Abstract
Hypertrophic cardiomyopathy (HCM) is a relatively common myocardial genetic disease having a wide variety of symptoms and prognoses. The most serious complications of HCM are sudden cardiac death induced by ventricular arrhythmia or inappropriate changes in blood pressure, and heart failure. Cardiac MR imaging is a valuable imaging method for detecting HCM because of its accurate measurement of wall thickness and myocardial mass without limited view and the unique ability of late gadolinium enhancement (LGE) to identify myocardial fibrosis related to the prognosis of HCM. Tagging and T1 or T2 mapping MR imaging techniques have emerged as quantitative methods for the evaluation of disease severity. In this review, we introduce the MR imaging techniques applied to HCM and demonstrate the typical phenotypes and some morphological characteristics of HCM. In addition, we discuss the clinical relevance of MR imaging for risk stratification and management of HCM.
Keywords: hypertrophic cardiomyopathy, cardiac magnetic resonance imaging, steady-state free precession, late gadolinium enhancement
Introduction
Hypertrophic cardiomyopathy (HCM) is a relatively common myocardial genetic disease, affecting approximately 0.2–0.5% of the general population.1–3 Hypertrophic cardiomyopathy presents a wide variety of symptoms and prognoses, ranging from no symptoms and normal life expectancy to the presence of ventricular arrhythmia, sudden cardiac death (SCD), or heart failure (HF).4–6 Accordingly, systemic reviews and guidelines have been reported for the appropriate management of patients with HCM.4,7,8
Hypertrophic cardiomyopathy is caused by the mutations of autosomal dominant transmitted genes encoding the cardiac sarcomere, including cardiac β-myosin heavy chain, cardiac myosin binding protein C, and troponin T, while the identification and diagnosis of this disease are largely based on clinical imaging findings, including asymmetrical myocardial hypertrophy of the left ventricle (LVH) without any underlying diseases leading to LVH.4,7,8 Echocardiography is used to observe HCM because of its easy accessibility, capability to measure the gradient across the left ventricular outflow tract (LVOT) at rest and under provocative maneuvers, capability to assess valvular dysfunction, and because there are no contraindications for any patient. Nonetheless, the use of cardiac MR imaging is strongly recommended when making a diagnosis and evaluating the severity of HCM because it has advantage over echocardiography: accurate measurement of wall thickness and myocardial mass using cine steady-state free precession (SSFP) MR imaging, detailed observation of cardiac structures without limited view, and the unique and important capability of late gadolinium enhancement (LGE) MR imaging to identify myocardial fibrosis related to the prognosis of HCM.2,5,9–11 In addition, T2-weighted imaging shows myocardial edema or inflammation related to chest pain or syncope associated with HCM.12,13 Cardiac MR imaging is also valuable for differentiating between HCM and other myocardial diseases showing LVH.14,15 There are some reports describing the usefulness of tagging, perfusion, or T1 or T2 mapping as quantitative MR imaging methods for the evaluation of disease severity.16–19 Thus, the combination of genetic tests and cardiac MR imaging can give us a new perspective on the frequency, management, and prognosis of HCM.2
In this review, we introduce the MR imaging sequences applied to HCM and demonstrate typical phenotypes of HCM as well as some non-hypertrophied characteristics associated with HCM. We also discuss the clinical relevance of cardiac MR imaging for risk stratification of HCM, and follow-up or therapeutic management of HCM.
Cardiac MR imaging techniques applied to HCM
SSFP
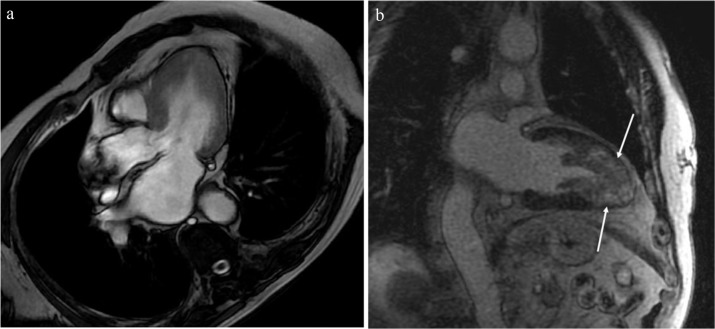
Cine SSFP MR imaging is used for morphological assessment of HCM and cardiac function measurement because of the high contrast between the myocardium and blood and high temporal resolution.20 Steady-state free precession is able to define all phenotypes of HCM because of its no limited view (Figs. 1a, 2a, 3, 4a, 5a). Apical hypertrophic cardiomyopathy (APH) or localized myocardial hypertrophy (e.g., inferior septum or lateral wall), which can be missed by echocardiography, is easily identified with SSFP (Figs. 5a, 6).9,10 Cine SSFP shows not only the wall motion but also the turbulence jet across the LVOT in patients with asymmetrical septal hypertrophy (ASH) HCM (Fig. 3). This MR imaging finding may be related to LVOT obstruction associated with HCM. Using retrospective gating, cine SSFP quantifies the myocardial thickness and mass accurately, which are related to the prognosis of HCM.21–24 It is unknown whether a maximum wall thickness ≥30 mm is a risk factor for SCD when using cardiac MR imaging because the difference in the measured maximum wall thickness can be 5 mm or more between echocardiography and MR imaging.23 The combination of a new threshold of the maximum wall thickness on cine SSFP and information about other risk factors may be valuable for the risk stratification of SCD associated with HCM (Fig. 7). Cine SSFP sometimes shows non-hypertrophied or thin myocardial regions, such as basal crypt, in HCM (Figs. 4a, 8, 9).25
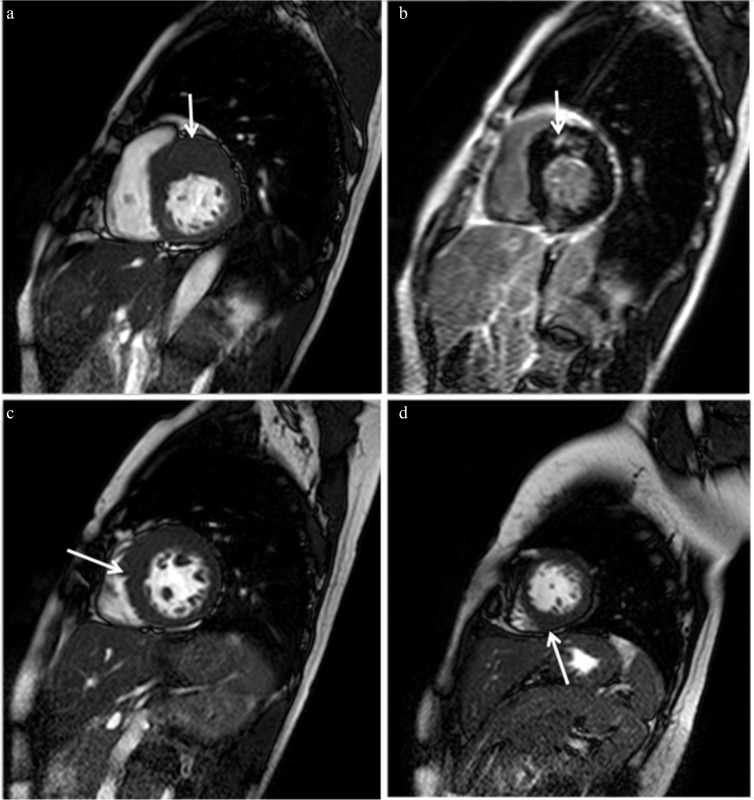
Fig. 1.
