Abstract
Rationale
Acute or chronic environmental enrichment (EE) reduces sucrose cue reactivity in rats. This effect may be mediated by dopamine receptors.
Objectives
We examined whether dopamine D1 or D2 receptor agonism could reverse the EE effect. We also examined whether any reversal effects would vary with the incubation of sucrose craving.
Methods
Following 10 days (2 h/day) of sucrose self-administration, rats experienced either 1 or 30 days of forced abstinence and either overnight (acute) or 29 day (chronic) EE. D1 (SKF 81297; 0, 0.3, or 1 mg/kg) or D2 (quinpirole; 0, 0.1, or 0.3 mg/kg) agonist was administered systemically immediately prior to a subsequent 2-h cue reactivity test the next day (n = 9–12 per group).
Results
Dose-dependent effects were limited to the day 1 test. High doses of the agonists increased day 1 acute EE cue reactivity to levels comparable to control animals. On the day 30 test, SKF 81297 increased cue reactivity in acute EE, chronic EE, and control rats. In contrast, quinpirole resulted in similar cue reactivity for control and enriched rats, more from a reduction in responding by controls vs. a recovery of responding by EE-experienced rats.
Conclusions
Both D1 and D2 receptors may be involved in the acute EE-mediated decrease in cue reactivity observed following 1 day of forced abstinence. In contrast, at 30 days of forced abstinence, D1 receptors may be critical in cue reactivity as SKF 81297 was effective at both restoring responding of enriched animals and potentiating responding of controls.
Keywords: Craving, Cuereactivity, Dopamine, Incubation, Relapse, Sucrose
Introduction
Craving is often defined clinically as a “strong desire” for a substance; this behavior has been modeled with rats responding for drug or food-associated stimuli (Grimm 2011). This seeking, or cue reactivity, increases over protracted abstinence (“incubation of craving”) (Grimm 2012; Venniro et al. 2016). We have reported previously that either acute (overnight) or prolonged (29 days) environmental enrichment (EE) significantly reduced sucrose seeking, including incubated sucrose seeking, and also reduced sucrose self-administration in rats (Grimm et al. 2008, 2013, 2016). EE has also been shown to decrease cocaine and ethanol seeking in rats (Chauvet et al. 2009; Thiel et al. 2009; Li et al. 2015).
Little is known about the neurotransmitter system(s) that may be involved with the decrease in reward seeking observed after exposure to enriched environments. Of the findings yet reported, there are data supporting a role for altered dopamine function following EE. For example, several weeks of EE was associated with decreased dopamine D1 receptor expression (Del Arco et al. 2007; Gill et al. 2013; Ferland et al. 2014), less D1 agonist-induced Fos protein expression (Mazarakis et al. 2014) in mesolimbic brain regions, and less D1 receptor-induced locomotion when a D1 agonist was microinjected into the medial prefrontal cortex (Del Arco et al. 2007).
Dopamine receptors and dopaminergic circuits, particularly those within the mesolimbic dopamine system, mediate incentive motivation and reinforcement (Wise 2004). Therefore, these findings indicate changes in dopamine-mediated reward processing, especially when taken in the context of previous findings with dopamine agonists and antagonists. Dopamine receptor agonism enhances responding for cues previously associated with water or food (Grimm et al. 2006; Gerdjikov et al. 2011; Saunders et al. 2013; du Hoffmann and Nicola, 2014). Dopamine antagonists reduce responding in the presence of, or for, cues associated with reinforcers including cocaine (Shalev et al. 2002) and sucrose (Grimm et al. 2011; Guy et al. 2011).
The EE effect of reducing reward seeking, along with a downregulation in dopamine receptor signaling, then fits with a hypothesis of a blunted incentive motivational response system following EE. For example, the overall locomotor effect of amphetamine is reduced following EE in both rats (Bowling et al. 1993) and mice (Heinla et al. 2014). Rats with prolonged exposure to EE also had decreased reward sensitivity (Kirkpatrick et al. 2013) and reduced amphetamine (Meyer and Bardo, 2015) and methylphenidate self-administration (Alvers et al. 2012). There are pharmacological explanations for the psychostimulant-mediated effects, including EE-mediated changes, in dopamine transporter levels and/or function (Darna et al. 2015). However, there may be other reasons for less reward-related responsivity in EE-exposed rats, in particular when not in the context of a psychostimulant challenge. One possibility is that EE or loss of EE produces a state of low motivation, perhaps reflecting decreased dopamine signaling (Ikemoto et al. 2015; Salamone et al. 2016).
To confirm a role for dopamine in the anti reward-seeking effect of EE, rats were trained for 10 days (2 h/ day) to self-administer a 10 % sucrose solution on a fixed-ratio (FR)1 schedule of reinforcement, where each sucrose delivery was accompanied by compound (light + tone) stimulus. Responding for the cue in the absence of sucrose (cue reactivity Test) was then assessed in a 2-h session after either 1 or 30 days of forced abstinence in either the home cage or in a large environment with two other rats and toys (EE). Immediately prior to the cue reactivity Test, rats were pre-treated with either the D1 receptor agonist SKF 81297 (D1 Ki 1.9 nM, D2 Ki >1000 nM; Neumeyer et al. 2003) or the D2 receptor agonist quinpirole (D2 Ki 48 nM, D1 Ki > 1000 nM; Mottola et al. 2002). This experimental design allowed us to examine the effects of D1 or D2 receptor agonism on cue reactivity after either one night (acute) or 29 days (chronic) EE and how any effect(s) might interact with the incubation of sucrose craving.
Materials and methods
Subjects
Three hundred one male Long-Evans rats (approximately 3.5 months old at start of study), bred in the Western Washington University vivarium, were used for this experiment. Experimental group sizes were n = 9–12 per group. This range was chosen based on our previous studies to account for attrition and to provide enough statistical power to detect effects of EE and incubation on sucrose seeking (Grimm et al. 2013). Prior to any enrichment treatment, rats were housed individually in Micro-Isolator chambers (20 × 32 × 20 cm; Lab Products, Inc., Seaford, DE, USA) under a 12-h reverse day/night cycle with lights off at 0700 h. All training and testing occurred between 0900 and 1100 h. Food (Purina Mills Inc. Mazuri Rodent Pellets, Saint Louis, MO, USA) and water were available ad libitum throughout the study except for a pre-training water deprivation 17 h prior to the first training session. Body weights were recorded every Monday, Wednesday, and Friday for the duration of the study. All procedures followed the “Public Health Service Policy on Humane Care and Use of Laboratory Animals” (PHS 2015) and were approved by the Western Washington University Institutional Animal Care and Use Committee.
