional variation in surgery rate among patients with disorders of sex development (DSD). We analyzed the Nationwide Inpatient Sample from 2001 to 2012 for patients younger than 21 years. DSD-related diagnoses and procedures were identified via International Classification of Diseases, Ninth Revision (ICD-9) codes. We identified a total of 43,968 DSD-related admissions. Of these, 73.4% of the admissions were designated as female and 642 (1.9%) were inpatient surgical admissions. Among neonates, less than 1% underwent any type of genital surgery. Nonsurgical admissions were associated with longer length of stay and higher cost. There was no significant regional variation in the rate of DSD surgeries, but we observed higher concentrations of DSD surgeries in states associated with tertiary referral centers.
Keywords: disorders of sex development, urology, pediatrics, epidemiology
Introduction
The term disorders of sex development (DSD) emerged from the 2005 Chicago Consensus as a new categorization for a broad constellation of congenital conditions resulting in atypical genetic, gonadal, or anatomical sex. Previously classified using a patchwork of historical and potentially stigmatizing terms, DSD-related conditions are now grouped into 3 broad categories: 46XX DSD, 46XY DSD, and sex-chromosome DSD. Most often diagnosed in the neonatal period, management of DSD can be technically challenging for clinicians and emotionally difficult for families. Current international consensus guidelines recommend emergent, multidisciplinary care for DSD neonates coordinated with pediatric urologists, endocrinologists, ethicists, geneticists, mental health professionals, and social workers at specialized, high-volume centers of excellence.1
Over the past decade in particular, advances in molecular biology have furthered our understanding of the genetic basis for many DSD conditions; similarly, a considerable body of literature has emerged on the psychosocial effects of DSD management and gender assignment both for patients and their families.2–5 However, much remains unknown about the epidemiology of DSD in the United States, with limited data currently available.6 Perhaps more important, little is known about the more controversial aspects of DSD management, particularly neonatal and surgical management patterns. Without knowing current treatment patterns, the ability to establish new best-practice guidelines for DSD is limited.
In this study, our objectives were (1) to describe the demographic characteristics, hospital utilizations (surgical vs nonsurgical), and patterns of inpatient surgical management for patients with DSD before versus after the 2005 consensus and (2) to evaluate state and regional variation in DSD surgical admissions from 2001 to 2011.
Methods
Data source
We used the Nationwide Inpatient Sample (NIS), an all-payer database managed by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). Derived from a 20% stratified probability sample of both pediatric and adult US hospitals, NIS includes post-stratification discharge weights that may be used to calculate national estimates.7
Selection of Patients
We identified all inpatient hospital encounters between 2001 and 2012 for patients (<21 years old) with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code(s) indicative of DSD, including: cervical/female genital anomaly, adrenogenital disorders, indeterminate sex, ambiguous genitalia, and androgen insensitivity (partial and total) (Appendix A). From this cohort, we abstracted which encounters had ICD-9-CM procedure codes for inpatient surgical procedures related to DSD including: hypospadias repair, chordee repair, vaginal construction/reconstruction, and so on. (Appendix 2).
Variable Definitions
When examining DSD surgical rates at the state level, we calculated the ratio of DSD surgical admissions to nonsurgical admissions for each state in groups: none (0%), low (>0%–1.6%), moderate (>1.6%–3.3%), and high (>3.3%–7.1%) based on the distribution of DSD-related surgical rates. We further defined differences in hospital utilizations between surgical and nonsurgical DSD admissions. Van Walraven comorbidity scores were calculated for each patient; these scores are derived from an Elixhauser comorbidity index and are specifically designed for use in administrative database research.8 NIS cost-to-charge files were used to convert hospital charges to costs.
Number of procedures was defined by HCUP as the total number of documented procedures, based on ICD-9 codes, present for the encounter whether valid or invalid operating room procedures (hcup-us.ahrq.gov). While DSD-related surgeries are counted in the number of procedures, this variable does not represent the number of DSD-related surgeries; rather, this count includes all procedures performed during a hospitalization, including both diagnostic and therapeutic procedures.