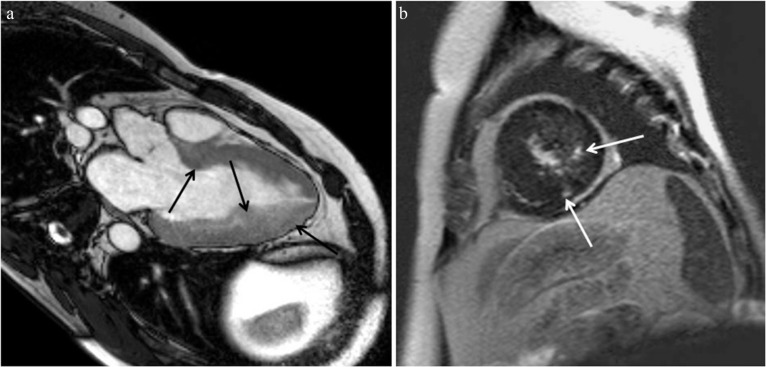
Typical MR images of asymmetrical septal hypertrophy (ASH) hypertrophic cardiomyopathy (HCM). (a) Steady-state free precession (SSFP) shows hypertrophied anterior and anterior septal myocardium at the basal level (arrow). (b) Late gadolinium enhancement (LGE) is identified in the hypertrophied anterior septal myocardium, the insertion point of the left and right ventricles (arrow). Myocardial hypertrophy is found in a spiral distribution from the basal anterior septum (a, arrow), through the middle septum at the midventricular level (c, arrow) to the inferior region at the apical level (d, arrow).
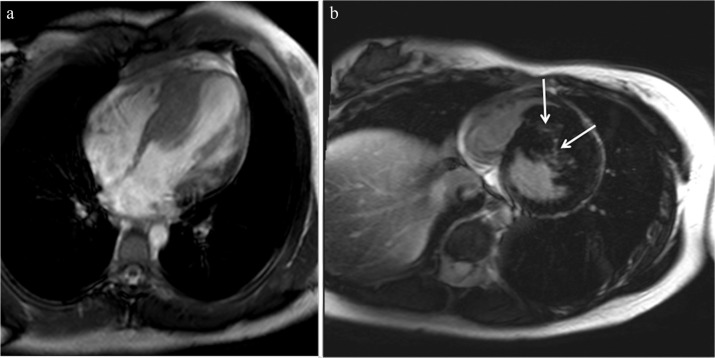
Fig. 2.
(a) Reverse curve type of hypertrophic cardiomyopathy (HCM) is demonstrated. (b) Late gadolinium enhancement (LGE) is identified in the most hypertrophied myocardium (arrows).
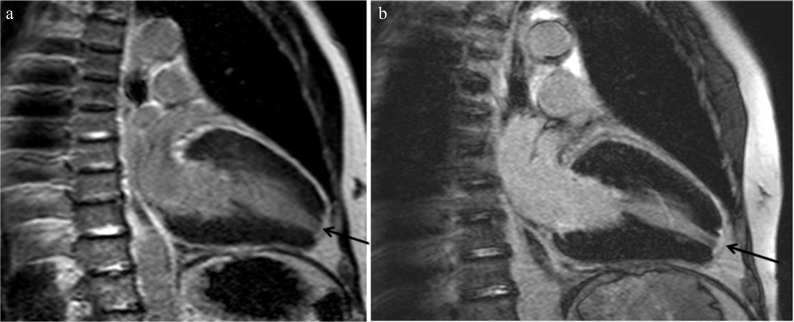
Fig. 3.
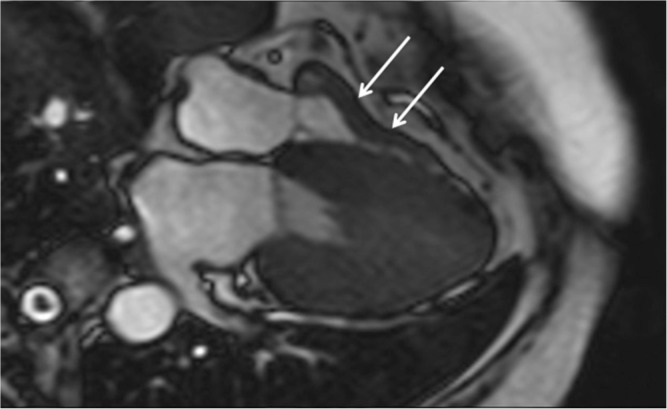
Cine steady-state free precession (SSFP) shows not only the high septal myocardial hypertrophy (arrowhead) but also the turbulence jet across the left ventricular outflow tract (arrow).
Fig. 4.
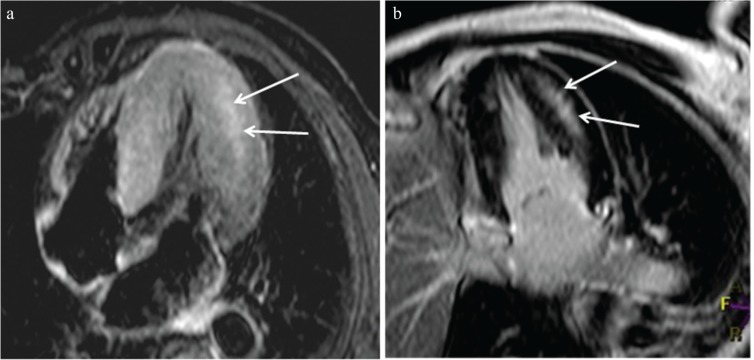
(a) Midventricular hypertrophy (arrowhead) associated with an apical aneurysm (arrow) is identified with steady-state free precession (SSFP). (b) Apical aneurysm associated with midventricular obstruction (MVO) is enhanced strongly (arrow), and the patient showed ventricular tachycardia.
Fig. 5.
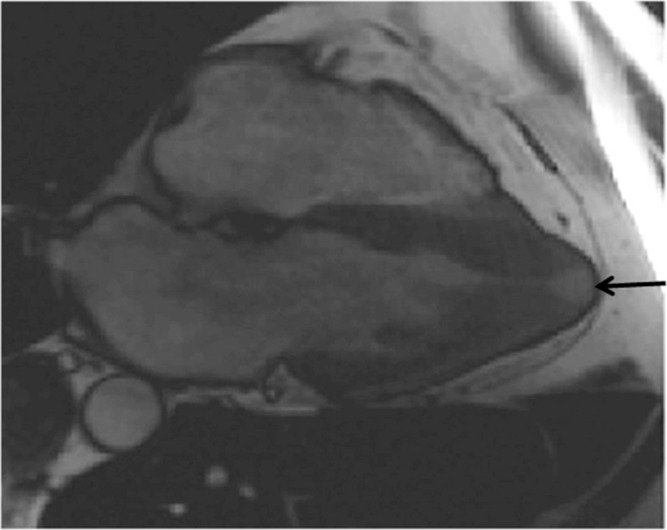
(a) Apical hypertrophic cardiomyopathy (APH) is identified with steady-state free precession (SSFP). (b) Apical myocardium is enhanced extensively (arrows), and the patient showed ventricular tachycardia.
Fig. 6.
