Abstract
After Kanda’s first report in 2014 on gadolinium (Gd) deposition in brain tissue, a considerable number of studies have investigated the explanation for the observation. Gd deposition in brain tissue after repeated administration of gadolinium-based contrast medium (GBCM) has been histologically proven, and chelate stability has been shown to affect the deposition. However, the mechanism for this deposition has not been fully elucidated. Recently, a hypothesis was introduced that involves the ‘glymphatic system’, which is a coined word that combines ‘gl’ for glia cell and ‘lymphatic’ system. According to this hypothesis, the perivascular space functions as a conduit for cerebrospinal fluid to flow into the brain parenchyma. The perivascular space around the arteries allows cerebrospinal fluid to enter the interstitial space of the brain tissue through water channels controlled by aquaporin 4. The cerebrospinal fluid entering the interstitial space clears waste proteins from the tissue. It then flows into the perivascular space around the vein and is discharged outside the brain. In addition to the hypothesis regarding the glymphatic system, some reports have described that after GBCM administration, some of the GBCM distributes through systemic blood circulation and remains in other compartments including the cerebrospinal fluid. It is thought that the GBCM distributed into the cerebrospinal fluid cavity via the glymphatic system may remain in brain tissue for a longer duration compared to the GBCM in systemic circulation. Glymphatic system may of course act as a clearance system for GBCM from brain tissue. Based on these findings, the mechanism for Gd deposition in the brain will be discussed in this review. The authors speculate that the glymphatic system may be the major contributory factor to the deposition and clearance of gadolinium in brain tissue.
Keywords: glymphatic system, cerebrospinal fluid, gadolinium-based contrast media, magnetic resonance imaging, tissue deposition
Introduction
Kanda’s report published in 2014 demonstrated signal enhancement in the dentate nucleus and globus pallidus on T1-weighted images that was increased with the number of times gadolinium-based contrast medium (GBCM) had been administered.1 This finding, indicating the possibility of tissue deposition of gadolinium (Gd) on unenhanced T1-weighted images has been confirmed in many clinical practices (Fig. 1). Kanda’s report caused a big sensation, and at the same time, generated concern regarding the mechanism of deposition. Although the mechanism for Gd accumulation in the brain is not clear, we will try to speculate on the theories according to current published research and generally accepted concepts.
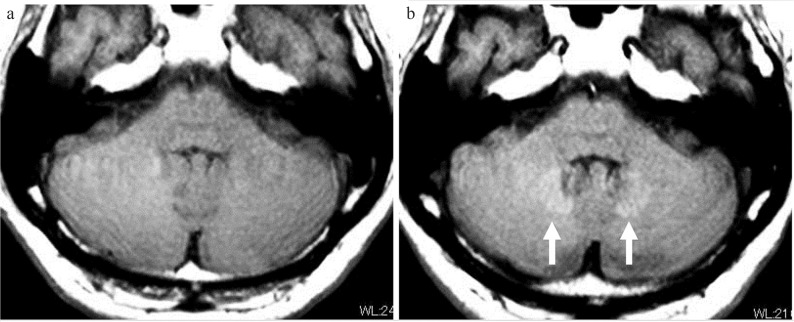
Fig. 1.
Deposition of gadolinium in the dentate nucleus. A case with multiple sclerosis between an initial MRI performed in 2009 (a) and an MRI performed in 2017 (b), repeated contrast-enhanced MRI examinations were performed 17 times using linear-type gadolinium-based contrast medium (GBCM) five times and macrocyclic GBCM 12 times. On the T1-weighted image obtained with fast spin echo imaging in 2017 (b: arrow), the bilateral dentate gyrus shows high-signal intensity. The signal intensity ratio between the pons and dentate nucleus was 1.02 in 2009 and 1.08 in 2017.
Classification of GBCM and stability
First, in this article we will review the classification of GBCM. Gadolinium is a heavy metal in the lanthanide series and has seven unpaired electrons. It is used as a contrast agent for MRI because it is strongly paramagnetic. Gadolinium ions alone are highly toxic and are coupled with chelating substances prior to medical use.2 The structure of this chelate determines the behavior of GBCM in the serum of living organisms and the degree of shortening of the relaxation time. Gadolinium-based contrast mediums are divided into linear and macrocyclic types depending on the structure of the chelates, and also divided into ionic and nonionic types depending on the charge state. In general, the macrocyclic chelate is more stable in vivo than the linear type, and the possibility of releasing the Gd ion is low. Ionic GBCM is more stable in vivo than the nonionic type.3 However, these are just comparison considerations, and all current contrast agents available commercially are considered clinically safe.
Gd deposition in the brain
The first report of Gd deposition in the brain was published in March of 2014 by Kanda et al.1 In this study, they evaluated the signal intensity ratio of the dentate nucleus to the pons and the signal intensity ratio of the globus pallidus to the thalamus according to GBCM administration history and compared to controls. Multivariate analysis was performed with factors such as the signal intensity ratios mentioned above, gender, age, original disease, and treatment history, and showed that only the number of doses of GBCM was correlated with the intensity ratios.