Statistical Analysis
We used weighted descriptive statistics to describe the characteristics of the DSD admissions by regions. To do this, we employed a univariate weighted logistic regression models for discrete variables (fitting binary, ordinal, or multinomial where appropriate). This allowed us to take into account the correlation structure of the data-set by allowing the variance within each hospital to change by year. For continuous variables we fit a weighted analysis of variance (ANOVA) to test for an association between region and outcomes. Because this was an exploratory analysis, we elected to not adjust for confounders in the regression and ANOVA models to keep the analysis descriptive.
We compared the rates of at least 1 DSD-related surgery admission (yes/no) by region using a weighted logistic regression model while accounting for the correlation structure of the data set. We excluded hospital births from this analysis since it was expected for neonates (newborns) with DSD to not have a DSD-related surgery. This facilitated an association test between region and DSD surgical admissions among admissions that were eligible for a surgery. We also reported the rates of each DSD-related surgery by time periods. We only tested for a difference in the rates of at least one DSD-related surgery.
We next created a heat map to plot the rates of surgical admissions at the state levels. Darker hues were used to represent higher rates of DSD-related surgical admissions compared with nonsurgical admissions. Year 2012 was excluded from the heat map because NIS stopped tracking hospital state after 2011 due to a database redesign.
We next compared DSD surgical admission rates (2001–2005 vs 2006–2012) using a weighted logistic regression model. These intervals were chosen to represent the time frames before and after the Chicago Consensus was released.
As a secondary analysis we compared hospital utilization between surgical admissions and non-surgical admissions. For this analysis, we did not exclude neonates. We used weighted descriptive statistics to describe in hospital deaths, hospital length of stay (LOS), and estimated cost of admission for surgical and nonsurgical admissions. We used a weighted logistic regression model to compare hospital deaths by admission type and weighted ANOVA for both hospital LOS and estimated cost by admissions type. Finally we used weighted descriptive statistics to describe the neonate characteristics, including hospital utilization from 2006 to 2012. We were unable to track neonatal births prior to 2006.
A 2-sided alpha of .05 was used as criteria for statistical significance. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Demographics
In total, we identified 43,968 (≤21 years) DSD-related encounters from 2001 to 2012 (Table 1), including newborn admissions. Of these, 17.6% were from the Northeast, 21.6% were from the Midwest, 36% were from the South, and 24.7% were from the West. Ages were skewed ranging between 0 and 21 years old with a mean age of 2.8 years (standard error [SE] 0.1) and a median age of <1 year (interquartile range [IQR] 0–1.4). Female was the assigned gender for most admissions (73.4%), with the Northeast region having the highest rates of female admissions (76.3% vs <74% in other regions; P < .01). There was an even distribution between publicly and privately/self-pay insured admissions. However, patients from the Midwest and Northeast had the highest rates of private/self-pay insurance (>54% in Midwest and Northeast vs <47% in other regions; P < .01). Most patients were seen at an urban teaching facility, though patients in the Midwest and Northeast had the highest rates (>77% in Midwest and Northeast vs <70% in other regions; P < .01). A total of 19.5% of patients had a comorbidity score of 1 or greater. Thirty-seven percent of our cohort had a median quartile income greater than the 50th percentile, though patients from the West region had the highest rates of income above the 50th percentile (43.1%; P < .01). The mean number of major operating room procedures per admission was 1.8 (SE 0.04).
Table 1.