Steady-state free precession (SSFP) is able to show the localized hypertrophy of the inferior septal myocardium because of its no limited view (arrow).
Fig. 7.
A case of sudden cardiac death and family history of hypertrophic cardiomyopathy (HCM). (a) The maximum wall thickness is 28 mm. (b) Late gadolinium enhancement (LGE) is observed in the hypertrophied anterior septal myocardium (arrow).
Fig. 8.
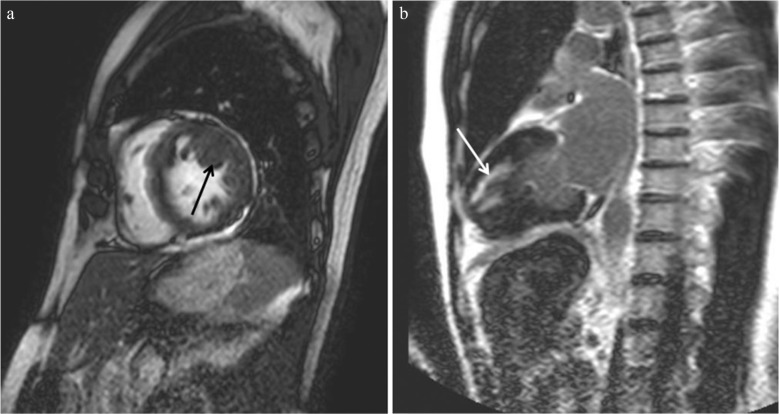
(a). Basal crypt is found at the basal inferior region in end-diastole (arrow). (b) At the end-systole, the crypt is not identified.
Fig. 9.
Apical pouching (i.e., focal thinning) is identified with steady-state free precession (SSFP) (arrow).
LGE
Late gadolinium enhancement is the most valuable MR imaging sequence for HCM, because it identifies myocardial replacement fibrosis or scarring that contributes to risk stratification for HCM (Figs. 4b, 7b).7,9,19,26–28 Late gadolinium enhancement is significantly related to ventricular tachyarrhythmia and SCD associated with HCM (Figs. 4b, 5b, 7).7,11 Late gadolinium enhancement is also useful for differentiating HCM from other cardiomyopathies with similar symptoms and LVH.14,15 The myocardial scarring shown by LGE usually locates at the insertion point of the left and right ventricles or the most hypertrophied myocardial regions (Figs. 1b, 2b, 5b), while endomyocardial LGE is observed in cardiac amyloidosis, inferior lateral patchy LGE is observed in Anderson-Fabry disease, and linear or patchy LGE is identified at the septal or inferior wall in hypertensive cardiomyopathy or aortic stenosis.14,15,29,30
T2-weighted imaging
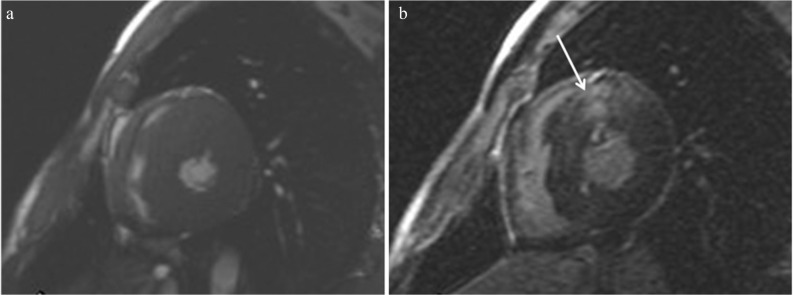
T2-weighted imaging in combination with double inversion-recovery black blood preparation shows myocardial edema or inflammation associated with HCM, which may be related to chest pain, syncope, or increase in troponin T.12,13,17,31 Myocardial hyperintensity on the T2-weighted image often locates within LGE areas (Fig. 10), but can locate outside LGE.13
Fig. 10.
T2-weighted imaging shows hyperintensity (a, arrows) of the hypertrophied midventricular and apical myocardium, which is consistent with late gadolinium enhancement (LGE) (b, arrows).
Cine phase contrast
Cine phase contrast MR imaging can be used to quantify the blood flow passing through the LVOT. The LVOT gradient, which reflects the severity of LVOT obstruction induced by HCM, can be estimated using this imaging technique and Bernoulli’s principle. Nonetheless, phase contrast imaging is not often used in the clinical setting because of its lengthy scan time, difficulty in setting an appropriate velocity encoding in cases of obstructive HCM, and the need for post-processing.
Tagging
Tagging MR imaging is useful for evaluating the myocardial wall motion and strain quantitatively. This technique shows regional strain abnormalities even in the non-hypertrophied myocardium of HCM, and it can be improved after the interventional procedure on the hypertrophied myocardium close to the LVOT.18 The hypertrophied myocardial region with LGE tends to show decreased wall motion on tagging,32 while the disconcordance between LGE and decreased circumferential strain is observed using 3D tagging.33 It is unknown whether the tagging has considerable advantages over cine SSFP and LGE MR imaging for identification of early-stage HCM and for risk stratification.
Perfusion
Perfusion MR imaging provides information about blood flow and myocardial circulation with high spatial and temporal resolution. Perfusion MR imaging is usually performed at rest in patients with HCM because the stress test can provoke a LVOT obstruction or sudden changes in blood pressure, which can lead to serious symptoms. Previous studies using perfusion MR imaging inpatients at rest show the close relationship between perfusion decrease and wall thickness, LGE, or hyperintensity on T2-weighted images.32,34 Therefore, the merits of perfusion MR imaging over LGE and T2-weighted imaging, which are performed more easily and which cover the whole heart, have not been validated in HCM.
T1 and T2 mapping
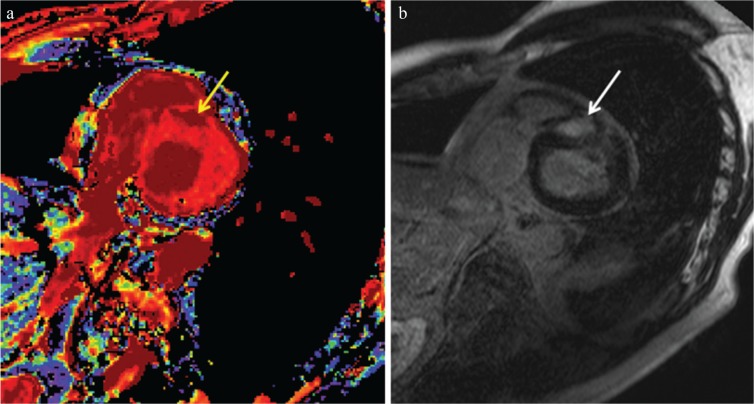
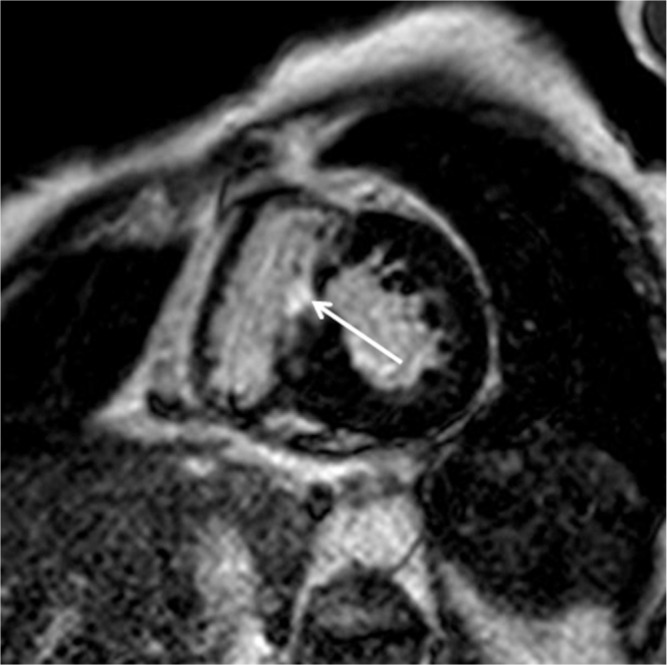
T1 and T2 mapping MR imaging techniques are able to identify myocardial injuries associated with HCM without gadolinium-based contrast agents. The native T1 values of the myocardium are increased not only in the regions showing LGE but also in those without LGE in HCM (Fig. 11).19,35 Therefore, T1 mapping without contrast may identify myocardial scarring as well as interstitial fibrosis in HCM. T1 mapping before and after gadolinium injection provides information about the extracellular volume fraction, which differs between HCM and the myocardial hypertrophy of athletes, and which is positively correlated with the maximum wall thickness in HCM.35 On the other hand, a previous study has indicated the closer relation of LGE to morphological and functional abnormalities in HCM compared with indications from the ratio of myocardial T1 to blood T1 values.36 T2 mapping is useful for confirming the presence of myocardial hyperintensity on T2-weighted images.17 Nonetheless, the clinical relevance of T1 and T2 mapping for risk stratification of HCM has not been confirmed so far.