We have summarized the time course of the studies following Kanda’s report in the subsequent paragraphs. Knowledge has accumulated rapidly during the past 3 to 4 years. The most important reports were published in June and July of 2015, and the existence of the phenomenon became widely accepted at that time.
October 2014; Errante et al.4: This group confirmed that the signal intensity in the dentate nucleus was linearly proportional to the number of times that linear-type GBCM was administered in patients with multiple sclerosis or metastatic brain tumors. A similar pattern of Gd deposition occurred in both conditions. Therefore, it was speculated that the high-signal intensity in the dentate nucleus was not a finding related to the multiple sclerosis disease process, but rather, seemed to be caused by a history of GBCM administration, which was common to both patient populations.
June 2015 (Epub 2015 Mar 5.); McDonald et al.5: This study compared autopsy cases between patients who were or were not administered a linear-type contrast agent (gadodiamide; Omniscan). In cases with a history of administration of gadolinium, the signal intensity on T1-weighted images of brain tissue including the globus pallidus and the dentate nucleus, and the concentration of Gd in tissue were correlated in a significant dose-dependent relationship. Thus, Gd deposition in brain tissue was proven histologically. In addition, not only the globus pallidus and dentate nucleus, but also many other tissues in the brain, showed an increase in Gd concentration.
June 2015 (Epub 2015 Apr 6.); Radbruch et al.6: This group compared the administration of linear-type GBCM (gadopentetate dimeglumine; Magnevist) with macrocyclic GBCM (gadoterate meglumine; Dotarem/Magnescope), and showed high-signal intensity in the dentate nuclei and globus pallidus on T1-weighted images with linear-type GBCM, but not with macrocyclic.
June 2015 (Epub 2015 May 5.); Kanda et al.7: This group also compared the administration of linear-type GBCM (gadopentetate dimeglumine; Magnevist) to macrocyclic (gadoteridol; ProHance). High-signal intensity in the dentate nuclei on T1-weighted images was seen with linear-type GBCM, but not with macrocyclic, indicating that chelate stability affects Gd deposition. This report comparing linear and macrocyclic types was published at approximately the same time as the Radbruch paper,6 giving additional confirmation to this finding.
June 2015 (Epub 2015 May 5.); Kanal et al.8: In a statement published as an editorial in the journal Radiology, Kanal et al. emphasized that no adverse symptoms are associated with GBCM brain deposition thus far. They reaffirmed the usefulness of GBCM in a clinical setting and called for ‘risk-aware’ use. There was also a concern raised about the possibility of depriving patients unnecessarily of crucial, even life saving, medical information from GBCA enhanced MR imaging. This statement created a sense of urgency at the time.
July 2015 (Epub 2015 May 5.); Kanda et al.7: This study reported the examination of autopsy cases without renal dysfunction. Comparisons were made between cases with and without administration of linear-type contrast agents. Analysis of the brain during autopsy by inductively coupled plasma mass spectrometry showed that Gd was present in all tissues of the brain in cases with a history of administration. In particular, the Gd concentration was high in the globus pallidus and dentate nuclei. Published at almost the same time as the above report by McDonald, Gd deposition was thus demonstrated using a different method.
During the time period above, clinicians widely recognized the existence of Gd deposition in the brain, after which the evaluation of each type of GBCM was reported.
November 2015; Weberling et al.9: This group measured the extent of the high-signal intensity in the dentate nuclei with gadobenate dimeglumine (MultiHance), which is listed as medium risk for nephrogenic systemic fibrosis (NSF). A signal intensity ratio in the dentate nucleus/pons that was equivalent to that found with linear-type GBCM (gadopentetate dimeglumine) was observed, and this signal intensity was significantly higher than that with macrocyclic GBCM (gadoterate meglumine; Dotarem/Magnescope). This was the first report on the type of GBCM with a medium risk for NSF.
March 2016 (Epub 2015 Jun 25.); Stojanov et al.10: This group reported increased signal intensity in the dentate nucleus in a case with macrocyclic GBCM (gadobutrol; Gadovist) administration. However, there was a problem with the signal intensity measurement in this report, because contradictory reports or letters were published subsequently.11,12
July 2016; Murata et al.13: This group examined autopsy cases administered GBCM who were in a risk group other than NSF risk. Gadoteridol, gadobutrol, and gadobenate dimeglumine, which are extracellular fluid contrast agents, and gadoxetate disodium (Eovist/Primovist), which is a liver-specific contrast medium, were examined. Gadolinium deposition in the dentate nucleus and globus pallidus was observed with all GBCMs examined. This was the first report of a liver-specific contrast medium. This report also showed that the bone deposition of Gd was 23-times higher than the deposition in brain tissue.
November 2016; Radbruch et al.14: When linear-type GBCM is administered five times or more, the dentate nucleus shows increased signal intensity on T1-weighted images. This report showed that when macrocyclic-type GBCM was subsequently administered the same number of times, the signal intensity on T1-weighted images of the dentate nucleus not only did not rise, but rather it decreased. Interestingly, this report suggests the presence of a washout effect by administering a macrocyclic contrast agent after Gd deposition.
