Abstract
Background
Few studies have examined the relationship between cardiometabolic risk factors linked to metabolic syndrome and mortality among women with breast cancer.
Methods
We used the Women’s Health Initiative to evaluate the relationship between cardiometabolic risk factors including, waist circumference (WC), blood pressure, high-cholesterol, and type-2 diabetes, and their relation with death from breast cancer, cardiovascular disease (CVD), and other causes among 8,641 women with local or regional stage invasive breast cancer. Cox proportional hazards models were used to estimate hazard ratios and 95% confidence intervals adjusted for important predictors of survival.
Results
After a median 11.3 years, there were 2,181 total deaths, 619 (28.4%) of which were due to breast cancer. Most participants (55.7%) had at least 2 cardiometabolic risk factors and 4.9% had 3 or 4. Having a larger number of risk factors was associated with higher risk of CVD and other-cause mortality (p trend <0.001 for both), but not with breast cancer mortality (p trend = 0.86). Elevated WC was associated with a higher risk of CVD (HR=1.28, 95% CI 1.05–1.57) and other cause mortality (HR=1.32, 95% CI 1.16–1.49) and only with a small and non-significant higher risk of breast cancer mortality (HR=1.19, 95% CI 0.93–1.52). Results did not differ in analyses stratified by race, hormone receptor status, or after an analysis of cases diagnosed within five years after baseline.
Conclusions
Among women with early stage breast cancer, cardiometabolic risk factors are significantly associated with cardiovascular and other-cause, but not breast cancer mortality.
Keywords: Cardiometabolic, Risk Factors, Survival, Breast, Cancer Women’s Health Initiative
INTRODUCTION
Metabolic syndrome (MetS) is a grouping of cardiometabolic risk factors linked to atherosclerotic heart disease (1;2), diabetes mellitus (3) and cancer (4;5). Different definitions of MetS have been proposed, and all include some measure of insulin resistance, dyslipidemia and increased abdominal obesity (6–10). The defining criteria for MetS have been harmonized by consensus from three international organizations and include three of the five following components: increased waist circumference (WC), elevated triglycerides, hypertension (HTN), elevated fasting glucose and low high density lipoprotein cholesterol (HDL-C) (11).
Several studies have demonstrated a relationship between cardiometabolic risk factors and post-menopausal breast cancer including two meta-analyses of BMI (12;13), a meta-analysis of other risk factors (5) and studies evaluating MetS as a whole (14–17). Others have described the relationship between cardiometabolic risk factors and breast cancer mortality (18–25) including studies evaluating the effects of insulin resistance (18;19), body weight (20;21;26), measures of central obesity (27) or the joint impact of one or more risk factors (22–25).
The increasing prevalence of MetS and evidence suggesting a relationship between cardiometabolic risk factors and breast cancer risk, potentially through a common shared pathway of altered insulin sensitivity and sex hormonal changes, highlights the importance of understanding the impact of these risk factors on outcomes after breast cancer (28). The Women’s Health Initiative (WHI) cohort provides an opportunity to explore the relationship between exposure to cardiometabolic risk factors and mortality following breast cancer.
METHODS
Study Population
The WHI consists of an observational cohort (OS) (N=93,676) and three clinical trials (CT) (N=68,132) including trials of hormone therapy (HT), dietary modification (DM), and Calcium + vitamin D (29;30). Eligible women were between the ages of 50 and 79 years, postmenopausal, and had a predicted survival of at least 3 years. Participants were recruited from 40 clinical centers in the US between October 1, 1993 and December 31, 1998 and initially followed through March, 2005. The participants subsequently provided consent for re-contact for follow-up and release of medical records in two 5-year extension studies lasting through September 30, 2015 https://www.whi.org/SitePages/WHI%20Home.aspx.
We included WHI participants who through 9/30/15 developed pathologically-confirmed invasive breast cancer after enrollment in either the OS or CT (N=9,651) and excluded women with a history of cancer (except non-melanomatous skin cancer) prior to enrollment (N=782) and distant stage disease at diagnosis (N=228) resulting in an analytic cohort of 8,641 (Supplementary figure 1).Women with distant stage disease were excluded in order to include a more homogenous study population with early stage breast cancer.