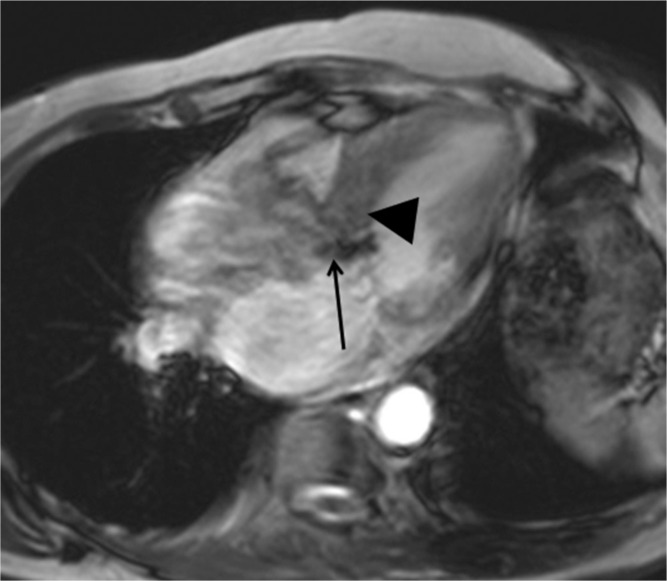
Fig. 11.
Non-contrast-enhanced T1 mapping (a) identifies a region with prolonged native T1 (arrow, 1370 ms), which is consistent with late gadolinium enhancement (LGE) in a patient with hypertrophic cardiomyopathy (HCM) (b, arrow). The native T1 of 1243 ms at the myocardium without LGE is higher than the normal myocardium of 1209 ms in a healthy volunteer at a 3T unit.
Cardiac MR imaging findings of HCM
Basal asymmetrical septal hypertrophy
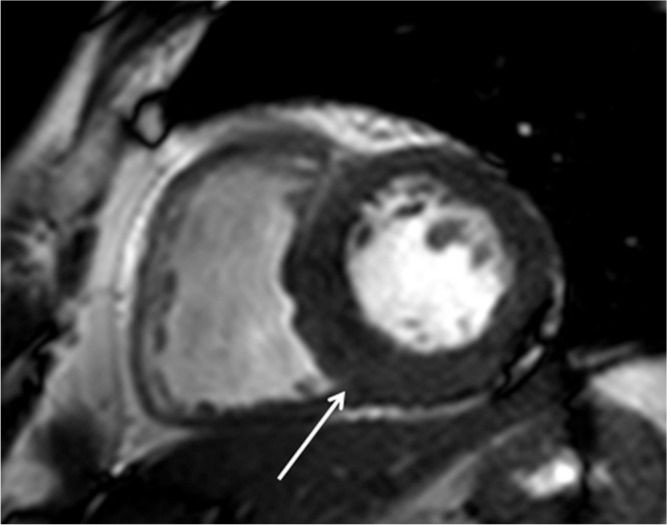
Basal ASH is the most common phenotype of HCM, at 60–70% of HCM cases. The basal anterior septal thickness is ≥ 15 mm at end-diastole and the ratio of septal to inferolateral wall thickness is ≥ 1.3, which can be measured using SSFP. In addition, the MR imaging is able to detect asymmetrical hypertrophy with a spiral configuration: basal anterior septal hypertrophy to apical inferior hypertrophy (Fig. 1a, c, d). In ASH, LGE is usually identified at the hypertrophied insertion points of the interventricular septum (Fig. 1b). The reverse-type ASH shows marked hypertrophy of the basal and midventricular septum protruding the left ventricular cavity and accompanies the frequent genetic mutations and LGE (Fig. 2).37 This ASH phenotype may be frequently related to serious ventricular arrhythmia. The localized hypertrophy of the high septum (i.e., sigmoid septum) or the inferior septum are other types of ASH (Figs. 3, 6). High septal ASH is the good indication for LVOT gradient reduction therapy such as myectomy and alcohol septal ablation (ASA).
Midventricular obstruction HCM
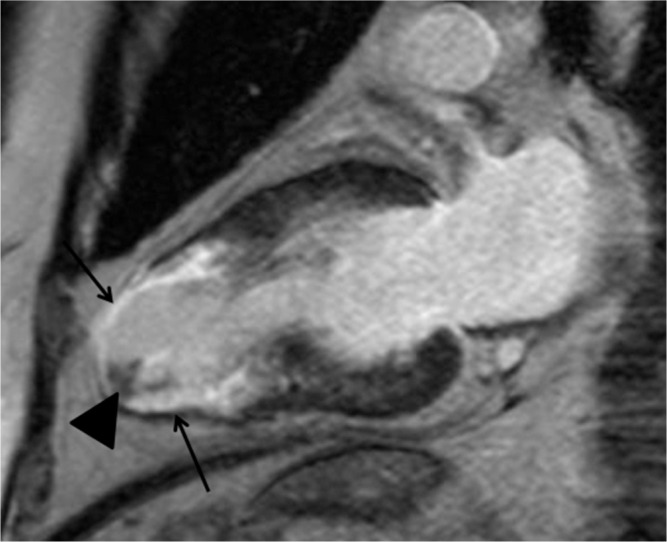
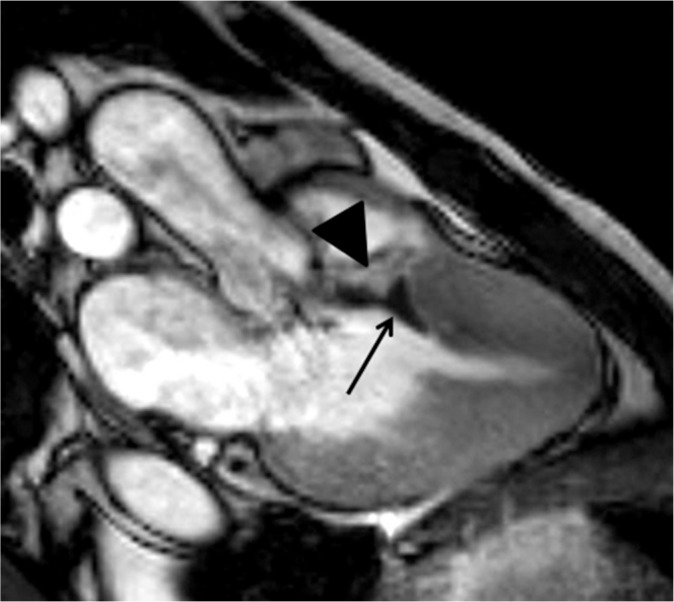
Midventricular obstruction (MVO) HCM is known to have a poor prognosis because of massive hypertrophy of the midventricular myocardium and associated apical aneurysm.38,39 Echocardiography can detect and quantify the turbulence or jet flow at the midventricular level in systole. Cine SSFP is also valuable for identifying the jet flow and midventricular hypertrophy in MVO HCM (Fig. 4a). In addition, LGE MR imaging shows where an apical aneurysm has been replaced by scar tissues, which is significantly related to adverse cardiac events (Fig. 4b).38 Apical thrombus, which can lead to cerebral infarction, is sometimes associated with an apical aneurysm in patients with MVO HCM (Fig. 12). Therefore, MVO HCM should be investigated carefully using cardiac MR imaging, and implantable cardioverter defibrillator or antithrombotic treatment should be performed based on the clinical and imaging findings.