Apparatus
Experimental procedures took place in operant conditioning chambers (30 × 20 × 24 cm; Med Associates, St. Albans, VT, USA) equipped with one retractable lever to the left side of the reward receptacle where sucrose solution was dispensed. A stationary lever was located on the opposite wall. The operant conditioning chambers included a red houselight on the wall opposite the retractable lever. Above the retractable lever was a white stimulus light and a sound generator (2 kHz, 15 dB over ambient noise). Each chamber was equipped with four infrared photobeam infrared emitters and detectors (Med Associates) to provide a measure of locomotion during training and testing sessions. This system did not differentiate between vertical or horizontal activity or stereotypy. The operant conditioning chambers were enclosed in sound-attenuating chambers equipped with fans to provide air flow and white noise.
Behavioral procedures
Training
Sessions began with illumination of the house light that remained on for the duration of the session and insertion of the retractable lever that remained extended for the duration of the session. Rats underwent 10 daily 2-h sessions wherein they learned to press the retractable lever for a 0.2 mL delivery of 10 % sucrose into the reward receptacle. These “active” lever presses were reinforced under a fixed-ratio 1 schedule with a 40 s “time-out.” Specifically, an active lever press was accompanied with a 5-s combined presentation of the white stimulus light and the tone along with reinforcer delivery. For this 5 s and the following 35 s, active lever responses were not reinforced but were recorded. Presses on the stationary (“in-active”) lever elicited no response and were recorded as a control for lever discrimination and a non-specific motor activity.
Forced abstinence
Following the tenth training session, rats were randomly assigned to a treatment condition consisting of a cross between duration of forced abstinence and a type of housing condition. The forced abstinence period was either from the end of the tenth training session to a testing session the next morning (22 h; “Day 1”) or to a testing session 30 days later (“Day 30”).
Environmental enrichment
EE consisted of a mixture of housing and social enrichment (Grimm et al. 2013). The EE housing was a large, 4-level wire-mesh environment (91 × 51 × 102 cm; Quality Cage Company, Portland, OR, USA) with novel toys replenished each M, W, F. Three rats were housed together in EE.
EE was either acute (EEACUTE) or chronic (EECHR). Acute groups were created so that rats experienced EE from the end of the tenth day training session or the 29th day of forced abstinence until testing the next morning (22 h). These were the Day 1 and Day 30 EEACUTE conditions. The one EECHR condition was exposure to EE from the end of the tenth day training session until testing on Day 30. All control (CON) rats remained single-housed.
Testing
Testing conditions were identical to training except the syringe containing sucrose solution was absent. Rats were randomly assigned to drug dose conditions within each experiment. As rats had already been randomly assigned to treatment conditions, this resulted in cohorts of rats (typically 8–12 subjects) each assigned to one of a variety of testing conditions. Drugs were stored at 4 °C in the dark and used within 48 h of preparation. SKF 81297 hydrobromide (Tocris Bioscience, Minneapolis, MN, USA) and (−) quinpirole hydrochloride (R & D Systems, Minneapolis, MN, USA) were dissolved in sterile 0.9 % saline and administered according to body weight (1 ml/ kg) to provide the doses indicated in Figs. 1–4. Doses were selected from the lowest range of each drug that has been shown to reinstate extinguished cocaine seeking in rats (Self et al. 1996; Alleweireldt et al. 2002). Rats received handling injections of saline (1 ml/kg) in the vivarium in the afternoon during the 2 days prior to testing. Testing day injections occurred immediately prior to testing in the room containing the operant conditioning chambers. SKF 81297 was administered SC; quinpirole, IP.
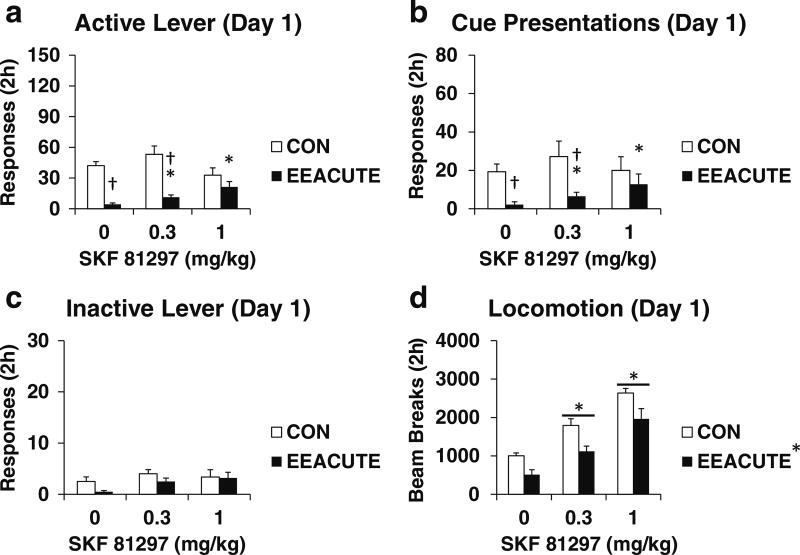
Fig. 1.