Cardiometabolic Risk Factors
We utilized information gathered on cardiometabolic risk factors collected prior to breast cancer diagnosis including WC and blood pressure (BP) measured during the WHI baseline clinic visit and history of diabetes and high cholesterol which were self-reported. There was a median of 7.1 years (range 0.003–20.6 years) between study entry and breast cancer diagnosis. High WC was defined as ≥ 88 cm which is considered to be clinically significant (31) and the suggested threshold for the definition of MetS for women in the US (11). High BP was defined as systolic BP > 130 mg Hg and/or diastolic BP > 85 mm Hg, as defined by others (11) or normal BP reading, and reported use of anti-hypertensive medications. Women were considered diabetic if they responded affirmatively to the question “Did a doctor ever say that you had sugar diabetes or high blood sugar when you were not pregnant?” or reported at baseline use of any anti-diabetic medication. Women were considered to have high cholesterol if they responded affirmatively to the question “Has a doctor ever told you that you had high cholesterol requiring medication?” or reported at baseline use of cholesterol-lowering medication. Height and weight were measured at baseline using a standardized protocol as was information on self-reported medication use. Body mass index (BMI) was computed as weight (in kg) / height (in m2).
Outcomes
Outcomes included mortality from breast cancer, CVD, and from other causes. Participants were followed for outcomes from breast cancer diagnosis until date of death or last contact prior to September 30, 2015. For those cases alive at last contact, 97% had at least one or more years of follow-up. CVD death included death due to definite coronary heart disease (CHD), cerebrovascular accident, pulmonary embolism, possible CHD, other cardiovascular and unknown CVD. Cause of death was determined by medical record review with central adjudication and linkage to the National Death Index. Agreement between local vs. central adjudication was 94% for death due to any cancer and 73% for death due to CVD or other causes (32).
Covariates
Standardized questionnaires were used at baseline to collect information on age, race/ethnicity, education, current health care provider, screening mammogram within the past 2 years, smoking, alcohol, recreational physical activity, postmenopausal hormone therapy (HT), and history of another cancer (except non-melanomatous skin cancer) diagnosed after study enrollment and before breast cancer diagnosis (29).
Information on breast cancer prognostic and clinical factors included the Surveillance, Epidemiology and End Results (SEER) summary stage, size, lymph node involvement, grade, hormone receptor status [estrogen receptor (ER), progesterone receptor (PR)] and HER2/neu overexpression, based on review of the pathology report using the SEER coding system (32;33). HER2/neu status was missing for 25% of participants and since this test was not commonly performed at baseline in the WHI, this variable was excluded from all of the analyses.
Data were collected on initial course of breast cancer therapy (surgery, radiation and chemotherapy) on a sub-set of WHI participants (N=4,094) who had Medicare A+B fee-for-service coverage at diagnosis and these data were utilized in a subset analysis.
Statistical Analysis
The frequency distribution of demographics, cardiometabolic abnormalities, and tumor characteristics at diagnosis were summarized overall and by number of cardiometabolic abnormalities. The association between demographics, tumor characteristics and presence of cardiometabolic abnormalities was assessed using either the Cochran-Armitage test for dichotomous variables, or the Mantel-Haenszel test for ordinal variables. Unknown categories and “other” race were excluded in any analysis of trend test.
The competing risks of death due to breast cancer, CVD, and other causes was first evaluated by plotting the cumulative incidence of death by the number of cardiometabolic abnormalities and compared using Gray’s test (34). Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CI) for the association between each of the four cardiometabolic abnormalities and death due to breast cancer, CVD, and other causes. The final models assessing individual cardiometabolic abnormalities were mutually adjusted for other cardiometabolic abnormalities, as well as age group, WHI trial, and other confounders that changed the HR estimate associated with cardiometabolic abnormalities (3+) by at least 5%. For breast cancer mortality, the model was also adjusted for race/ethnicity, alcohol, episodes of moderate to strenuous recreational physical activity, and BMI as a categorical variable. For CVD mortality the model was adjusted for alcohol, episodes of moderate to strenuous recreational physical activity, and HT use. For other cause mortality, the model was adjusted for WHI trial and age-group. Separate sensitivity analyses were conducted to determine whether the relationship between cardiometabolic abnormalities and breast cancer-specific, CVD, and other cause mortality varied by race (white vs. African American), obesity (BMI ≥ 30kg/m2 vs. < 30kg/m2), ER status (+ vs. −), and after exclusion of participants in the intervention arms of the WHI hormone therapy and dietary modification trials. To account for the length of time from exposure at baseline to breast cancer diagnosis, a sensitivity analysis was conducted excluding women diagnosed 5 or more years from baseline. A separate sub-set analysis adjusting for first course of cancer treatment was performed for 4,094 participants for whom cancer treatment information was available.