Demographics of Disorders of Sex Development Cohort by Region.
| Northeast (n = 7745) | Midwest (n = 9517) | South (n = 15 847) | West (n = 10 858) | Total (n = 43 968) | P | |
|---|---|---|---|---|---|---|
| Age, y mean (SE) | 3.0 (0.2) | 3.0 (0.2) | 2.6 (0.1) | 2.8 (0.1) | 2.8 (0.1) | .11a |
| Race/Ethnicity, n (%) | <.01b | |||||
| White | 3855 (49.8) | 3263 (34.3) | 6122 (38.6) | 3509 (32.3) | 16 749 (38.1) | |
| Black | 1356 (17.5) | 809 (8.5) | 3120 (19.7) | 480 (4.4) | 5765 (13.1) | |
| Hispanic | 1136 (14.7) | 323 (3.4) | 3011 (19.0) | 3939 (36.3) | 8409 (19.1) | |
| Asian | 269 (3.5) | 103 (1.1) | 321 (2.0) | 739 (6.8) | 1432 (3.3) | |
| Native American | —c | 47 (0.5) | 103 (0.6) | 218 (2.0) | 376 (0.9) | |
| Other | 775 (10) | 443 (4.6) | 768 (4.8) | 372 (3.4) | 2,357 (5.4) | |
| Missing | 346 (4.5) | 4529 (47.6) | 2404 (15.2) | 1601 (14.7) | 8880 (20.2) | |
| Sex, n (%) | <.01b | |||||
| Male | 1749 (22.6) | 2401 (25.2) | 4149 (26.2) | 2692 (24.8) | 10 991 (25.0) | |
| Female | 5911 (76.3) | 7003 (73.6) | 11498 (72.6) | 7866 (72.4) | 32 279 (73.4) | |
| Missing | 85 (1.1) | 113 (1.2) | 200 (1.3) | 300 (2.8) | 698 (1.6) | |
| Admission year, n (%) | .91b | |||||
| 2001–2005 | 3273 (42.3) | 3911 (41.1) | 6550 (41.3) | 4199 (38.7) | 17 932 (40.8) | |
| 2006–2011 | 4473 (57.7) | 5606 (58.9) | 9298 (58.7) | 6659 (61.3) | 26 036 (59.2) | |
| Insurance, n (%) | <.01b | |||||
| Public | 3321 (42.9) | 4006 (42.1) | 8743 (55.2) | 5061 (46.6) | 21 130 (48.1) | |
| Private/Self pay | 4305 (55.6) | 5204 (54.7) | 6426 (40.6) | 5060 (46.6) | 20 995 (47.8) | |
| Other/Missing | 120 (1.6) | 307 (3.2) | 678 (4.3) | 738 (6.8) | 1843 (4.2) | |
| Hospital type, n (%) | <.01b | |||||
| Rural | 465 (6.0) | 660 (6.9) | 1094 (6.9) | 318 (2.9) | 2537 (5.8) | |
| Urban nonteaching | 917 (11.8) | 1349 (14.2) | 3813 (24.1) | 3262 (30.0) | 9 341 (21.2) | |
| Urban teaching | 6363 (82.2) | 7414 (77.9) | 10835 (68.4) | 7190 (66.2) | 31 802 (72.3) | |
| Missing | 0 (0.0) | 94 (1.0) | 106 (0.7) | 88 (0.8) | 288 (0.7) | |
| Hospital bed size, n (%) | <.012 | |||||
| Small | 1105 (14.3) | 1883 (19.8) | 1621 (10.2) | 653 (6.0) | 5262 (12.0) | |
| Medium | 1779 (23.0) | 1922 (20.2) | 4039 (25.5) | 3519 (32.4) | 11 259 (25.6) | |
| Large | 4861 (62.8) | 5618 (59.0) | 10082 (63.6) | 6598 (60.8) | 27 159 (61.8) | |
| Missing | 0 (0.0) | 94 (1.0) | 106 (0.7) | 88 (0.8) | 288 (0.7) | |
| Household income percentile, n (%) | <0.012 | |||||
| 0–25th | 1853 (23.9) | 1963 (20.6) | 5325 (33.6) | 2034 (18.7) | 11 175 (25.4) | |
| 26th to 50th | 1213 (15.7) | 2465 (25.9) | 3174 (20.0) | 2399 (22.1) | 9252 (21.0) | |
| 51st to 75th | 1365 (17.6) | 1980 (20.8) | 2727 (17.2) | 2443 (22.5) | 8515 (19.4) | |
| 76th to 100th | 1884 (24.3) | 1516 (15.9) | 2080 (13.1) | 2241 (20.6) | 7722 (17.6) | |
| Missing | 1430 (18.5) | 1594 (16.7) | 2541 (16.0) | 1740 (16.0) | 7305 (16.6) |
Weighted analysis of variance.