Testing measures on Day 1 of forced abstinence. Groups experienced 10 days of sucrose self-administration and then either CON or EEACUTE housing prior to testing. Immediately prior to testing, rats were pre-treated with SKF 81297 (0, 0.3, 1 mg/kg SC; n = 9–10 per group). Testing measures are indicated in panels. a Active lever responses. b Cue presentations. c Inactive lever responses. d Photobeam breaks (locomotion). Means ± SEMs indicated on Figure. Asterisk indicates significant difference from 0 dose, P < 0.0073. Dagger indicates significant difference from CON, P < 0.0073
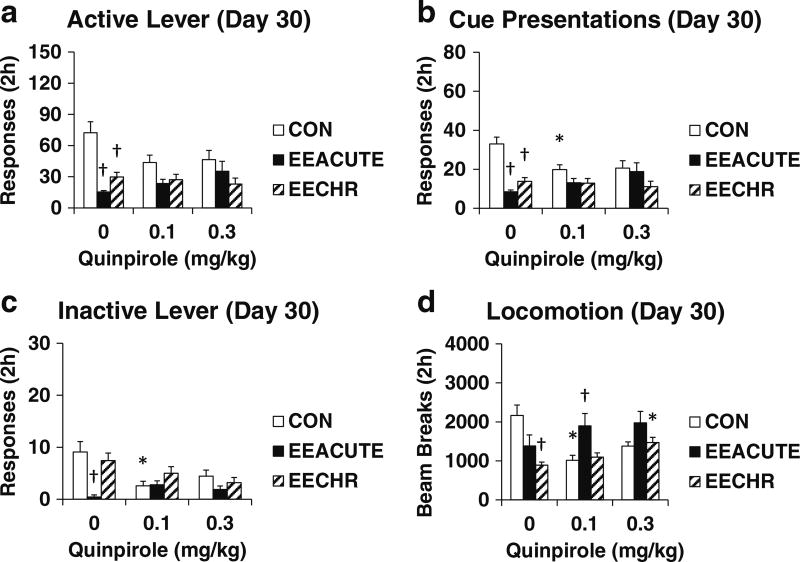
Fig. 4.
Testing measures on Day 30 of forced abstinence. Groups experienced 10 days of sucrose self-administration and then either CON, EEACUTE, or EECHR housing prior to testing. Immediately prior to testing, rats were pre-treated with quinpirole (0, 0.1, 0.3 mg/kg SC; n = 10–12 per group). Testing measures are indicated in panels. a Active lever responses. b Cue presentations. c Inactive lever responses. d Photobeam breaks (locomotion). Means ± SEMs indicated on Figure. Asterisk indicates significant difference from 0 dose, P < 0.0043. Dagger indicates significant difference from CON, P < 0.0043
Statistical analyses
Data were segregated by drug and by day of forced abstinence. The reasons for segregating data were to control for the fact that each experiment (SKF 81297 Day 1, SKF 81297 Day 30, quinpirole Day 1, and quinpirole Day 30) was run separately and sequentially over several months and also to facilitate interpretation of the effects of the drugs on EE at either the early or late forced abstinence time points. For Day 1 of forced abstinence, there were two housing conditions (CON, EEACUTE). For Day 30 of forced abstinence, there were three housing conditions (CON, EEACUTE, EECHR). Data were analyzed using ANOVA and, subsequently for testing active lever presses, ANCOVA. For the ANOVAs, active lever presses, sucrose deliveries (infusions) during training (or cue presentations during testing), inactive lever presses, and photobeam breaks (locomotion) were analyzed separately. Training and testing data were also analyzed separately. Two ANCOVAs were conducted for testing active lever pressing in each experiment. One ANCOVA was conducted using inactive lever presses as the covariate, and another was conducted using photobeam breaks as the covariate. These were conducted to determine if inactive lever responding or locomotor activity covaried with changes in active lever responding on the test day. Pre-training body weight and training data were analyzed to determine if groups differed prior to assignment to treatment conditions. Body weights were compared between Day 30 testing groups to determine if chronic EE affected body weight.
For the analyses, main factors were HOUSING (2 or 3 levels for Day 1 or Day 30, respectively) and DOSE of either SKF 81297 or quinpirole (3 levels). Training ANOVA included the repeated-measures factor TIME (10 days of training). Statistically significant main effects from ANOVA were followed by the Tukey’s HSD post hoc tests. The criterion for statistical significance was P < 0.05. Following a significant interaction, t tests were done between each CON group at each dose, between each EE group at each dose, and between each CON and EE group at each dose. This resulted in seven comparisons for Days 1 and 12 and for Day 30. Family-wise error for these tests was reduced by using a Šidák correction; this resulted in the conservative criterion for statistical significance to be P < 0.0073 for Day 1 and P < 0.0043 for Day 30. To identify whether incubation of craving occurred for CON conditions across experiments, and also to determine whether CON responding varied across experiments, 2-way ANOVA (DAY X DRUG) was conducted on CON vehicle active lever responding from all four experiments. For brevity, in most instances, only statistics for significant main effects and interactions of ANOVA are noted in the text. Means ± standard error of the mean (SEM) are indicated in the text and on the figures. The IBM SPSS Statistics 22 was used for all statistical calculations except t tests. These were calculated in Excel 2013.
Results
Body weight
Within each experiment, average body weights did not differ between treatment conditions prior to the start of the study. Weights in grams for each experiment were SKF 81297 Day 1, 388.3 ± 5.6; SKF 81297 Day 30, 399.6 ± 6.3; quinpirole Day 1, 399.4 ± 4.3; and quinpirole Day 30, 414.0 ± 3.9. Final body weights for rats in the Day 30 SKF 81297 or quinpirole experiments did not differ according to the treatment conditions. Final post forced abstinence weights in grams for the Day 30 SKF 81297 experiment were 451.4 ± 6.0 and for the Day 30 quinpirole experiment, 467.0 ± 4.0.
Behavioral procedures
Training
There were no significant differences between groups in Training measures. Statistically significant F values for training behavior data ANOVAs are indicated in Table 1. As noted in the Table, only the repeated measure TIME was significant for each behavior. For each experiment, active lever responding and infusions increased, while inactive lever responding and locomotion decreased, over the 10 days of training. The mean ± SEM of each of these measures on the tenth day of Training for each experiment is provided in Table 2.
Table 1.