November 2016; Bauer et al.15: This report showed that the maximum standardized uptake value (SUVmax) of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) decreased in the dentate nucleus or globus pallidus in cases in which Gd deposition is seen. The measurement method and other problems with the study design have been indicated.16
March 2017 (Epub 2016 Dec 7.); Radbruch et al.17: When an examination with macrocyclic-type GBCM (gadoterate meglumine; Dotarem/Magnescope, and gadobutrol; Gadovist) was performed, no increased signal intensity in the dentate nucleus was observed with administration of contrast agent 20 or more times at intervals of 3 months on average. This result indicated that the gadolinium deposition risk might be minimized when macrocyclic-type GBCAs are used.
March 2017 (Epub 2017 Jan 11.); Kahn et al.18: This study reported a case of administration of gadoxetate disodium, which is a hepatocyte-specific contrast medium. A correlation was found between the number of times the medium was administered and increased signal intensity in the dentate nucleus. A significant increase was not observed when the number of doses was less than 10. Following the report by Murata et al.13, increased signal intensity with a liver-specific contrast agent was confirmed.
May 2017; Rasschaert et al.19: An animal experiment was performed to show the impact of renal failure on Gd deposition in the cerebellar nuclei. They compared renally impaired (subtotal nephrectomy) rats versus rats with normal renal function using linear-type GBCM (gadodiamide; Omniscan). They observed that the subtotal nephrectomy group had significantly higher T1 signal enhancement in the deep cerebellar nuclei and a major increase in the total Gd concentration in cerebellum, plasma, cerebrospinal fluid, parietal bone, and femur after administration of gadodiamide. Their result indicates that renal impairment substantially increases T1 signal enhancement in the cerebellar nuclei and the total tissue concentration of Gd after linear-type GBCM administration.
July 2017 (Epub 2017 May 11.); Forslin et al.20: Recently, an interesting report that requires careful interpretation was published. This study investigated the relationship between administration of multiple doses of Gd-based contrast agent and the signal intensity in the dentate nucleus and globus pallidus, as well as the association with cognitive function in multiple sclerosis cases. The study showed that increased signal intensity in the dentate nucleus among patients with multiple sclerosis was associated with lower verbal fluency scores. This association remained significant even after correction for several aspects of disease severity. These results must be interpreted with caution because this study was only exploratory and does not prove causality. However, this is the first report to correlate clinical symptoms with Gd deposition. Thus, to understand the clinical implications, future studies that further investigate cognitive indices and other clinical outcome variables in other cohorts with repeated administration of linear-type GBCM compared to macrocyclic agents, will be important.
Mass transportation in the brain: Cerebrospinal fluid and the glymphatic system
Obviously, blood flow plays a very large role in substance transportation into the brain. Oxygen and glucose, which are required for metabolism, are carried to the brain by blood flow and are transported into brain tissue across the blood-brain barrier, which is formed by the end feet of glial cells. However, substances in the brain are not only transported by blood flow. In recent years, mass transport in the brain by cerebrospinal fluid or interstitial fluid has been shown. The lymphatic system is involved in protein waste removal from the body. However, researchers conventionally thought that no lymphatic system was present in the brain. Nedergaard and Iliff et al. hypothesized that the perivascular space constitutes a system in the brain corresponding to the lymphatic system and named this the ‘glymphatic system’, which is a coined word that combines ‘g1’ for glia cell and ‘lymphatic’ system.21–23 (Fig. 2). An outline of their hypothesis is as follows. The perivascular space functions as a conduit for cerebrospinal fluid to flow into the brain parenchyma. The driving force of this conduit is arterial pulsation. Cerebrospinal fluid is led to the perivascular space around the artery and enters the interstitial space of the brain tissue through water channels controlled by aquaporin 4 (AQP4), which is distributed in the foot processes of astrocytes that make up the outer wall of the perivascular space. Cerebrospinal fluid entering the interstitial space washes out waste proteins, such as amyloid β, from the tissue. Cerebrospinal fluid that has washed out from between the cells in this way flows into the perivascular space around the veins and is discharged outside the brain.
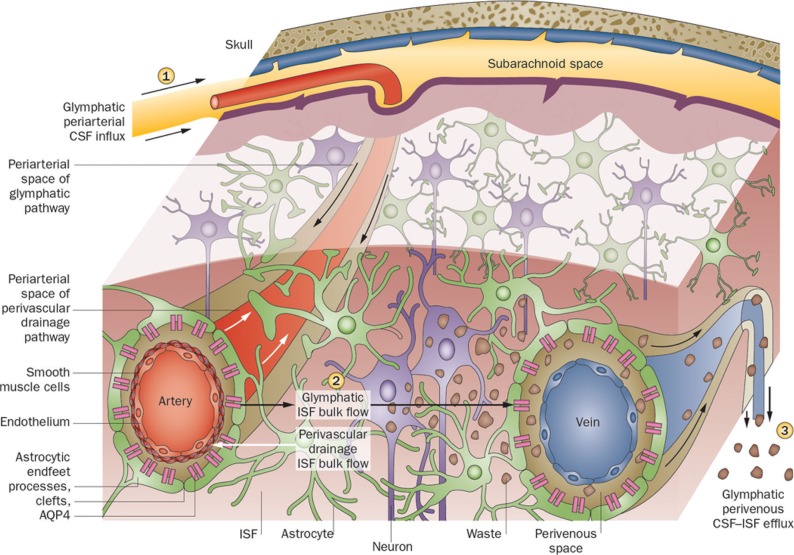
Fig. 2.