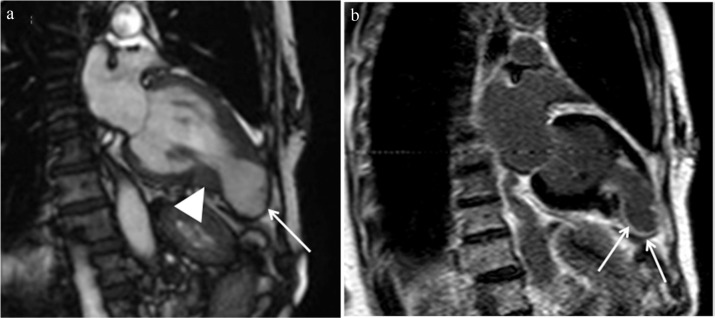
Fig. 12.
Apical aneurysm showing late gadolinium enhancement (LGE) (arrows) and associated intraventricular thrombus (arrowhead) is observed in midventricular obstruction (MVO) hypertrophic cardiomyopathy (HCM).
Apical HCM
Apical hypertrophic cardiomyopathy is characteristic of localized hypertrophy of the apical myocardium and spade-like deformity of the left ventricular cavity (Fig. 5a). It is well known that the APH shows a giant T wave on electrocardiogram and is more frequent in the Japanese population than in Western populations.40 Cardiac MR imaging identifies this phenotype of HCM more commonly than does echocardiography because of its no limited view.10 Apical hypertrophic cardiomyopathy is reported to have a better prognosis than other types of HCM. However, one-third of patients with APH present with ventricular tachyarrhythmia and have a worse outcome.41 A previous study has indicated that extensive LGE is related to ventricular tachyarrhythmia even in cases of APH.42
Combined hypertrophy
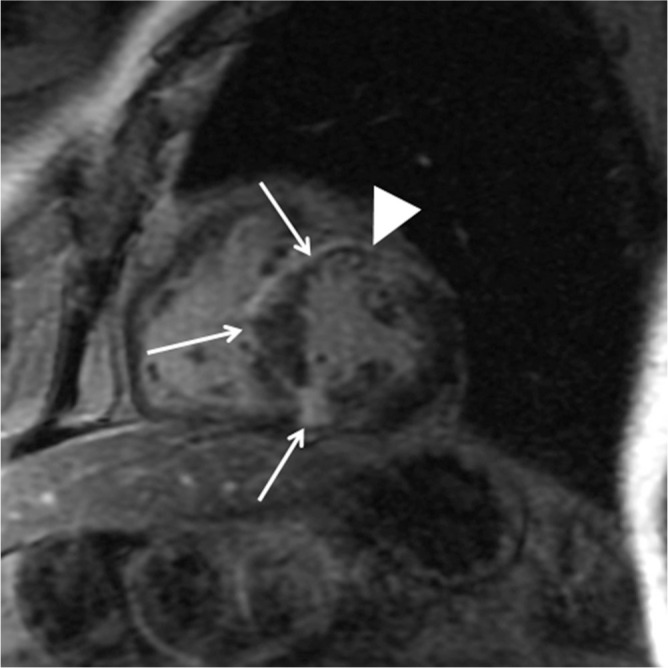
It is not uncommon that several of the phenotypes noted above are combined in HCM (Figs. 10, 13).43 SSFP is able to detect multiple hypertrophied regions correctly because of its high spatial resolution and no limited view (Fig. 13a). Late gadolinium enhancement MR imaging indicates the dominant phenotype by demonstrating myocardial scarring (i.e., the most damaged myocardium) in those patients with combined hypertrophy (Figs. 10b, 13b).
Fig. 13.
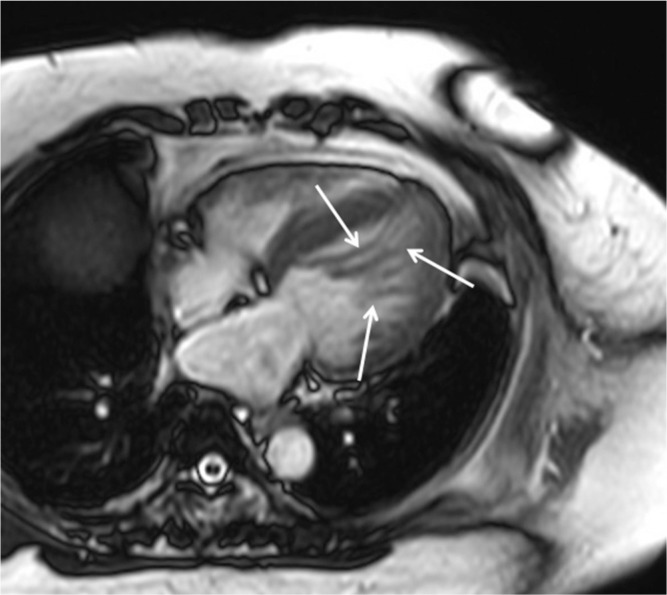
(a) Steady-state free precession (SSFP) shows hypertrophied basal septal, midventricular lateral, and apical lateral myocardium (arrows). (b) Late gadolinium enhancement (LGE) is found only in the lateral and inferior myocardium at the apical level (arrows).
Right ventricular hypertrophy
Right ventricular hypertrophy occurs in one-third of patients with HCM (Fig. 14).9,44 Compared with echocardiography, cardiac MR imaging accurately shows some types of right ventricular hypertrophy, including hypertrophy of the septal insertion point and the entire hypertrophy. Although a right ventricular outflow obstruction may occur in association with right ventricular myocardial hypertrophy, its clinical significance remains unknown in HCM.
Fig. 14.
Right ventricular hypertrophy is identified in addition to hypertrophy of the left ventricle (LVH) (arrows).