Descriptive analyses and proportional hazards models were completed using SAS version 9.4 (Carey, NC) and cumulative incidence plots were created using the R package “cmprsk” (35). To test the proportionality assumption for the Cox proportional hazards regression analysis, an interaction term between the log of time and the ordinal cardiometabolic abnormalities was added to each model, and the interaction terms were not statistically significant.
RESULTS
Table 1 describes demographic and clinical characteristics of breast cancer cases in the WHI stratified by number of cardiometabolic risk factors (none, 1–2, 3–4). Overall, mean age at WHI study enrollment was 62.9 (SD 6.9) years; the majority of participants were non-Hispanic white (88%), educated beyond high school (82%) and had a health care provider (95%). The most common cardiometabolic risk factors were high WC (41%) and elevated blood pressure (40%). History of high cholesterol and diabetes were observed in only 13% and 5% respectively. About 64% of the women had at least one cardiometabolic risk factor, 28% had 2 or more and 4.9% had three or more (data not shown). The majority of participants presented with local stage (77%), tumors < 2 cm in size (73%), lymph node negative (78%), grade 1 or 2 (68%) and ER+ and/or PR+ (80%). Variables associated with a greater number of cardiometabolic risk factors included: older age, less education, African-American race, participation in a WHI clinical trial, having a current health care provider, having no mammogram in the past 2 years, being a non-drinker, no physical activity, no use of HT, higher BMI and higher tumor grade. The mean body mass index (BMI) at enrollment was 28.2 kg/m2 (SD 5.9).
Table 1.
Demographic and clinical characteristics of incident breast cancer cases in the WHI by the number of cardiometabolic abnormalities present at baseline
| All Women | Number of Cardiometabolic Abnormalities at Baseline(a) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| None | 1–2 | 3–4 | |||||||
| Characteristics(b) | N | % | N | % | N | % | N | % | p trend(c) |
| Total | 8,641 | 2,821 | 4,812 | 427 | |||||
|
| |||||||||
| Age group at baseline | <.001 | ||||||||
|
| |||||||||
| 50–54 | 1,077 | 12% | 497 | 18% | 446 | 9% | 22 | 5% | |
|
| |||||||||
| 55–59 | 1,824 | 21% | 719 | 25% | 890 | 18% | 76 | 18% | |
|
| |||||||||
| 60–64 | 2,152 | 25% | 670 | 24% | 1236 | 26% | 106 | 25% | |
|
| |||||||||
| 65–69 | 1,910 | 22% | 518 | 18% | 1177 | 24% | 116 | 27% | |
|
| |||||||||
| 70+ | 1,678 | 19% | 417 | 15% | 1063 | 22% | 107 | 25% | |
|
| |||||||||
| Education | <.001 | ||||||||
|
| |||||||||
| High school or less | 1,569 | 18% | 374 | 13% | 943 | 20% | 133 | 31% | |
|
| |||||||||
| > High school | 7,016 | 82% | 2424 | 87% | 3840 | 80% | 291 | 69% | |
|
| |||||||||