Significant F values for training measures
| Experiment | Active lever responses |
Sucrose deliveries | Inactive lever responses |
Photobeam breaks |
|---|---|---|---|---|
| SKF Day 1 | TIME (9,468) = 11.5 | TIME (9,468) = 24.6 | TIME (9,468) = 20.7 | TIME (9,468) = 20.7 |
| SKF Day 30 | TIME (9,702) = 19.8 | TIME (9,702) = 42.1 | TIME (9,702) = 39.8 | TIME (9,702) = 45.9 |
| quin Day 1 | TIME (9,495) = 6.3 | TIME (9,495) = 10.8 | TIME (9,495) = 59.3 | TIME (9,495) = 21.5 |
| quin Day 30 | TIME (9,774) = 3.6 | TIME (9,774) = 19.8 | TIME (9,774) = 125.6 | TIME (9,774) = 52.7 |
F value and degrees of freedom indicated. All F values were statistically significant at P < 0.001
SKF SKF 81297, quin quinpirole
Table 2.
Means ± SEMs for the tenth day of training measures for each experiment
| Experiment | Active lever responses |
Sucrose deliveries |
Inactive lever responses |
Photobeam breaks |
|---|---|---|---|---|
| SKF 81297 Day 1 | 131.1 ± 9.1 | 70.9 ± 3.5 | 5.4 ± 0.8 | 1724.3 ± 72.1 |
| SKF 81297 Day 30 | 157.0 ± 7.6 | 78.4 ± 2.5 | 9.9 ± 1.8 | 1807.9 ± 53.6 |
| Quinpirole Day 1 | 121.7 ± 8.4 | 68.4 ± 3.3 | 5.4 ± 0.7 | 1465.4 ± 63.0 |
| Quinpirole Day 30 | 123.5 ± 6.8 | 66.5 ± 2.5 | 5.1 ± 0.5 | 1739.0 ± 63.8 |
Testing
Active lever responses in the CON vehicle conditions incubated comparing Day 1 to Day 30 (Day F(1,38) = 14.4, P < 0.001) but did not differ by experiment (DRUG main effect and DRUG X DAY interaction both not significant). These results also indicate that the CON vehicle, or baseline, level of active lever responding was similar between the two experiments (Experiments 1 and 3) examining drug effects on Day 1 and between the two experiments examining drug effects on Day 30 (Experiments 2 and 4). Significant F values for testing ANOVAs are indicated for each experiment in Table 3. Data, with significant post hoc tests indicated, are presented in Figs. 1–4.
Table 3.
ANOVA significant F values for testing measures
| Experiment | Active lever responses |
Cue deliveries | Inactive lever responses |
Photobeam breaks |
|---|---|---|---|---|
| SKF Day 1 | H (1,52) = 13.6*** | H (1,52) = 51.9*** | n.s. | H (1,52) = 21.5*** |
| HXD (2,52) = 5.2** | D (2,52) = 3.6* | D (2,52) = 45.6*** | ||
| HXD (2,52) = 3.7* | ||||
| SKF Day 30 | H (2,78) = 11.7*** | H (2,78) = 21.4*** | D (2,78) = 11.7*** | H (2,78) = 18.0*** |
| D (2,78) = 13.7*** | D (2,78) = 18.4*** | D (2,78) = 31.3*** | ||
| quin Day 1 | H (1,55) = 75.9*** | H (1,55) = 55.8*** | H (1,55) = 15.8*** | H (1,55) = 7.5** |
| HXD (2,55) = 8.3** | HXD (2,55) = 4.9* | D (2,55) = 89.6** | HXD (2,55) = 3.8* | |
| HXD (2,55) = 11.7*** | ||||
| quin Day 30 | H (2,86) = 16.3*** | H (2,86) = 15.8*** | H (2,86) = 9.3*** | H (2,86) = 6.1** |
| HXD (4,86) = 3.1* | HXD (4,86) = 4.4** | D (2,86) = 4.0* | HXD (4,86) = 5.8*** | |
| HXD (4,86) = 4.3** |
F value and degrees of freedom indicated. Factors are indicated by H (housing) and D (dose) with a cross indicating an interaction
SKF SKF 81297, quin quinpirole, n.s. not significant
P < 0.05,
P < 0.01, and
P < 0.001
SKF 81297 Day 1 (Experiment 1). As indicated in Table 3 and Fig. 1, EEACUTE reduced sucrose seeking (both active lever responses and cue presentations). The low dose of SKF 81297 attenuated this effect while the high dose reversed it. EEACUTE reduced locomotion while both doses of dopamine D1 receptor agonist increased locomotion. The active lever results were not explained by covariance of either inactive lever responses or locomotion as the overall ANOVA effects were unchanged examining either covariation of inactive lever responses or locomotion (Table 3 vs. 4).
Table 4.
ANCOVA significant F values for active lever responses during testing (housing and dose effects)
| Experiment | Inactive lever responses as covariate | Photobeam breaks as covariate |
|---|---|---|
| SKF Day 1 | H (1,51) = 49.1*** | H (1,51) = 27.0*** |
| HXD (2,51) = 4.6* | HXD (2,51) = 6.0** | |
| SKF Day 30 | H (1,77) = 9.7*** | D (2,77) = 15.1*** |
| D (2,77) = 9.0*** | ||
| quin Day 1 | H (1,54) = 51.3*** | H (1,54) = 59.7*** |
| HXD (2,54) = 6.1** | HXD (2,54) = 7.7** | |
| quin Day 30 | H (2,85) = 13.7*** | H (2,85) = 16.4*** |
F value and degrees of freedom indicated. Factors are indicated by H (housing) and D (dose) with a cross indicating an interaction
SKF SKF 81297, quin quinpirole
P < 0.05,
P < 0.01, and
P < 0.001
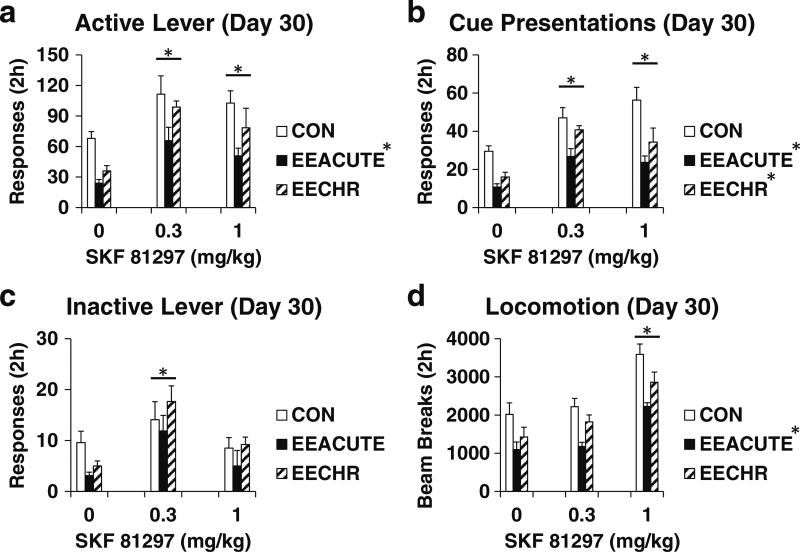
SKF 81297 Day 30 (Experiment 2). As indicated in Table 3 and Fig. 2, both EEACUTE and EECHR reduced sucrose seeking (although EECHR active lever P = 0.04 did not achieve the Šidák threshold of P < 0.0043). EEACUTE reduced locomotion. Both doses of SKF 81297 significantly reversed the EE effects on sucrose seeking indicated by responding similar or greater than CON vehicle-treated rats. Both doses of SKF 81297 also increased sucrose seeking by CON rats. The low dose of SKF 81297 increased inactive lever responding for all conditions while the high dose increased locomotion for all conditions. The ANOVA results for active lever responding were not changed when examining the covariance of inactive lever (Table 4). However, when examining the covariance of locomotion, the main effect of HOUSING was no longer statistically significant (Table 3 vs. 4).