Univariate weighted logistic regression.
Count less than 15.
DSD Surgical Admissions Before and After Chicago Consensus
There was a significant difference between the time period (2001–2005 vs 2006–2012) and DSD-related surgical admissions. After 2005, there was a higher rate of DSD-related surgical admissions (1.5% between 2001 and 2005 vs 2.4% between 2006 and 2012; P = .01). We observed that the rates of each DSD surgery were higher in all DSD surgeries except for hysterectomy (Table 2).
Table 2.
Disorders of Sex Development Surgical Admissions by Treatment Year.a
| 2001–2005 (n = 17 932), n (%) | 2006–2012 (N = 15 597), n (%) | P | |
|---|---|---|---|
| Any genital surgery | 261 (1.5) | 381 (2.4) | .01b |
| Reconstruction of penis | 0 (0.0) | 30 (0.2) | |
| Urethral construction | 97 (0.5) | 131 (0.8) | |
| Hypospadias | 78 (0.4) | 201 (1.3) | |
| Chordee repair | 24 (0.1) | 77 (0.5) | |
| Orchiectomy | —c | 15 (0.09) | |
| Hysterectomy | 76 (0.4) | 41 (0.3) | |
| Penile repair | —c | 23 (0.1) |
We only modeled any transgender surgery. The remainder procedures are what we screened to determine if a patient had a transgender surgery and we only report the distribution.
Univariate weighted logistic regression.
Count less than 15.
DSD Hospital Utilization by Admission Type
Nonsurgical admissions had a significantly higher inhospital death compared to surgical admissions (1.8% vs 0%; P < .01). Similarly, nonsurgical admissions had a significantly higher LOS compared with surgical admissions (mean 8.2 days [SE 0.2] vs 4.6 days [SE 0.5]; P < .01). There were extreme outliers present among non-surgical admissions (longest LOS = 298 days), and there were significantly more nonsurgical admissions compared with surgical (43,317 vs 651 admissions, respectively). This likely biased the mean and median LOS to be longer among the nonsurgical group. The mean cost was similar between the 2 groups ($14 985 [SE 628.9] for nonsurgical vs $14 846 [SE 1306.7] for surgical), but the median cost of surgical admissions was almost double the nonsurgical admissions ($9325.9; IQR $6786.6 to $17 370 vs $4153.5; IQR $1473.4 to $12 240). However, there was no significant difference in the overall cost between the 2 admission types (P = .92).
Neonatal Characteristics and Surgical Rate
We identified a subcohort of 10 444 DSD neonates between the years 2006 and 2012. Female gender was assigned in 75% of admissions. Most neonates were publicly insured (52.5%). In all, 8.1% of neonates had a Van Walraven comorbidity score ≥1 with a mean score of 0.4 (SE 0.03). Overall, 62.3% of neonates were seen in an urban teaching facility. Forty-three percent of the neonates’ families had a median income greater than the 50th percentile which was higher than the full cohort. The mean number of major operations performed per encounter was 1.9 (SE 0.1).
Less than 1% of neonates had a DSD-related surgery. A total of 2.9% of neonates died in the hospital. Neonates stayed between 0 and 180 days, with 10.9 mean number of days (SE 0.5), and a median of 2.4 days (IQR 1.3–9.8 days). The cost of neonate admissions ranged between $34 and $1 295 634 with a mean cost of $16 567 (SE $1258.6) and a median cost of $1881.01 (IQR $872.1 to $12 429).