| Race | <.001 | ||||||||
|
| |||||||||
| White | 7,609 | 88% | 2582 | 92% | 4183 | 87% | 325 | 76% | |
|
| |||||||||
| African American | 537 | 6% | 85 | 3% | 350 | 7% | 68 | 16% | |
|
| |||||||||
| Other | 495 | 6% | 154 | 5% | 279 | 6% | 34 | 8% | |
|
| |||||||||
| WHI Study | <.001 | ||||||||
|
| |||||||||
| Clinical Trial | 3,886 | 45% | 1000 | 35% | 2238 | 47% | 199 | 47% | |
|
| |||||||||
| Observational | 4,755 | 55% | 1821 | 65% | 2574 | 53% | 228 | 53% | |
|
| |||||||||
| Current Health Care Provider | 0.010 | ||||||||
|
| |||||||||
| No | 400 | 5% | 145 | 5% | 225 | 5% | 6 | 1% | |
|
| |||||||||
| Yes | 8,180 | 95% | 2657 | 95% | 4549 | 95% | 420 | 99% | |
|
| |||||||||
| Mammography | <.001 | ||||||||
|
| |||||||||
| Mammogram within 2 years | 7,355 | 87% | 2470 | 90% | 4045 | 86% | 350 | 85% | |
|
| |||||||||
| No mammogram within 2 years | 1,062 | 13% | 275 | 10% | 642 | 14% | 64 | 15% | |
|
| |||||||||
| Body Mass Index (BMI) kg/m2 | <.001 | ||||||||
|
| |||||||||
| < 25.0 kg/m2 | 2,882 | 34% | 1648 | 59% | 1044 | 22% | 14 | 3% | |
|
| |||||||||
| 25.0 – 29.9 kg/m2 | 2,968 | 35% | 1023 | 37% | 1624 | 34% | 112 | 26% | |
|
| |||||||||
| 30.0 – 34.9 kg/m2 | 1,653 | 19% | 107 | 4% | 1270 | 27% | 154 | 36% | |
|
| |||||||||
| ≥35.0 kg/m2 | 1,069 | 12% | 16 | 1% | 843 | 18% | 146 | 34% | |
|
| |||||||||
| Developed another cancer between WHI enrollment and breast cancer | 0.736 | ||||||||
|
| |||||||||
| No | 8,154 | 94% | 2,653 | 94% | 4,541 | 94% | 405 | 95% | |
|
| |||||||||
| Yes | 487 | 6% | 168 | 6% | 271 | 6% | 22 | 5% | |
|
| |||||||||
| Cardio-Metabolic Abnormalities | |||||||||
|
| |||||||||
| Waist Circumference | |||||||||
|
| |||||||||
| < 88 cm | 5,083 | 59% | 2821 | 100% | 1929 | 40% | 15 | 4% | |
|
| |||||||||
| > 88 cm | 3,543 | 41% | 0 | - | 2883 | 60% | 412 | 96% | |
|
| |||||||||
| History of high cholesterol | |||||||||
|
| |||||||||
| No | 7,021 | 87% | 2821 | 100% | 4067 | 85% | 115 | 27% | |
|
| |||||||||
| Yes | 1,059 | 13% | 0 | - | 745 | 15% | 312 | 73% | |
|
| |||||||||
| History of Diabetes | |||||||||
|
| |||||||||
| No | 8,224 | 95% | 2821 | 100% | 4637 | 96% | 215 | 50% | |
|
| |||||||||
| Yes | 414 | 5% | 0 | - | 175 | 4% | 212 | 50% | |
|
| |||||||||
| Blood Pressure(d) | |||||||||
|
| |||||||||
| Normal | 5,176 | 60% | 2821 | 100% | 1989 | 41% | 46 | 11% | |
|
| |||||||||
| Elevated | 3,459 | 40% | 0 | - | 2823 | 59% | 381 | 89% | |
|
| |||||||||
|
| |||||||||
| Tumor Characteristics | |||||||||
|
| |||||||||
| Tumor size | 0.739 | ||||||||
|
| |||||||||
| < 2cm | 6,136 | 73% | 2,017 | 73% | 3,365 | 72% | 312 | 75% | |
|
| |||||||||
| ≥ 2 cm | 2,246 | 27% | 728 | 27% | 1,290 | 28% | 103 | 25% | |
|
| |||||||||
| Lymph node involvement | 0.270 | ||||||||
|
| |||||||||
| No | 6,624 | 78% | 2,193 | 79% | 3,659 | 77% | 329 | 79% | |