Fig. 2.
Testing measures on Day 30 of forced abstinence. Groups experienced 10 days of sucrose self-administration and then either CON, EEACUTE, or EECHR housing prior to testing. Immediately prior to testing, rats were pre-treated with SKF 81297 (0, 0.3, 1 mg/kg SC; n = 9–10 per group). Testing measures are indicated in panels. a Active lever responses. b Cue presentations. c Inactive lever responses. d Photobeam breaks (locomotion). Means ± SEMs indicated on Figure. Asterisk indicates significant difference from 0 dose, P < 0.0043. Dagger indicates significant difference from CON, P < 0.0043
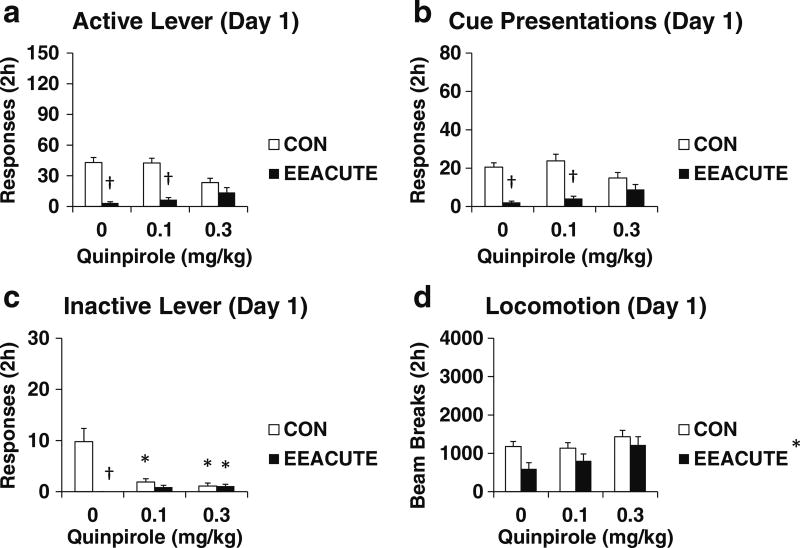
Quinpirole Day 1 (Experiment 3). The results for this experiment were similar to those from the SKF 81297 Day 1 Experiment. As indicated in Table 3 and Fig. 3, EEACUTE reduced sucrose seeking (both active lever responses and cue presentations) and this effect was reversed by the high dose of quinpirole. EEACUTE reduced inactive lever responding and locomotion. Unlike following SKF 81297, quinpirole reduced inactive lever responding by CON rats and slightly increased inactive lever responding by EEACUTE rats. The active lever results were not explained by the covariance of either inactive lever responses or locomotion (Table 3 vs. 4).
Fig. 3.
Testing measures on Day 1 of forced abstinence. Groups experienced 10 days of sucrose self-administration and then either CON or EEACUTE housing prior to testing. Immediately prior to testing, rats were pre-treated with quinpirole (0, 0.1, 0.3 mg/kg IP; n = 10–11 per group). Testing measures are indicated in panels. a Active lever responses. b Cue presentations. c Inactive lever responses. d Photobeam breaks (locomotion). Means ± SEMs indicated on Figure. Asterisk indicates significant difference from 0 dose, P < 0.0073. Dagger indicates significant difference from CON, P < 0.0073
Quinpirole Day 30 (Experiment 4). As indicated in Table 3 and Fig. 4, both EEACUTE and EECHR reduced sucrose seeking (both active lever responses and cue presentations). EEACUTE reduced inactive lever responding, and EECHR reduced locomotion. Both doses of quinpirole resulted in CON and EE rats responding at similar rates. This was driven by a combination of a drug-induced decrease in responding by CON rats and increased responding by EEACUTE rats. Statistical significance to support this effect is found with the significant decrease in cue presentations for CON rats following the low dose of quinpirole and the near significant increase in cue presentations by EEACUTE rats (P = 0.03 but not meeting the Šidák cutoff of P < 0.0043 following the high dose). The low dose of quinpirole reduced inactive lever responding and locomotion by CON rats. For EE rats, the low dose increased locomotion by EEACUTE rats; the high dose increased locomotion by EECHR rats. The active lever ANOVA results were changed when accounting for covariance of either inactive lever responding or locomotion. In both ANCOVAs, the Housing X Dose interaction was no longer significant (Table 3 vs. 4).