Outline of the glymphatic system. This figure illustrates that perivascular clearance comprises perivascular drainage and glymphatic pathways. (1) Cerebrospinal fluid flows into the brain parenchyma via the periarterial space, which is the perivascular space surrounding the parenchymal arteries. From this perivascular space surrounding the artery, cerebrospinal fluid enters the interstitium of the brain tissue via aquaporin 4 (AQP4)-controlled water channels. These are distributed in the end feet of astrocytes that constitute the outer wall of the perivascular space. (2) Cerebrospinal fluid entering the interstitial fluid flows by convection, and the cerebral spinal fluid (CSF)– interstitial fluid (ISF) exchange within the brain parenchyma. (3) After washing the waste proteins from the tissue, it flows into the perivenous space, which is the perivascular space around the deep-draining vein, and is subsequently discharged outside the brain.22,23 (Reprinted by permission from Macmillan Publishers Ltd: Nat Rev Neurol [11:457–470], copyright [2015]).
To investigate this hypothesis, Iliff et al. observed the subcortical region (100 μm depth) of the mouse brain in vivo using a two-photon laser-scanning microscope. This technique is capable of observing the living brain through the dura using infrared laser light with excellent permeability.21 They visualized the movement of intracisternally injected fluorescent dextrans into the cerebral cortex after labeling the cerebral vasculature with blood–brain barrier impermeant fluorescent dextran. After intracisternal injection, the tracer rapidly entered the brain along the outside of cortical surface arteries and penetrating arterioles through a pathway immediately surrounding the vascular smooth muscle cells. Rapid tracer movement along the margins of surface arteries was consistent with the presence of paravascular sheaths surrounding the cerebral surface arteries. Although small molecular weight tracers distributed into the interstitial space shortly after intracisternal administration, large molecular weight tracers remained confined to the paravascular space. In the analysis of the distribution of moderate molecular weight tracer, the tracer was not observed around veins at early time points (<10 min after injection). At longer time points (>1 h), the tracer that had been injected intracisternally accumulated along capillaries and parenchymal venules.21 Recently, there have been several reports visualizing or estimating the activity of the glymphatic system in human subjects. These reports include a trial for evaluating cerebrospinal fluid pulsations in the human brain with ultra-fast magnetic resonance encephalography,24 a trial for analyzing the contrast enhancement pattern of the perivascular space on heavily T2-weighted 3D-fluid attenuated inversion recovery (FLAIR) images 4 h after intra-venous administration of GBCA,25 and a trial for evaluating diffusivity along the direction of the perivascular space.26
Here, we would like to define the terms ‘para-vascular’ and ‘peri-vascular’. The existence of waste drainage pathways, which are paths through the arterial walls, was observed and reported before the glymphatic system was hypothesized. This pathway was called the ‘perivascular drainage pathway’.27 Although this pathway is termed ‘perivascular’, it refers to the pathway in the arterial wall and not the Virchow-Robin space, which is the space outside of the vessel wall. In the paper by Iliff et al., the term ‘paravascular pathway’ was used in the title to indicate the pathway through the Virchow-Robin space, which in conventional anatomy is termed the ‘perivascular space’.21 Also in another paper by the same group, the definition of paravascular space is described as the cavity between the basement membrane of the blood vessel and the end feet of the surrounding astrocytes.28 The above-mentioned ‘perivascular drainage pathways’, which pass through the arterial wall, are distinct from the ‘glymphatic system’, which is also called the ‘paravascular pathway’ by Nedergaard and Iliff et al.21 Again, the corresponding anatomical structure, the ‘paravascular pathway’ by Nedergaard and Iliff et al.21 refers to the Virchow-Robin space, which is the ‘perivascular space’ as recognized by clinicians, including radiologists.