Papillary muscular abnormality
Papillary muscular abnormalities, including hypertrophied papillary muscles, increase in the number of muscles, and abnormal attachment of the muscles to the mitral valves or interventricular septum, are often investigated in patients with HCM (Figs. 15a, 16).45–47 Late gadolinium enhancement may be observed in hypertrophied papillary muscles (Fig. 15b). When hypertrophied papillary muscles induce a LVOT obstruction and consequent clinical symptoms, ASA is not effective, and surgical intervention, such as myectomy combined with papillary muscle reorientation, should be selected for the release of the obstruction.47
Fig. 15.
Hypertrophied papillary muscle (a, arrow) shows late gadolinium enhancement (LGE) (b, arrow).
Fig. 16.
An increase in papillary muscles is associated with hypertrophic cardiomyopathy (HCM) (arrows).
Basal crypt and apical pouching
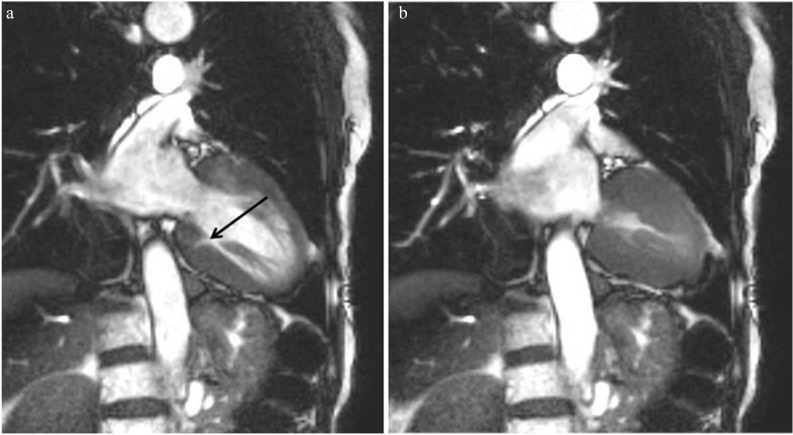
Basal crypt and apical pouching or local thinning are often observed in patients with HCM (Figs. 8, 9).25,48,49 The basal crypt is considered one of the morphological signs of genotype positive/phenotype negative subjects.48 Cine SSFP can identify a basal crypt clearly in the basal inferior region because of its high contrast, sufficient temporal resolution, and wide range of view (Fig. 8). Apical pouching may be enhanced, but its clinical significance remains unknown because of the lack of clinical symptoms and no progression (Fig. 17).
Fig. 17.
Apical pouching is enhanced (a, arrow), and had not changed at the follow-up late gadolinium enhancement (LGE) MR imaging performed 2 years later (b, arrow).
End-stage HCM
Hypertrophic cardiomyopathy usually shows normal or supernormal systolic function of the left ventricle, possibly representing a left ventricular ejection fraction > 75%. However, approximately 5–10% of patients with HCM show an ejection fraction < 50%, which is called “end-stage”, “dilated phase”, or “burn-out” HCM.4,6,50,51 End-stage HCM has a poor prognosis in the 5 years at its initial diagnosis because of SCD and progressive and decompensated HF.51 Cardiac MR imaging is able to measure the ejection fraction accurately as well as identify extensive myocardial scarring in end-stage HCM (Fig. 18). Extensive LGE, reflecting myocardial scarring, leads to systolic dysfunction in HCM. Cardiac MR imaging also demonstrates the coexistence of myocardial hypertrophy and thinning in end-stage HCM (Fig. 18). In addition, an ejection fraction of 50–65% indicates impairment of systolic function in patients with HCM.28 Therefore, close clinical and MR imaging follow-up may be required in those HCM patients with an ejection fraction <65%.28
Fig. 18.
Ejection fraction is 33.7% on cine steady-state free precession (SSFP) in this hypertrophic cardiomyopathy (HCM) patient. Extensive late gadolinium enhancement (LGE) (arrows) and myocardial wall thinning (arrowhead) are depicted.
Clinical Relevance of Cardiac MR Imaging to HCM
Differential diagnosis
Myocardial hypertrophy is observed in some cardiomyopathies including Anderson-Fabry disease, amyloidosis, hypertensive cardiomyopathy, and aortic valvular diseases. Even athlete’s LVH should be differentiated from HCM, because SCD can occur in young athletes with HCM.1,52 Cardiac MR imaging is valuable for differentiating between HCM and other myocardial diseases showing LVH because of its accurate identification of the patterns of myocardial hypertrophy and LGE.9,14,15,29,30 Although even athletes’ LVH can show LGE, the frequency of LGE and geometric indices acquired by cardiac MR imaging differ between athletes and HCM patients.52,53 In addition, basal crypt and apical pouching may be suggestive of the diagnosis of HCM.25,48,49
Risk stratification
The risk stratification for HCM, especially the risks for SCD, is generally assessed based on the clinical and family history, patient’s age, and electrocardiogram findings.4,7,8 Cardiac MR imaging is valuable because of its ability to measure myocardial thickness accurately and identify myocardial fibrosis noninvasively. Massive hypertrophy is considered as the risk factor for SCD,4,7,24 and maximum wall thickness and myocardial mass can be measured using cine SSFP (Fig. 2a). Midventricular obstruction is the “high-risk” type of HCM, especially when it is associated with apical aneurysm, possibly leading to ventricular tachyarrhythmia or systemic thrombosis (Figs. 4, 12).38,39 Extensive LGE can indicate risks for the ventricular tachyarrhythmia or systolic impairment associated with HCM (Figs. 4b, 5b, 7, 18).7,11,27,28,54–56 Because more than 60% patients show LGE, myocardial LGE is not always associated with risks for serious arrhythmia, SCD, or HF. Nonetheless, the assessment of presence, extent, and progression of LGE should be evaluated carefully for the risk stratification of HCM, because LGE reflects replacement and interstitial myocardial fibrosis and associated coronary artery dysplasia.11,28,42,56,57
Indication and assessment of ASA
Surgical myectomy is considered the standard treatment for LVOT obstruction associated with HCM,7 while ASA is a less invasive alternative to myectomy in some patients, including elderly patients and those who may not tolerate surgery.58 Cardiac MR imaging is able to play an important role in the choice of these treatments, because whereas localized septal hypertrophy is a good candidate for both myectomy and ASA (Fig. 3), the LVOT obstruction caused by papillary muscle abnormalities should be treated by surgical intervention such as septal myectomy and papillary muscle replacement (Figs. 15a, 16). Hypertrophy of the left ventricle or non-septal hypertrophy may be reduced following release of the LVOT obstruction, which can be assessed with SSFP.59 Late gadolinium enhancement MR imaging shows the ablated region in the hypertrophied septum clearly (Fig. 19). The location and extent of LGE induced by ASA and non-anteroseptal myocardial hypertrophy are predictive of the success rate of ASA.60,61 Contrast-enhanced cine SSFP can also be used to evaluate the ablated myocardium, including the microvascular obstruction and enhanced region (Fig. 20).62 Therefore, cardiac MR imaging is useful for treatment planning and follow-up in patients with HCM with LVOT obstruction.
Fig. 19.
The septal myocardium after alcohol septal ablation (ASA) shows late gadolinium enhancement (LGE) and reduced thickness (arrow).
Fig. 20.
Contrast-enhanced steady-state free precession (SSFP) imaging identifies the ablated myocardium as the microvascular obstruction showing marked low intensity (arrow) and enhanced region (arrowhead).