DSD Surgical Admissions by Region and State
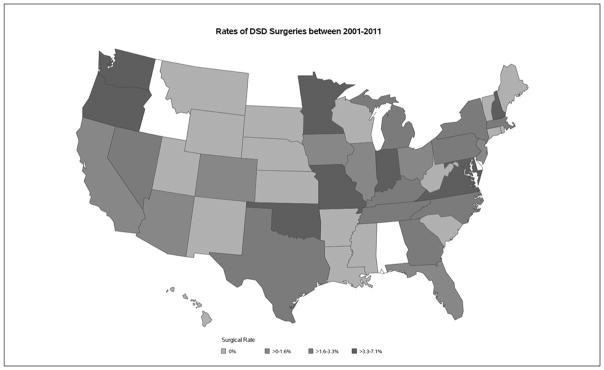
There was no evidence of a significant difference between regions (Midwest, Northeast, South, and West) and surgical admissions. The overall rate of surgical admissions from year 2001 to 2012 was 1.9% (Table 3). When looking at the distribution of state level DSD surgical rates, we observed concentrations of surgeries in states. Washington, Oregon, Minnesota, Oklahoma, Missouri, Indiana, Virginia, Maryland, and New Hampshire had the highest rates of DSD surgical admissions from 2001 to 2011, ranging between 3.3% and 7.1%. Alabama, Delaware, and Idaho were not present in our sample (Figure 1).
Table 3.
Disorders of Sex Development Surgical Admissions by Region.
| Midwest (n = 7230) | Northeast (n = 5824) | South (n = 12 105) | West (n = 8369) | Total (n = 33 529) | P | |
|---|---|---|---|---|---|---|
| Any genital surgery | 145 (2.0%) | 126 (2.2%) | 244 (2.0%) | 127 (1.5%) | 642 (1.9%) | .75a |
Univariate weighted logistic regression.
Figure 1.
Heat map for surgical admissions by state. 0%, no surgeries; >0% to 1.6%, low surgical rate; >1.6% to 3.3%, moderate surgical rate; >3.3% to 7.1%, high surgical rate.
Discussion
Awareness of DSD among physicians, policy makers, and the lay public has increased over the past decade as the biological basis and psychosocial implications of genital ambiguity have become clearer and better publicized.9 The best approaches to medical management of children with DSD remain controversial and are subject to debate. A decade after the Chicago Consensus, confusion persists over how, when, and where best to treat patients with DSD, and recommendations published by advisory bodies capture this uncertainty.10,11 Limited pediatric and urologic literature currently exists about whether the recommendations set forth in 2006 are being implemented consistently and effectively. This study, to our knowledge, represents the first contemporary, national-level investigation of DSD patient characteristics, distributions (both before and after the 2005 consensus), and inpatient surgical management patterns in the United States.
We found a small but significant increase in DSD surgical admissions from 2001–2005 (1.5%) to 2006–2012 (2.4%). We initially hypothesized a decrease in DSD surgical admissions after the 2005 consensus as new recommendations continue to caution against unnecessary genital surgeries until an age of patient informed consent due to the related adverse outcomes. However, the increase in surgical admissions seen in our study may be due to the fact that clinicians are more attuned to who and when to treat given the better-defined DSD nomenclature, new DSD categorization, and more specific management guidelines after the 2005 consensus. This shift in DSD surgical practices may provides support for this more selective surgical practice pattern.
We found that nonsurgical admissions had a longer LOS and higher mortality. The overall cost was similar between the two admission types. The longer LOS and higher mortality associated with non-surgical admissions could be due to increased comorbidity among non-surgical patients (possibly why they were non-surgical); likewise these patients could require more intensive medical management compared to the surgical patients. One would expect that surgical admissions would have a higher cost than non-surgical admissions due to the complexity of genital reconstruction. However, we found a longer LOS in the nonsurgical group, in large part due to the presence of extreme outliers among non-surgical admissions (longest LOS = 298 days); thus, this has likely biased the mean LOS and hospital charge rates to be longer among the nonsurgical group.