Amyloid β, a protein associated with Alzheimer’s disease, aggregates interstitially to form amyloid plaques and contributes to disease progression. The above-mentioned paper describing the glymphatic system also evaluated the clearance of amyloid β in healthy and AQP4-knockout mice.21 Evaluation of the time course after injection of amyloid β directly into brain tissue showed that the clearance of amyloid β is delayed in the AQP4-knockout mouse, and that the glymphatic system including the water channels formed by AQP4 is involved. They also showed that the flow rate of cerebrospinal fluid and/or interstitial fluid in tissues related to this system increases during sleep compared to the awake state.29
Although the glymphatic system theory suggests involvement of the perivascular space in substance transport in the brain, the theory is still a hypothesis that involves a function rather than an obvious anatomical structure. This theory currently cannot be confirmed, especially in humans. Several papers have been published that argue against this theory. For example, although Nedergaard and Iliff et al.21 speculate that the distribution of cerebrospinal fluid-interstitial fluid in tissues is caused by interstitial flow and its driving force is supplied by arterial pulsation, Asgari et al. used a mathematical model to show that the driving force due to arterial pulsation may result in transport through the perivascular space by diffusion rather than bulk flow such as interstitial flow.30 Spector et al. have questioned many points regarding the hypothesis of Nedergaard and Iliff et al.21 in their review. They maintain that the theory ignores the transport of substances to the brain parenchyma via the pia matter and the ependyma. They also argue that the cerebrospinal fluid in the perivascular space is reported to be almost stagnant or in a state of ‘to and fro,’ and that observation with a two-photon microscope is different from the physiological environment.31 They assert that the term ‘glymphatic’ is not suitable for a name because lymph fluid and cerebrospinal fluid have different protein concentrations and different immune functions. Regarding the concept of the ‘glymphatic system’, objections and rebutting evidence may emerge in the future, and thus, researchers should continue to pay attention to this concept. However, with respect to a substance transport system in the brain, the idea that not only blood flow but also including flow of cerebrospinal and interstitial fluid is very significant. In 1925, Cushing had already pointed out the importance of the circulation of interstitial fluid as a second type of circulation, and he also introduced the concept of ‘perivascular lymphatics’.32 In late 20th century, there were also several studies on substance transport system by cerebrospinal fluid. Ohata et al. reported an observation which indicates that cerebral spinal fluid (CSF) pathway is the major route of protein-rich edema clearance.33 Weller et al. made continuous study on waste removal system in the brain, and they reported that there is a pathway for interstitial and cerebrospinal fluid from the brain into cervical lymphatics. They indicated drainage of fluid could occur along perivascular spaces from the grey matter into perivascular spaces of the leptomeningeal arteries.34
Behavior of GBCM hours after injection
To evaluate endolymphatic hydrops of the inner ear, Naganawa et al. introduced an imaging method called HYbriD of Reversed image Of Positive endolymph signal and native image of positive perilymph Signal (HYDROPS) which involves a heavily T2-weighted 3D-FLAIR (hT2W-3D-FLAIR) image at 4 h after injection of contrast medium.35 This HYDROPS method is designed to evaluate the presence or absence and the extent of expansion of the inner ear endolymph in Meniere’s disease by obtaining an image that shows contrast in which the perilymph is enhanced by GBCM and the endolymph is not enhanced. Although these images focus on the bilateral inner ear, intracranial structures including the brain and other structures are also visualized on sets of HYDROPS images. In a study of endolymphatic HYDROPS images in healthy volunteers after intravenous GBCM, positive signal enhancement was observed on hT2W-3D-FLAIR in the anterior eye segment, optic nerve sheath, cerebrospinal fluid of Meckel’s cave, cerebrospinal fluid in the internal auditory canal, cerebrospinal fluid of the ambient cistern, and perilymph fluid, indicating transfer of GBCM to these fluid compartments (Fig. 3). Signal enhancement was not observed in the cerebrospinal fluid of the lateral ventricle, brain parenchyma, or endolymph in the inner ear.36 Another report showed signal enhancement in the perivascular space of the basal ganglia on hT2W-3D-FLAIR images, in addition to the cerebrospinal fluid in the ambient cistern25 (Fig. 4).
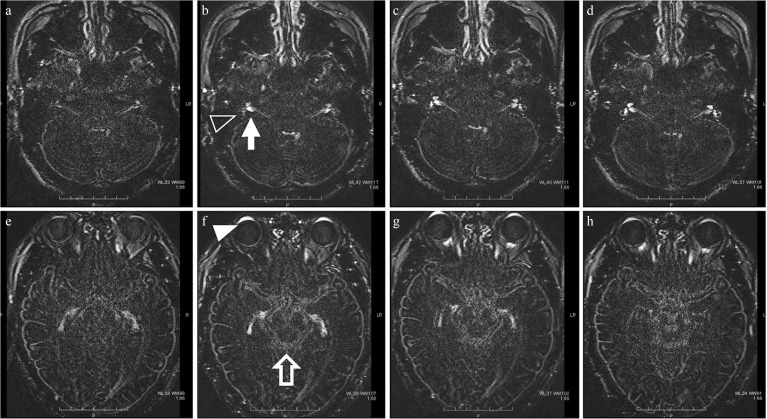
Fig. 3.
Distribution of gadolinium-based contrast medium (GBCM) over time after intravenous injection. Heavily T2-weighted fluid attenuated inversion recovery (FLAIR) imaging over time after GBCM administration to normal volunteers. Images at 30 minutes (a and e), 1.5 h (b and f), 3 h (c and g), and 6 h (d and h) after administration are shown. Signal enhancement was observed in the anterior eye segment (empty arrow head), the perilymph of the inner ear (white arrow head), the cerebrospinal fluid in the internal auditory canal (arrow), Meckel’s cave, and the suprasellar cistern to the ambient cistern (empty arrow), indicating the distribution of GBCM. The peak enhancement after administration was 1.5 h in the anterior eye segment and Meckel’s cave, 3 h in the internal auditory canal and ambient cistern, and 4.5 h (not shown) in the perilymph in the inner ear and optic nerve sheath.