Conclusions
Cardiac MR imaging is useful for making a diagnosis of HCM and identifying the phenotypes of HCM because of its ability to show the cardiac morphologies clearly. Cardiac MR imaging can also contribute to risk stratification for HCM because of its accurate measurement of LVH, detection of “high-risk” phenotypes, and identification of myocardial fibrosis (i.e., LGE). In addition, cardiac MR imaging is useful for treatment planning and follow-up in patients with HCM associated with LVOT obstruction. Therefore, cardiac MR imaging should be applied to patients with HCM or suspicious HCM in clinical practice.
Acknowledgments
The authors thank Yoshio Matsumura, RT (Nippon Medical School) and Hiroshi Yamamoto, RT (Nihon University Hospital) for their technical support. The contents of this review were partly presented at the 45th annual meeting of the Japanese Society for Magnetic Resonance in Medicine at the recommendation of Noriko Manabe, MD (Hokkaido University).
Footnotes
Conflicts of Interest
All authors have declared no conflict of interest related to this article.
References
- 1.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation 1995;92:785–789. [DOI] [PubMed] [Google Scholar]
- 2.Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015; 65:1249–1254. [DOI] [PubMed] [Google Scholar]
- 3.Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol 2008; 19:104–110. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002; 287:1308–1320. [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ, Roberts WC, McAllister HA, Rosing DR, Epstein SE. Sudden death in young athletes. Circulation 1980; 62:218–229. [DOI] [PubMed] [Google Scholar]
- 6.Houston BA, Stevens GR. Hypertrophic cardiomyopathy: a review. Clin Med Insights Cardiol 2015;8(Suppl 1):53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gersh BJ, Maron BJ, Bonow RO, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. American Association for Thoracic Surgery. American Society of Echocardiography. American Society of Nuclear Cardiology. Heart Failure Society of America. Heart Rhythm Society. Society for Cardiovascular Angiography and Interventions. Society of Thoracic Surgeons 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation 2011; 124:2761–2796. [DOI] [PubMed] [Google Scholar]
- 8.Authors/Task Force members. Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 9.Maron MS. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2012; 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 2004; 90:645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amano Y, Kitamura M, Tachi M, Takeda M, Mizuno K, Kumita S. Delayed enhancement magnetic resonance imaging in hypertrophic cardiomyopathy with Basal septal hypertrophy and preserved ejection fraction: relationship with ventricular tachyarrhythmia. J Comput Assist Tomogr 2014; 38:67–71. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Aty H, Cocker M, Strohm O, Filipchuk N, Friedrich MG. Abnormalities in T2-weighted cardiovascular magnetic resonance images of hypertrophic cardiomyopathy: regional distribution and relation to late gadolinium enhancement and severity of hypertrophy. J Magn Reson Imaging 2008; 28:242–245. [DOI] [PubMed] [Google Scholar]
- 13.Amano Y, Aita K, Yamada F, Kitamura M, Kumita S. Distribution and clinical significance of high signal intensity of the myocardium on T2-weighted images in 2 phenotypes of hypertrophic cardiomyopathy. J Comput Assist Tomogr 2015; 39:951–955. [DOI] [PubMed] [Google Scholar]
- 14.De Cobelli F, Esposito A, Belloni E, et al. Delayed-enhanced cardiac MRI for differentiation of Fabry’s disease from symmetric hypertrophic cardiomyopathy. AJR Am J Roentgenol 2009; 192:W97–W102. [DOI] [PubMed] [Google Scholar]
- 15.Takeda M, Amano Y, Tachi M, Tani H, Mizuno K, Kumita S. MRI differentiation of cardiomyopathy showing left ventricular hypertrophy and heart failure: differentiation between cardiac amyloidosis, hypertrophic cardiomyopathy, and hypertensive heart disease. Jpn J Radiol 2013; 31:693–700. [DOI] [PubMed] [Google Scholar]
- 16.Dass S, Suttie JJ, Piechnik SK, et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging 2012; 5:726–733. [DOI] [PubMed] [Google Scholar]
- 17.Amano Y, Yanagisawa F, Tachi M, Hashimoto H, Imai S, Kumita S. Myocardial T2 mapping in patients with hypertrophic cardiomyopathy. J Comput Assist Tomogr 2017; 41:344–348. [DOI] [PubMed] [Google Scholar]
- 18.van Dockum WG, Kuijer JP, Götte MJ, et al. Septal ablation in hypertrophic obstructive cardiomyopathy improves systolic myocardial function in the lateral (free) wall: a follow-up study using CMR tissue tagging and 3D strain analysis. Eur Heart J 2006; 27:2833–2839. [DOI] [PubMed] [Google Scholar]
- 19.Kato S, Nakamori S, Bellm S, et al. Myocardial native T1 time in patients with hypertrophic cardiomyopathy. Am J Cardiol 2016; 118:1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr JC, Simonetti O, Bundy J, Li D, Pereles S, Finn JP. Cine MR angiography of the heart with segmented true fast imaging with steady-state precession. Radiology 2001; 219:828–834. [DOI] [PubMed] [Google Scholar]
- 21.Rickers C, Wilke NM, Jerosch-Herold M, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005; 112:855–861. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Kaushikkar S, Haacke EM, et al. Coronary arteries: three-dimensional MR imaging with retrospective respiratory gating. Radiology 1996; 201:857–863. [DOI] [PubMed] [Google Scholar]
- 23.Bois JP, Geske JB, Foley TA, Ommen SR, Pellikka PA. Comparison of maximal wall thickness in hypertrophic cardiomyopathy differs between magnetic resonance imaging and transthoracic echocardiography. Am J Cardiol 2017; 119:643–650. [DOI] [PubMed] [Google Scholar]
- 24.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 2000; 342:1778–1785. [DOI] [PubMed] [Google Scholar]
- 25.Deva DP, Williams LK, Care M, et al. Deep basal inferoseptal crypts occur more commonly in patients with hypertrophic cardiomyopathy due to disease-causing myofilament mutations. Radiology 2013; 269:68–76. [DOI] [PubMed] [Google Scholar]
- 26.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology 2001; 218:215–223. [DOI] [PubMed] [Google Scholar]
- 27.Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 2003; 41:1561–1567. [DOI] [PubMed] [Google Scholar]
- 28.Olivotto I, Maron BJ, Appelbaum E, et al. Spectrum and clinical significance of systolic function and myocardial fibrosis assessed by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol 2010; 106:261–267. [DOI] [PubMed] [Google Scholar]
- 29.Andersen K, Hennersdorf M, Cohnen M, Blondin D, Mödder U, Poll LW. Myocardial delayed contrast enhancement in patients with arterial hypertension: initial results of cardiac MRI. Eur J Radiol 2009; 71:75–81. [DOI] [PubMed] [Google Scholar]
- 30.Rudolph A, Abdel-Aty H, Bohl S, et al. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol 2009; 53:284–291. [DOI] [PubMed] [Google Scholar]