Children diagnosed with DSD were more likely to be assigned female gender at birth, consistent with prior studies.12,13 A plurality of DSD patients were publicly insured or paid out of pocket for their care, and represented approximately 50% from households in lower income quartiles. Costs associated with DSD—both in terms of resources and hospital charges—were high, particularly for neonates. Average LOS exceeded 1.5 weeks, and inpatient costs exceeded $16 000 per admission. In light of these findings, as well as the broader financial impact felt by families of pediatric patients as a result of lost time and productivity when caring for a hospitalized child, it is likely the economic impact associated with DSD is significant, warranting future investigation.14
Optimal timing of surgical management for children with DSD is unclear. Some authors have suggested that early surgical intervention, particularly during infancy, may be warranted due to beneficial hormonal effects on healing and development of the reconstructed genitalia, as well as psychosocial benefits for patients and families.3,13,15,16 However, as noted by Houk et al, direct comparisons between surgical outcomes of early versus childhood or pubescent DSD surgeries are lacking, and other authors have described poor functional, cosmetic, and patient-satisfaction results experienced by children later in life as a result of surgery during infancy.1, 17–20
With the aforementioned controversy regarding early surgical intervention, less parental inclination for surgery for less severe forms of clitoromegaly, neonatal anesthesia risks, and emphasis on a multi-disciplinary management approach, we hypothesized that the rate for infancy/neonatal DSD surgery would decrease or remain constant over the study period. Indeed, we found that less than 1% of DSD neonates underwent DSD-related surgery during their initial hospital admission. However, nonneonatal DSD surgical admissions increased since 2005; whether this is due to an increased numerator (surgical admissions) or a decreased denominator (nonsurgical admissions) is unclear and, based on these data at least, unknowable. Despite the persistent progress in DSD management, its inherent complexity continues to pose challenges on the indications, timing, and evaluation for DSD surgery.21
Our analysis of 43 968 encounters over a 12-year study period did not reveal associations between DSD surgical admissions and region, although we did observe high rates of surgical admissions within certain states. Further investigations accounting for confounders are necessary to understand these possible discrepancies.
Of the 9 states in our study that had the highest DSD surgical admissions, 5 of the states had an accredited pediatric urology fellowship program (WA, OK, MO, IN, and MD). The other four states (OR, MN, VA, and NH) were all in close proximity to large referral centers in their region as well. This observation is in line with the recommendations for care of such patients at tertiary care centers experienced in both medical and surgical DSD management.4,13,22
The findings of our study must be interpreted in the context of its design limitations. NIS represents a 20% stratified sample of U.S. hospital admissions; as such, our reported results may not be generalizable to all US hospitalizations. HCUP is a retrospective administrative database, which limits our inference to associations. While NIS allows us to draw inference at the national level, we are unable to account for multiple observations from the same patient. However, NIS provides rigorous tracking of discharge and hospital weights in order to minimize the risk of sampling bias. Additionally, NIS might be affected by miscoding bias. Our analysis is sensitive to the accuracy of diagnostic and procedure coding in NIS; while the accuracy level of NIS is quite high for an administrative database, it is possible that at least some portion of our cohort may be incorrectly coded. Despite these limitations, the NIS database is rigorously monitored and audited for coding accuracy and has long been reliably used to evaluate patterns of both adult and pediatric care, and represents a reliable panorama of the characteristics of an inpatient surgical cohort.
We did not perform any formal statistical analysis at the state level for DSD surgical admissions, and our observations will need to be validated with an appropriate statistical model. When comparing non-surgical to surgical admissions, we found significantly more non-surgical admissions. This makes the surgical group more susceptible to outliers. Our modeling approaches did not adjust for confounders, but we still accounted for the complex survey design present in NIS. Despite these limitations to our statistical approach, to our knowledge, no other article has described this population using a national administrative database.