Discussion
Abstinence-specific effects
Previous studies support roles for dopamine and glutamate neurotransmission in the incubation of sucrose craving (Grimm et al. 2006, 2011; Uejima et al. 2007), but there has yet to be a report describing sucrose abstinence-dependent changes in specific dopamine receptor subtypes. The finding of a likely role for dopamine, noted above, was our report (Grimm et al. 2006) of an abstinence-dependent effect of systemically delivered cocaine in potentiating sucrose cue reactivity. A low dose of cocaine (5 mg/kg) increased responding for a sucrose-paired cue on Day 1, but not Day 30 of forced abstinence. Higher doses (10 and 20 mg/kg) potentiated responding on Day 30 only. In a subsequent study, we did not find an abstinence-dependent effect with D1 antagonism when injecting the drug directly into the nucleus accumbens core or shell (Grimm et al. 2011), but found that systemically administered D1 antagonist was more effective on Day 1 of forced abstinence than on Day 30. In the present study, SKF 81297 and quinpirole only affected the responding of CON rats on Day 30 with SKF 81297 in some instances increasing and quinpirole decreasing responding. These findings generally fit with a rate-dependency description of the incubation of active lever responding. That is, the baseline rate of responding predicts the rate-changing effects of psychostimulant or pharmacological challenges. This rate dependency has been hypothesized to be due to the tone of the dopamine system of the individual (Anghelescu and Heuser 2008). This proposed mechanism for rate-dependent effects of psychostimulants, in the context of our current findings with SKF 81297 and quinpirole and our previous findings with cocaine (indirect dopamine agonist) and dopamine antagonist, supports a hypothesis that dopamine tone changes over the course of forced abstinence from sucrose self-administration. This may lead to enhanced motivation to seek and consume sucrose (Grimm 2012) although further research is needed to better elucidate the neurobiology of the incubation of sucrose craving.
EE-specific effects
Overall, chronic EE is associated with decreased D1 receptor availability and signaling in limbic brain regions. Chronic EE results decrease in D1 receptor expression in the prefrontal cortex (Del Arco et al. 2007) and striatum (Gill et al. 2012, 2013). Chronic daily exposure to EE also results in decreased D1 receptor mRNA in the dorsal hippocampus (Ferrland et al. 2014) and less D1 agonist-induced locomotion in EE-exposed rats compared to controls when D1 agonist was infused into the prefrontal cortex (Del Arco et al. 2007). Finally, chronic EE results in less systemically administered D1 agonist-induced Fos in the striatum (Mazarakis et al. 2014). If D1 receptors are downregulated in our chronic EE procedure, then adding SKF 81297 could have brought back the diminished D1 signaling. As for acute EE resulting in changes in D1 signaling, we are not aware of any published findings. Our present behavioral pharmacology data support a role for both receptor subtypes in the effects of acute EE on sucrose cue reactivity. The D1 effect was observed after 1 or 30 days of forced abstinence (Figs. 1 and 2) while the D2 effect was limited to the Day 1 forced abstinence time point (Fig. 3). Keeping with the logic in the section above regarding receptor adaptations related to chronic EE, the fact that SKF 81297 and quinpirole could increase responding that had been reduced by EE could indicate that acute EE also leads to diminished signaling via D1 receptors on either Day 1 or Day 30 and D2 receptors only on Day 1.
EE mechanisms
The present results indicate that there may be changes in dopamine D1 and D2 receptor signaling following either acute or chronic EE, with effects of acute EE being present both after 1 or 30 days of forced abstinence from sucrose self-administration. Our working hypothesis is that EE affects incentive motivation. Alexander et al. (1978, 1981) found that rats living in a large colony drank less morphine solution than rats living alone. It was hypothesized that the enriched environment resulted in less drug intake as the drug effects interfered with social behavior (Alexander et al. 1981), but also that the intake of morphine in the isolated environment was due to a need to cope with isolation (Alexander 2010). This could be restated that the enriched environment provides reinforcement that exceeds that of morphine. For the present study where rats were moved from EE to the operant conditioning chamber, rats may be finding that the reinforcement provided by the operant conditioning chamber (with or without access to sucrose) is now, in contrast to EE, less reinforcing. This shift in incentive motivation defines a negative contrast.
Little has been published regarding the neurobiology of negative contrast although there is some evidence for a role of dopamine and GABA neurotransmission (Flaherty et al. 1992; Torres et al. 1996; Phelps et al. 2015). For example, amphetamine (indirect dopamine agonist) partially reversed negative contrast (Phelps et al. 2015). In this procedure, rats were trained to respond for four food pellets and then were switched to respond for one pellet; negative contrast was seen as a decrease in responding for one pellet, especially compared to a control condition of rats trained and “tested” with only one pellet. In this particular study, amphetamine administration just prior to the contrast test session resulted in a significant attenuation of the contrast effect. Dopamine receptor antagonism exacerbated negative contrast (Flaherty et al. 1992; Torres et al. 1996; Phelps et al. 2015). In addition, in a very different paradigm, the dopamine receptor antagonist pimozide attenuated both positive and negative contrast created by increasing or decreasing current in a brain self-stimulation procedure (Phillips and LePiane 1986). Even more, Genn et al. (2004) observed a lack of dopamine efflux in the nucleus accumbens of rats experiencing negative contrast (switched from 32 to 4 % sucrose) whereas controls (4 to 4 %, no switch) experienced a significant increase in dopamine. These results connect decreased dopamine signaling with negative contrast and support the hypothesis that negative contrast might be “overcome” by restoring an appropriate balance of stimulation at dopamine receptors. Further study is required to test this EE-mediated negative contrast hypothesis. However, this will require development of procedures to better isolate negative contrast effects of EE from other potential mechanisms including EE-mediated changes in stress (Solinas et al. 2010), learning (Will et al. 1977; Murtha et al. 1990; Daniel et al. 1999; Pham et al. 1999; Hellemans et al. 2004), and impulsivity (Kirkpatrick et al. 2013).