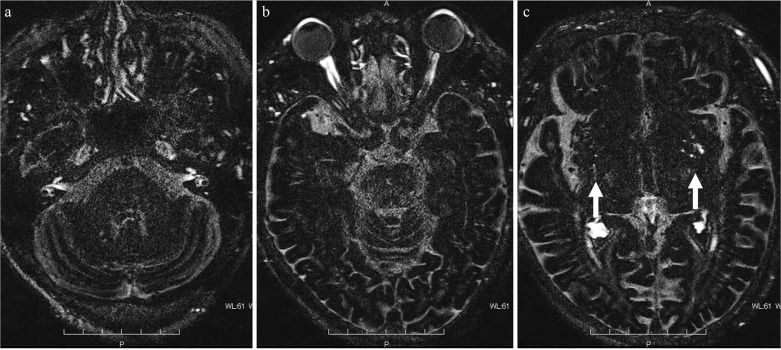
Fig. 4.
Distribution of gadolinium-based contrast medium (GBCM) to the perivascular space. Heavily T2-weighted fluid attenuated inversion recovery (FLAIR) 4 h after intravenous GBCM administration. As in Fig. 3, in addition to the internal auditory canal (a), Meckel’s cave (a), anterior eye segment (b), and optic nerve sheath (b), a wide range of the cerebrospinal fluid space shows high-signal intensity indicating the distribution of GBCM. Markedly high-signal intensity is seen in the enlarged perivascular space of the basal ganglia (c: arrow) compared to the other cerebrospinal fluid cavities.
Most intravenously administered GBCM is excreted through systemic circulation by the kidneys. However, the hT2W-3D-FLAIR observations have shown that a certain amount of GBCM leaks out from the blood in systemic circulation and remains in other compartments such as the anterior part of the eyeball or areas surrounding the cranial nerves, even several hours after GBCM administration. For example, the aqueous humor, which is in the aqueous chamber located in the anterior eye segment, is produced by the ciliary epithelium and flows from the trabecular meshwork through Schlemm’s canal to the outside of the eye. Because the ciliary processes where the aqueous humor is formed have fenestrated blood vessels and high vascular permeability,37 GBCM may leak into the aqueous humor at a relatively early stage after administration as shown in Fig. 3f. A gradual decrease of GBCM shown in Fig. 3g and h seems to represent wash out process of GBCM from the aqueous humor in the anterior eye segment. This anterior eye segment, which is a relatively closed system, shows unique GBCM dynamics compared to other areas. In rodent experiments, CSF may move directly from the subarachnoid space into the submucosal lymphatics, which emerge at the level of the cribriform plate (CSF-lymphatic connection). In experiments of other animals including dogs, various tracers injected into the CSF or brain parenchyma made their way into the lymphatic vessels external to the cranium and into a variety of lymph nodes in the head and neck.38 As shown above, there are various routes for clearance of GBCM other than systemic blood circulation and the kidneys, including routes via the aqueous humor or via the CSF-lymphatic connection and the dynamics of GBCM are different from that in systemic circulation. Interestingly, the contrast agent distributes in areas related to cranial nerves, such as the optic nerve sheath, Meckel’s cave, and internal auditory canal. Details regarding the mechanism of GBCM distribution in these areas are unknown. However, there would be similar clearance routes as mentioned above in these areas.
Hypothesis for Gd deposition in the brain
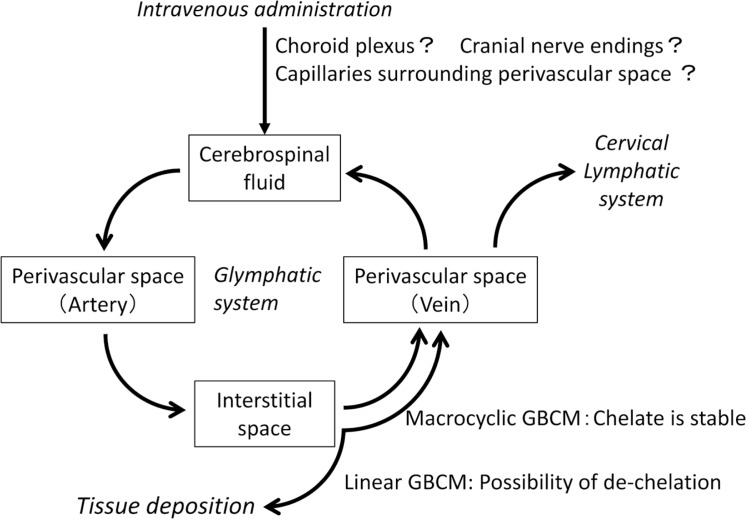
No theory or mechanism has been established regarding the cause of Gd deposition in the globus pallidus and dentate nucleus of the cerebellum, although several hypotheses have been considered. Kanda et al. suspects that Gd distribution across the blood–brain barrier may occur due to dechelation and subsequent transmetallation, coupled with the existence of a metal transporter.39
An article entitled ‘Intrathecal Contrast-Enhanced Magnetic Resonance Imaging–Related Brain Signal Changes: Residual Gadolinium Deposition?’ was published from an institute in Turkey. The researchers performed systematic intrathecal administration of GBCM.40 This report is a comparative study of MRI before and after intrathecal administration of GBCM in six patients with normal renal function who had a history of intrathecal administration of linear-type GBCM but had never undergone intravenous injection of GBCM. This study provides very important information regarding the cause of Gd deposition. Visual assessment and quantitative evaluation were carried out in the globus pallidus, putamen, and dentate nuclei. In the visual assessment, increased