Perhaps most important, given the number of DSD-related surgeries that are typically performed on an outpatient basis, it is likely that our findings significantly underreport the total number of DSD-related surgeries that occurred during the study period. We believe the use of NIS is justifiable, however, given that its considerable depth of data capture is unmatched in other pediatric datasets.
Our analysis adds a richer and in-depth look at important factors surrounding DSD management in the United States that have not previously been analyzed on a national level. However, it ultimately is only a preliminary view that touches the surface of this highly complex issue. Future investigations are needed to better understand the reasons underlying these phenomena.
Conclusion
Neonates diagnosed with DSD were most commonly assigned as female gender and were managed without immediate surgery. DSD surgical admission rates in the United States were evenly distributed regionally, but we observed concentrations of surgical rates at the state level. Since the 2005 DSD consensus meeting, the rate of nonneonatal DSD surgical admissions has increased.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Routh is supported in part by grant K08-DK100534 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The Duke Biostatistics Core’s support of this project was made possible (in part) by Grant Number UL1TR001117 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Appendix A. ICD-9 Codes for Diagnoses of DSD
| ICD-9 | Diagnosis |
|---|---|
| 259.51, 259.50, 259.5 | Androgen insensitivity |
| 259.52 | Part androgen insensitivity |
| 255.2 | Adrenogenital disorders |
| 752.49 | Cervix/fem gen anom NEC |
| 752.7 | Indeterminate sex |
Abbreviations: ICD-9, International Classification of Diseases, Ninth Revision; DSD, disorders of sexual development; NEC, not elsewhere classified.
Appendix B. ICD-9 Codes for Inpatient DSD-Related Procedures
| ICD-9 | Surgery |
|---|---|
| 64.5 | Sex transformation NOS |
| 64.44 | Reconstruction of penis |
| 64.45 | Replantation of penis |
| 58.46 | Urethral construction/recon |
| 58.45 | Hypospadias |
| 64.43 | Construction of penis |
| 64.49 | Penile repair NEC |
| 64.42 | Chordee repair |
| 64.94, 64.95, 64.97 | Penile prosthesis insertion |
| 62.3, 62.4 | Orchiectomy |
| 62.7 | Testicular prosthesis |
| 68.3, 68.4, 68.5, 68.6, 68.7, 68.9 | Hysterectomy |
| 65.3, 65.4, 65.5, 65.6 | Oophorectomy |
| 70.6 | Vaginal construction |
Abbreviations: ICD-9, International Classification of Diseases, Ninth Revision; DSD, disorders of sexual development; NOS, not otherwise specified; NEC, not elsewhere classified.
Footnotes
Author Contributions
RT and RJ contributed towards manuscript draft. SW and GMP performed statistical analysis. DWA, BJY, MA, JSW and JTP contributed to editing. JCR contributed to project oversight and editing.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The article’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH. The funding source had no role in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
References
- 1.Houk CP, Hughes IA, Ahmed SF, Lee PA Writing Committee for the International Intersex Consensus Conference Participants. Summary of consensus statement on intersex disorders and their management. International Intersex Consensus Conference. Pediatrics. 2006;118:753–757. doi: 10.1542/peds.2006-0737. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Buritica D. Overview of genetics of disorders of sexual development. Curr Opin Pediatr. 2015;27:675–684. doi: 10.1097/MOP.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 3.Warne G, Grover S, Hutson J, et al. Murdoch Childrens Research Institute Sex Study Group. A long-term outcome study of intersex conditions. J Pediatr Endocrinol Metab. 2005;18:555–567. doi: 10.1515/jpem.2005.18.6.555. [DOI] [PubMed] [Google Scholar]