Motor vs. motivation
Finally, acute or chronic EE reduced motor activity in the operant conditioning chamber during the test session. Locomotor behavior has been shown in many previous studies to be decreased in environmentally enriched animals (Bowling et al. 1993; Grimm et al. 2013) either in a novel environment or in the operant conditioning chamber. This could be taken as an indication of several states including diminished motor capacity, increased anxiety, or a lack of motivation to respond to novel or conditioned cues. As this effect occurs without pharmacological manipulation, the motor capacity hypothesis is ruled out. An increase in anxiety is an unlikely explanation for two reasons. First, if there is a change in anxiety due to EE, evidence from some studies suggest EE would be anxiolytic (Solinas et al. 2010). This would likely be observed as increased locomotion. Second, in a previous study, we did not find a consistent relationship between acute or chronic EE and plasma corticosterone levels (Grimm et al. 2016).
Following SKF 81297 or quinpirole injection, there were some instances where a drug-induced change in active lever responding was accompanied by a change in locomotor activity in the same direction. It is difficult to rule out motor vs. motivational explanations for such changes in locomotion. This is a complication arising from the fact that forward locomotion may represent a motivated action (Wise 1987). Even so, we also observed several dissociations between drug-induced active lever responding and locomotion in the present study. For example, both doses of SKF 81297 increased Day 1 CON locomotion but not active lever responding, the low dose of SKF 81297 increased active lever responding for all housing conditions but did not affect locomotion, the high dose of quinpirole resulted in similar active lever responding between CON and EEACUTE rats on Day 1 but had no effect on locomotion, and the high dose of quinpirole resulted in similar active lever responding across housing conditions but only elevated locomotor responding in the EECHR group.
We further examined the potential confounding role for potentially non-specific activity in interpreting active lever responding by using ANCOVA. Comparing Table 4 and Table 3, there was some decreased sensitivity to detect main effects and/or interactions when inactive lever responding was a covariate (Experiment 4) and when locomotor activity was a covariate (Experiments 2 and 4). However, the overall findings were that drug-induced changes in these behaviors varied with drug-induced changes in active lever responding in some, but not all instances. In addition, it is possible that the apparent suppressive effects of D2 agonism for Day 30 responding (CON rats) were related to an abstinence-dependent effect on D2 autoreceptors. Our dose range (0, 0.1, 0.3 mg/kg) shown to increase drug seeking (Self et al. 1996) overlapped with that of Linthorst et al. (1991) who reported that 0.1 mg/kg decreased striatal dopamine levels.
Concluding remarks
The present results fit with a hypothesis of the anti-craving effect of EE and the pro-craving effect of incubation as being mediated by plasticity in mesolimbic dopamine pathways, resulting in changes in motivated behavior. The receptor specificity of the effects we observed, however, needs to be more precisely evaluated by extending the range of doses of agonists and incorporating reversal studies using receptor-specific antagonists. In addition, further research should include examination of dopamine receptor transduction pathway molecular indicators of plasticity. A better understanding of how these effects are mediated may inform treatment approaches for behaviors characterized by relapse such as drug seeking or diet recidivism.
Acknowledgments
The authors wish to thank Fiona Griffin, Neil Ingermann, Helena Reisterer, and Matthew Kroll for the help with the data collection. This study was supported by the National Institutes of Health grant DA016285-04 and Western Washington University.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- Alexander BK. The Globalization of Addiction. Oxford University Press; 2010. [Google Scholar]
- Alexander BK, Beyerstein BL, Hadaway PF, Coambs RB. Effect of early and later colony housing on oral ingestion of morphine in rats. Pharmacol Biochem Behav. 1981;15:571–576. doi: 10.1016/0091-3057(81)90211-2. [DOI] [PubMed] [Google Scholar]
- Alexander BK, Coambs RB, Hadaway PF. The effect of housing and gender on morphine self-administration in rats. Psychopharmacology. 1978;58:175–179. doi: 10.1007/BF00426903. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2002;159:284–293. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- Alvers KM, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behav Pharmacol. 2012;23:650–657. doi: 10.1097/FBP.0b013e3283584765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anghelescu I, Heuser I. Psychostimulants. In: Offermanns S, Rosenthal W, editors. Encyclopedia of molecular pharmacology. 2. Springer; New York: 2008. pp. 1038–1044. [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Darna M, Beckmann JS, Gipson CD, Bardo MT, Dwoskin LP. Effect of environmental enrichment on dopamine and serotonin transporters and glutamate neurotransmission in medial prefrontal and orbitofrontal cortex. Brain Res. 2015;1599:115–125. doi: 10.1016/j.brainres.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, Mora F. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm (Vienna) 2007;114:43–48. doi: 10.1007/s00702-006-0565-8. [DOI] [PubMed] [Google Scholar]
- du Hoffmann J, Nicola SM. Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci. 2014;34:14349–14364. doi: 10.1523/JNEUROSCI.3492-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland JM, Zeeb FD, Yu K, Kaur S, Taves MD, Winstanley CA. Greater sensitivity to novelty in rats is associated with increased motor impulsivity following repeated exposure to a stimulating environment: implications for the etiology of impulse control deficits. Eur J Neurosci. 2014;40:3746–3756. doi: 10.1111/ejn.12748. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Becker HC, Checke S, Rowan GA, Grigson PS. Effect of chlorpromazine and haloperidol on negative contrast. Pharmacol Biochem Behav. 1992;42:111–117. doi: 10.1016/0091-3057(92)90455-o. [DOI] [PubMed] [Google Scholar]
- Genn RF, Ahn S, Phillips AG. Attenuated dopamine efflux in the rat nucleus accumbens during successive negative contrast. Behav Neurosci. 2004;118:869–873. doi: 10.1037/0735-7044.118.4.869. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Baker TW, Beninger RJ. Amphetamine-induced enhancement of responding for conditioned reward in rats: interactions with repeated testing. Psychopharmacology. 2011;214:891–899. doi: 10.1007/s00213-010-2099-x. [DOI] [PubMed] [Google Scholar]