- 31.Simonetti OP, Finn JP, White RD, Laub G, Henry DA. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology 1996; 199:49–57. [DOI] [PubMed] [Google Scholar]
- 32.Soler R, Rodríguez E, Monserrat L, Méndez C, Martínez C. Magnetic resonance imaging of delayed enhancement in hypertrophic cardiomyopathy: relationship with left ventricular perfusion and contractile function. J Comput Assist Tomogr 2006; 30:412–420. [DOI] [PubMed] [Google Scholar]
- 33.Amano Y, Yamada F, Hashimoto H, Obara M, Asai K, Kumita S. Fast 3-breath-hold 3-dimensional tagging cardiac magnetic resonance in patients with hypertrophic myocardial diseases: a feasibility study. Biomed Res Int 2016; 2016:3749489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hueper K, Zapf A, Skrok J, et al. In hypertrophic cardiomyopathy reduction of relative resting myocardial blood flow is related to late enhancement, T2-signal and LV wall thickness. PLoS One 2012; 7:e41974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swoboda PP, McDiarmid AK, Erhayiem B, et al. Assessing myocardial extracellular volume by T1 mapping to distinguish hypertrophic cardiomyopathy from athlete’s heart. J Am Coll Cadiol 2016; 67:2189–2190. [DOI] [PubMed] [Google Scholar]
- 36.Chu LC, Corona-Villalobos CP, Halushka MK, et al. Structural and functional correlates of myocardial T1 mapping in 321 patients with hypertrophic cardiomyopathy. J Comput Assist Tomogr 2017; 41:653–660. [DOI] [PubMed] [Google Scholar]
- 37.Binder J, Ommen SR, Gersh BJ, et al. Echocardiography-guided genetic testing in hypertrophic cardiomyopathy: septal morphological features predict the presence of myofilament mutations. Mayo Clin Proc 2006; 81:459–467. [DOI] [PubMed] [Google Scholar]
- 38.Minami Y, Kajimoto K, Terajima Y, et al. Clinical implications of midventricular obstruction in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2011; 57:2346–2355. [DOI] [PubMed] [Google Scholar]
- 39.Maron MS, Finley JJ, Bos JM, et al. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation 2008; 118:1541–1549. [DOI] [PubMed] [Google Scholar]
- 40.Kitaoka H, Doi Y, Casey SA, Hitomi N, Furuno T, Maron BJ. Comparison of prevalence of apical hypertrophic cardiomyopathy in Japan and the United States. Am J Cardiol 2003; 92:1183–1186. [DOI] [PubMed] [Google Scholar]
- 41.Eriksson MJ, Sonnenberg B, Woo A, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 39:638–645. [DOI] [PubMed] [Google Scholar]
- 42.Amano Y, Takayama M, Fukushima Y, Kitamura M, Kumita S. Delayed-enhancement MRI of apical hypertrophic cardiomyopathy: assessment of the intramural distribution and comparison with clinical symptoms, ventricular arrhythmias, and cine MRI. Acta Radiol 2011; 52:613–618. [DOI] [PubMed] [Google Scholar]
- 43.Minami Y, Haruki S, Hagiwara N. Phenotypic overlap in hypertrophic cardiomyopathy: apical hypertrophy, midventricular obstruction, and apical aneurysm. J Cardiol 2014; 64:463–469. [DOI] [PubMed] [Google Scholar]
- 44.Maron MS, Hauser TH, Dubrow E, et al. Right ventricular involvement in hypertrophic cardiomyopathy. Am J Cardiol 2007; 100:1293–1298. [DOI] [PubMed] [Google Scholar]
- 45.Harrigan CJ, Appelbaum E, Maron BJ, et al. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol 2008; 101:668–673. [DOI] [PubMed] [Google Scholar]
- 46.Kwon DH, Setser RM, Thamilarasan M, et al. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart 2008; 94:1295–1301. [DOI] [PubMed] [Google Scholar]
- 47.Patel P, Dhillon A, Popovic ZB, et al. Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy patients without severe septal hypertrophy: implications of mitral valve and papillary muscle abnormalities assessed using cardiac magnetic resonance and echocardiography. Circ Cardiovasc Imaging 2015; 8:e003132. [DOI] [PubMed] [Google Scholar]
- 48.Germans T, Wilde AA, Dijkmans PA, et al. Structural abnormalities of the inferoseptal left ventricular wall detected by cardiac magnetic resonance imaging in carriers of hypertrophic cardiomyopathy mutations. J Am Coll Cardiol 2006; 48:2518–2523. [DOI] [PubMed] [Google Scholar]
- 49.Amano Y, Takayama M, Kumita S. Magnetic resonance imaging of apical left ventricular aneurysm and thinning associated with hypertrophic cardiomyopathy. J Comput Assist Tomogr 2008; 32:259–264. [DOI] [PubMed] [Google Scholar]
- 50.Harris KM, Spirito P, Maron MS, et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 2006; 114:216–225. [DOI] [PubMed] [Google Scholar]
- 51.Kawarai H, Kajimoto K, Minami Y, Hagiwara N, Kasanuki H. Risk of sudden death in end-stage hypertrophic cardiomyopathy. J Card Fail 2011; 17:459–464. [DOI] [PubMed] [Google Scholar]
- 52.Erz G, Mangold S, Franzen E, et al. Correlation between ECG abnormalities and cardiac parameters in highly trained asymptomatic male endurance athletes: evaluation using cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 2013; 29:325–334. [DOI] [PubMed] [Google Scholar]
- 53.Petersen SE, Selvanayagam JB, Francis JM, et al. Differentiation of athlete’s heart from pathological forms of cardiac hypertrophy by means of geometric indices derived from cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2005; 7:551–558. [DOI] [PubMed] [Google Scholar]
- 54.Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014; 130:484–495. [DOI] [PubMed] [Google Scholar]
- 55.Kwon DH, Setser RM, Popović ZB, et al. Association of myocardial fibrosis, electrocardiography, and ventricular tachyarrhythmia in hypertrophic cardiomyopathy: a delayed contrast enhanced MRI study. Int J Cardiovasc Imaging 2008; 24:617–625. [DOI] [PubMed] [Google Scholar]
- 56.Kwon DH, Smedira NG, Rodriguez ER, et al. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol 2009; 54:242–249. [DOI] [PubMed] [Google Scholar]
- 57.Moravsky G, Ofek E, Rakowski H, et al. Myocardial fibrosis in hypertrophic cardiomyopathy: accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging 2013; 6:587–596. [DOI] [PubMed] [Google Scholar]
- 58.Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 1995; 346:211–214. [DOI] [PubMed] [Google Scholar]
- 59.Amano Y, Takayama M, Kumita S, Kumazaki T. MR imaging evaluation of regional, remote, and global effects of percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy. J Comput Assist Tomogr 2007; 31:600–604. [DOI] [PubMed] [Google Scholar]
- 60.van Dockum WG, ten Cate FJ, ten Berg JM, et al. Myocardial infarction after percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: evaluation by contrast-enhanced magnetic resonance imaging. J Am Coll Cardiol 2004; 43:27–34. [DOI] [PubMed] [Google Scholar]
- 61.Kitamura M, Amano Y, Takayama M, et al. Usefulness of non-anteroseptal region left ventricular hypertrophy using cardiac magnetic resonance to predict repeat alcohol septal ablation for refractory obstructive hypertrophic cardiomyopathy. Am J Cardiol 2017; 120:124–130. [DOI] [PubMed] [Google Scholar]
- 62.Amano Y, Yamada F, Kitamura M, et al. Contrast-enhanced steady-state free precession in the assessment of hypertrophic obstructive cardiomyopathy after alcohol septal ablation. Magn Reson Med Sci 2016; 15:130–136. [DOI] [PubMed] [Google Scholar]