signal intensity was confirmed in all cases including remarkably high-signal intensity in three cases. With quantitative evaluation, the increased signal intensity was confirmed in all cases when compared to pre-intrathecal administration images. This means that Gd deposition in the basal ganglia can occur only through the cerebrospinal fluid, and the results support the hypothesis that the glymphatic system may be at least partially involved in the route of Gd distribution into brain tissue. Naganawa et al. reported that there were enhancement of the perivascular spaces at 4 h after intra venous GBCA injection even in subjects without renal insufficiency, and speculated that the GBCA in the blood vessels might have permeated into the cerebrospinal fluid space and the perivascular spaces.25 Taken together with the information that GBCM distributes in the cerebrospinal fluid cavity and perivascular spaces within several hours after administration as described in the previous section, one hypothesis arises regarding the fate of Gd after intravenous injection. Most intravenously administered GBCM is excreted via the kidneys through systemic circulation, and the concentration of GBCM in the cerebral blood vessels is also rapidly decreasing. However, some GBCM distributes from the choroid plexus, parenchymal blood vessels or other areas to the cerebrospinal fluid. Although the concentration of GBCM that is transferred to the cerebrospinal fluid is very low, unlike GBCM in the systemic circulation, clearance of GBCM takes a considerable amount of time. Therefore, the brain tissue is exposed to cerebrospinal fluid containing GBCM at a relatively low concentration over a longer period of time compared to the blood flow. In the meantime, when dechelation of linear-type GBCM occurs via an unknown mechanism, deposition of Gd in the brain tissue may occur (Fig. 5). In particular, because the dentate nuclei and globus pallidus express receptors and transporters for many types of metals, Gd may accumulate at a higher concentration in these regions than in other regions. This theory is our hypothesis regarding the cause of Gd deposition in the basal ganglia. We consider that this hypothesis may explain the distribution of Gd within brain tissue even with an intact blood-brain-barrier. However, at the present, to our knowledge, there are no published studies indicating the exact dynamics of GBCM in the brain parenchyma leading to Gd deposition, including information such as the biological half-life of GBCM in tissue.
Fig. 5.
Hypothesis of the mechanism for gadolinium deposition via the glymphatic system. Introducing our hypothesis for gadolinium deposition. Compared with gadolinium-based contrast medium (GBCM) in the systemic circulation, GBCM distributed into the cerebrospinal fluid cavity via glymphatic system can remain in brain tissue for a long time. The authors of this review speculate that the glymphatic system may be involved in the tissue deposition of gadolinium.
Conclusion
In this review, mass transport systems in the brain other than blood flow were discussed with the phenomenon of Gd deposition in the brain as a key factor. The theory of the ‘glymphatic system’ is not yet established. However, many findings are accumulating from animal experiments, and the detailed pathophysiology of the glymphatic system may be clarified in clinical cases in relation to clinical disease in the future. However, it is necessary to evaluate by experiments or clinical observation whether the glymphatic system hypothesis is correct or needs to be altered.
Footnotes
Conflicts of Interest
Toshiaki Taoka received lecture fees from Bayer Yakuhin, Ltd., Fuji Pharma Co., Ltd., Daiichi-Sankyo Co. Ltd., and Eisai Co. Ltd.
Shinji Naganawa received lecture fees from Bayer Yakuhin, Ltd., Fuji Pharma Co., Ltd., Daiichi-Sankyo Co. Ltd., and Eisai Co. Ltd, and non-purpose research funds from Daiichi-Sankyo Co. Ltd. and Eisai Co. Ltd.