- Gill KE, Beveridge TJ, Smith HR, Porrino LJ. The effects of rearing environment and chronic methylphenidate administration on behavior and dopamine receptors in adolescent rats. Brain Res. 2013;1527:67–78. doi: 10.1016/j.brainres.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW. Animal models of craving. In: Olmstead C, Walz W, editors. Animal models of drug addiction. Humana Press; USA: 2011. [Google Scholar]
- Grimm JW. Incubation of sucrose craving in animal models. In: Brownell K, Gold M, editors. Handbook of food and addiction. Oxford University Press; Oxford: 2012. [Google Scholar]
- Grimm JW, Barnes JL, Koerber J, Glueck E, Ginder D, Hyde J, Eaton L. Effects of acute or chronic environmental enrichment on regional Fos protein expression following sucrose cue-reactivity testing in rats. Brain Struct Funct. 2016;221:2817–2830. doi: 10.1007/s00429-015-1074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Buse C, Manaois M, Osincup D, Fyall A, Wells B. Time-dependent dissociation of cocaine dose-response effects on sucrose craving and locomotion. Behav Pharmacol. 2006;17:143–149. doi: 10.1097/01.fbp.0000190686.23103.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Harkness JH, Ratliff C, Barnes J, North K, Collins S. Effects of systemic or nucleus accumbens-directed dopamine D1 receptor antagonism on sucrose seeking in rats. Psychopharmacology. 2011;216:219–233. doi: 10.1007/s00213-011-2210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, Harkness JH. Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol. 2008;19:777–785. doi: 10.1097/FBP.0b013e32831c3b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Weber R, Barnes J, Koerber J, Dorsey K, Glueck E. Brief exposure to novel or enriched environments reduces sucrose cue-reactivity and consumption in rats after 1 or 30 days of forced abstinence from self-administration. PLoS One. 2013;8:e54164. doi: 10.1371/journal.pone.0054164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy EG, Choi E, Pratt WE. Nucleus accumbens dopamine and muopioid receptors modulate the reinstatement of food-seeking behavior by food-associated cues. Behav Brain Res. 2011;219:265–272. doi: 10.1016/j.bbr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Heinla I, Leidmaa E, Visnapuu T, Philips MA, Vasar E. Enrichment and individual housing reinforce the differences in aggressiveness and amphetamine response in 129S6/SvEv and C57BL/6 strains. Behav Brain Res. 2014;267:66–73. doi: 10.1016/j.bbr.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res. 2004;150:103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Yang C, Tan A. Basal ganglia circuit loops, dopamine and motivation: a review and enquiry. Behav Brain Res. 2015;290:17–31. doi: 10.1016/j.bbr.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick K, Marshall AT, Clarke J, Cain ME. Environmental rearing effects on impulsivity and reward sensitivity. Behav Neurosci. 2013;127:712–724. doi: 10.1037/a0034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Meng L, Huang K, Wang H, Li D. Environmental enrichment blocks reinstatement of ethanol-induced conditioned place preference in mice. Neurosci Lett. 2015;599:92–96. doi: 10.1016/j.neulet.2015.05.035. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, De Lang H, De Jong W, Versteeg DH. Effect of the dopamine D2 receptor agonist quinpirole on the in vivo release of dopamine in the caudate nucleus of hypertensive rats. Eur J Pharmacol. 1991;201:125–133. doi: 10.1016/0014-2999(91)90335-n. [DOI] [PubMed] [Google Scholar]
- Mazarakis NK, Mo C, Renoir T, van Dellen A, Deacon R, Blakemore C, Hannan AJ. Super-enrichment’ reveals dose-dependent therapeutic effects of environmental stimulation in a transgenic mouse model of Huntington’s disease. J Huntingtons Dis. 2014;3:299–309. doi: 10.3233/JHD-140118. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Bardo MT. Amphetamine self-administration and dopamine function: assessment of gene x environment interactions in Lewis and Fischer 344 rats. Psychopharmacology. 2015;232:2275–2285. doi: 10.1007/s00213-014-3854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottola D, Kilts J, Lewis M, Connery H, Walker Q, Jones S, Booth R, Hyslop D, Piercey M, Wightman R, Lawler C. Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J Pharmacol Exp Ther. 2002;301:1166–1178. doi: 10.1124/jpet.301.3.1166. [DOI] [PubMed] [Google Scholar]
- Murtha S, Pappas BA, Raman S. Neonatal and adult forebrain norepinephrine depletion and the behavioral and cortical thickening effects of enriched/impoverished environment. Behav Brain Res. 1990;39:249–261. doi: 10.1016/0166-4328(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Neumeyer J, Kula N, Bergman J, Baldessarini R. Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. Eur J Pharmacol. 2003;474:137–140. doi: 10.1016/s0014-2999(03)02008-9. [DOI] [PubMed] [Google Scholar]
- Pham TM, Soderstrom S, Winblad B, Mohammed AH. Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav Brain Res. 1999;103:63–70. doi: 10.1016/s0166-4328(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Phelps CE, Mitchell EN, Nutt DJ, Marston HM, Robinson ES. Psychopharmacological characterisation of the successive negative contrast effect in rats. Psychopharmacology. 2015;232:2697–2709. doi: 10.1007/s00213-015-3905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, LePiane FG. Effects of pimozide on positive and negative incentive contrast with rewarding brain stimulation. Pharmacol Biochem Behav. 1986;24:1577–1582. doi: 10.1016/0091-3057(86)90488-0. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Pardo M, Yohn S, López-Cruz L, SanMiguel N, Correa M. Mesolimbic dopamine and the regulation of motivated behavior. Curr Top Behav Neurosci. 2016;27:231–257. doi: 10.1007/7854_2015_383. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol. 2010;92:572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009;12:1151–1156. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres C, Morales A, Candido A, Maldonado A. Successive negative contrast in one-way avoidance: effect of thiopental sodium and chlorpromazine. Eur J Pharmacol. 1996;314:269–275. doi: 10.1016/s0014-2999(96)00564-x. [DOI] [PubMed] [Google Scholar]
- Uejima JL, Bossert JM, Poles GC, Lu L. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res. 2007;181:292–296. doi: 10.1016/j.bbr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52. doi: 10.1016/bs.pbr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Will BE, Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Relatively brief environmental enrichment aids recovery of learning capacity and alters brain measures after postweaning brain lesions in rats. J Comp Physiol Psychol. 1977;91:33–50. doi: 10.1037/h0077306. [DOI] [PubMed] [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35:227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]