- 4.Palmer BW, Wisniewski AB, Schaeffer TL, et al. A model of delivering multi-disciplinary care to people with 46 XY DSD. J Pediatr Urol. 2012;8:7–16. doi: 10.1016/j.jpurol.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Allen L. Disorders of sexual development. Obstet Gynecol Clin North Am. 2009;36:25–45. doi: 10.1016/j.ogc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd JC, Wiener JS, Gargollo PC, Inman BA, Ross SS, Routh JC. Contemporary epidemiological trends in complex congenital genitourinary anomalies. J Urol. 2013;190(4 suppl):1590–1595. doi: 10.1016/j.juro.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 9.Khosla R, Say L, Temmerman M. Sexual health, human rights, and law. Lancet. 2015;386:725–726. doi: 10.1016/S0140-6736(15)61449-0. [DOI] [PubMed] [Google Scholar]
- 10.Mouriquand P, Caldamone A, Malone P, Frank JD, Hoebeke P. The ESPU/SPU standpoint on the surgical management of disorders of sex development (DSD) J Pediatr Urol. 2014;10:8–10. doi: 10.1016/j.jpurol.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Evaluation of the newborn with developmental anomalies of the external genitalia. American Academy of Pediatrics. Committee on Genetics. Pediatrics. 2000;106(1 pt 1):138–142. doi: 10.1542/peds.106.1.138. [DOI] [PubMed] [Google Scholar]
- 12.Mazur T. Gender dysphoria and gender change in androgen insensitivity or micropenis. Arch Sex Behav. 2005;34:411–421. doi: 10.1007/s10508-005-4341-x. [DOI] [PubMed] [Google Scholar]
- 13.Romao RL, Salle JL, Wherrett DK. Update on the management of disorders of sex development. Pediatr Clin North Am. 2012;59:853–869. doi: 10.1016/j.pcl.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Prosser LA, Hammitt JK, Keren R. Measuring health preferences for use in cost-utility and cost-benefit analyses of interventions in children: theoretical and methodological considerations. Pharmacoeconomics. 2007;25:713–726. doi: 10.2165/00019053-200725090-00001. [DOI] [PubMed] [Google Scholar]
- 15.Lee PA, Witchel SF. Genital surgery among females with congenital adrenal hyperplasia: changes over the past five decades. J Pediatr Endocrinol Metab. 2002;15:1473–1477. doi: 10.1515/jpem.2002.15.9.1473. [DOI] [PubMed] [Google Scholar]
- 16.Migeon CJ, Wisniewski AB, Gearhart JP, et al. Ambiguous genitalia with perineoscrotal hypospadias in 46,XY individuals: long-term medical, surgical, and psychosexual outcome. Pediatrics. 2002;110:e31. doi: 10.1542/peds.110.3.e31. [DOI] [PubMed] [Google Scholar]
- 17.Creighton SM, Liao LM. Changing attitudes to sex assignment in intersex. BJU Int. 2004;93:659–664. doi: 10.1111/j.1464-410x.2003.04694.x. [DOI] [PubMed] [Google Scholar]
- 18.Alizai NK, Thomas DF, Lilford RJ, Batchelor AG, Johnson N. Feminizing genitoplasty for congenital adrenal hyperplasia: what happens at puberty? J Urol. 1999;161:1588–1591. [PubMed] [Google Scholar]
- 19.Bailez MM, Gearhart JP, Migeon C, Rock J. Vaginal reconstruction after initial construction of the external genitalia in girls with salt-wasting adrenal hyperplasia. J Urol. 1992;148(2 pt 2):680–682. doi: 10.1016/s0022-5347(17)36691-0. [DOI] [PubMed] [Google Scholar]
- 20.Eroğlu E, Tekant G, Gündoğdu G, et al. Feminizing surgical management of intersex patients. Pediatr Surg Int. 2004;20:543–547. doi: 10.1007/s00383-004-1208-5. [DOI] [PubMed] [Google Scholar]
- 21.Mouriquand PD, Gorduza DB, Gay CL, et al. Surgery in disorders of sex development (DSD) with a gender issue: if (why), when, and how? J Pediatr Urol. 2016;12:139–149. doi: 10.1016/j.jpurol.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Lee PA, Houk CP, Ahmed SF, Hughes IA. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics. 2006;118:e488–e500. doi: 10.1542/peds.2006-0738. [DOI] [PubMed] [Google Scholar]