References
- 1.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270:834–841. [DOI] [PubMed] [Google Scholar]
- 2.Kanda T, Nakai Y, Aoki S, et al. Contribution of metals to brain MR signal intensity: review articles. Jpn J Radiol 2016; 34:258–266. [DOI] [PubMed] [Google Scholar]
- 3.Kanda T, Oba H, Toyoda K, Kitajima K, Furui S. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol 2016; 34:3–9. [DOI] [PubMed] [Google Scholar]
- 4.Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 2014; 49:685–690. [DOI] [PubMed] [Google Scholar]
- 5.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275:772–782. [DOI] [PubMed] [Google Scholar]
- 6.Radbruch A, Weberling LD, Kieslich PJ, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 2015; 275:783–791. [DOI] [PubMed] [Google Scholar]
- 7.Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276:228–232. [DOI] [PubMed] [Google Scholar]
- 8.Kanal E, Tweedle MF. Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology 2015; 275:630–634. [DOI] [PubMed] [Google Scholar]
- 9.Weberling LD, Kieslich PJ, Kickingereder P, et al. Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol 2015; 50:743–748. [DOI] [PubMed] [Google Scholar]
- 10.Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 2016; 26:807–815. [DOI] [PubMed] [Google Scholar]
- 11.Radbruch A, Weberling LD, Kieslich PJ, et al. High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Invest Radiol 2015; 50:805–810. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y, Huang DQ, Shih G, Prince MR. Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol 2016; 206:414–419. [DOI] [PubMed] [Google Scholar]
- 13.Murata N, Gonzalez-Cuyar LF, Murata K, et al. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol 2016; 51:447–453. [DOI] [PubMed] [Google Scholar]
- 14.Radbruch A, Weberling LD, Kieslich PJ, et al. Intraindividual analysis of signal intensity changes in the dentate nucleus after consecutive serial applications of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol 2016; 51:683–690. [DOI] [PubMed] [Google Scholar]
- 15.Bauer K, Lathrum A, Raslan O, et al. Do gadolinium-based contrast agents affect the 18F-FDG PET/CT uptake in the dentate nucleus and the globus pallidus? A pilot study. J Nucl Med Technol 2017;45:30–33. [DOI] [PubMed] [Google Scholar]
- 16.Naganawa S. Effect of gadolinium deposition on 18F-FDG PET/CT of dentate nucleus and globus pallidus. J Nucl Med Technol 2017;45:173. [DOI] [PubMed] [Google Scholar]
- 17.Radbruch A, Haase R, Kieslich PJ, et al. No signal intensity increase in the dentate nucleus on unenhanced T1-weighted MR images after more than 20 serial injections of macrocyclic gadolinium-based contrast agents. Radiology 2017; 282:699–707. [DOI] [PubMed] [Google Scholar]
- 18.Kahn J, Posch H, Steffen IG, et al. Is there long-term signal intensity increase in the central nervous system on T1-weighted images after MR imaging with the hepatospecific contrast agent gadoxetic acid? A cross-sectional study in 91 patients. Radiology 2017; 282:708–716. [DOI] [PubMed] [Google Scholar]
- 19.Rasschaert M, Idée JM, Robert P, et al. Moderate renal failure accentuates T1 signal enhancement in the deep cerebellar nuclei of gadodiamide-treated rats. Invest Radiol 2017; 52:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forslin Y, Shams S, Hashim F, et al. Retention of gadolinium-based contrast agents in multiple sclerosis: retrospective analysis of an 18-year longitudinal study. AJNR Am J Neuroradiol 2017; 38:1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012;4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nedergaard M, Goldman SA. Brain drain. Sci Am 2016; 314:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarasoff-Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 2015; 11:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiviniemi V, Wang X, Korhonen V, et al. Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 2016; 36:1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naganawa S, Nakane T, Kawai H, Taoka T. Gd-based contrast enhancement of the perivascular spaces in the basal ganglia. Magn Reson Med Sci 2017; 16:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol 2017; 35:172–178. [DOI] [PubMed] [Google Scholar]
- 27.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol 2008; 18:253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rangroo Thrane V, Thrane AS, Plog BA, et al. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci Rep 2013; 3:2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asgari M, de Zélicourt D, Kurtcuoglu V. Glymphatic solute transport does not require bulk flow. Sci Rep 2016; 6:38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol 2015; 273:57–68. [DOI] [PubMed] [Google Scholar]
- 32.Cushing H. The third circulation and its channels (Cameron Lecture). Lancet 1925;2:851–857. [Google Scholar]
- 33.Ohata K, Marmarou A, Povlishock JT. An immunocytochemical study of protein clearance in brain infusion edema. Acta Neuropathol 1990; 81:162–177. [DOI] [PubMed] [Google Scholar]
- 34.Weller RO, Kida S, Zhang ET. Pathways of fluid drainage from the brain—morphological aspects and immunological significance in rat and man. Brain Pathol 1992; 2:277–284. [DOI] [PubMed] [Google Scholar]
- 35.Naganawa S, Yamazaki M, Kawai H, Bokura K, Sone M, Nakashima T. Imaging of Ménière’s disease after intravenous administration of single-dose gadodiamide: utility of subtraction images with different inversion time. Magn Reson Med Sci 2012; 11:213–219. [DOI] [PubMed] [Google Scholar]
- 36.Naganawa S, Suzuki K, Yamazaki M, Sakurai Y. Serial scans in healthy volunteers following intravenous administration of gadoteridol: time course of contrast enhancement in various cranial fluid spaces. Magn Reson Med Sci 2014; 13:7–13. [DOI] [PubMed] [Google Scholar]
- 37.Freddo TF. A contemporary concept of the blood-aqueous barrier. Prog Retin Eye Res 2013; 32:181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res 2005; 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanda T, Nakai Y, Oba H, Toyoda K, Kitajima K, Furui S. Gadolinium deposition in the brain. Magn Reson Imaging 2016; 34:1346–1350. [DOI] [PubMed] [Google Scholar]
- 40.Öner AY, Barutcu B, Aykol Ş, Tali ET. Intrathecal contrast-enhanced magnetic resonance imaging-related brain signal changes: residual gadolinium deposition? Invest Radiol 2017; 52:195–197. [DOI] [PubMed] [Google Scholar]