|
| |||||||||
| Yes | 1,895 | 22% | 594 | 21% | 1,085 | 23% | 90 | 21% | |
|
| |||||||||
| SEER Summary Stage | 0.131 | ||||||||
|
| |||||||||
| Local | 6,622 | 77% | 2,189 | 78% | 3,662 | 76% | 323 | 76% | |
|
| |||||||||
| Regional | 2,019 | 23% | 632 | 22% | 1,150 | 24% | 104 | 24% | |
|
| |||||||||
| Grade | 0.002 | ||||||||
|
| |||||||||
| 1 | 2,272 | 26% | 786 | 30% | 1,245 | 28% | 88 | 22% | |
|
| |||||||||
| 2 | 3,605 | 42% | 1,182 | 45% | 1,978 | 45% | 194 | 49% | |
|
| |||||||||
| 3–4 | 2,096 | 24% | 658 | 25% | 1,201 | 27% | 113 | 29% | |
|
| |||||||||
| Unknown | 668 | 8% | - | - | - | ||||
|
| |||||||||
| Hormone Receptor Status | 0.524 | ||||||||
|
| |||||||||
| ER or PR + | 6,952 | 80% | 2,290 | 87% | 3,852 | 87% | 335 | 86% | |
|
| |||||||||
| ER and PR − | 1,068 | 12% | 345 | 13% | 601 | 13% | 55 | 14% | |
|
| |||||||||
| ER or PR unknown | 621 | 7% | - | - | - | ||||
|
| |||||||||
| HER2neu status | 0.598 | ||||||||
|
| |||||||||
| Positive | 910 | 11% | 286 | 13% | 525 | 14% | 38 | 12% | |
|
| |||||||||
| Negative | 5,599 | 65% | 1870 | 87% | 3123 | 86% | 274 | 88% | |
|
| |||||||||
| Unknown | 2,132 | 25% | - | - | - | ||||
|
| |||||||||
| Lifestyle Factors | |||||||||
|
| |||||||||
| Smoking Status | 0.092 | ||||||||
|
| |||||||||
| Never Smoked | 4,171 | 49% | 1,347 | 48% | 2,347 | 49% | 209 | 50% | |
|
| |||||||||
| Past Smoker | 3,840 | 45% | 1249 | 45% | 2149 | 45% | 189 | 45% | |
|
| |||||||||
| Current Smoker | 522 | 6% | 191 | 7% | 268 | 6% | 20 | 5% | |
|
| |||||||||
| Alcohol intake | <.001 | ||||||||
|
| |||||||||
| Non drinker | 799 | 9% | 193 | 7% | 488 | 10% | 68 | 16% | |
|
| |||||||||
| Past drinker | 1,386 | 16% | 335 | 12% | 831 | 17% | 134 | 32% | |
|
| |||||||||
| <1 drink per month | 1,040 | 12% | 298 | 11% | 621 | 13% | 48 | 11% | |
|
| |||||||||
| <1 drink per week | 1,790 | 21% | 574 | 20% | 1029 | 22% | 70 | 17% | |
|
| |||||||||
| 1 to <7 drinks per week | 2,337 | 27% | 940 | 33% | 1173 | 25% | 74 | 17% | |
|
| |||||||||
| 7+ drinks per week | 1,224 | 14% | 469 | 17% | 638 | 13% | 29 | 7% | |
|
| |||||||||
| Episodes moderate to strenuous activity | <.001 | ||||||||
|
| |||||||||
| No activity | 1,213 | 15% | 305 | 11% | 805 | 17% | 86 | 20% | |
|
| |||||||||
| Some activity of limited duration | 3,290 | 40% | 1000 | 35% | 2025 | 42% | 209 | 50% | |
|
| |||||||||
| 2 – <4 episodes per week | 1,550 | 19% | 570 | 20% | 893 | 19% | 63 | 15% | |
|
| |||||||||
| 4 episodes per week | 2,126 | 26% | 944 | 33% | 1,076 | 22% | 64 | 15% | |
|
| |||||||||
| HT usage status | <.001 | ||||||||
|
| |||||||||
| Never Used | 3,371 | 39% | 864 | 31% | 2057 | 43% | 220 | 52% | |
|
| |||||||||
| Past or current user | 5,263 | 61% | 1954 | 69% | 2751 | 57% | 207 | 48% | |
581 women had an unknown number of cardio-metabolic abnormalities
Unknown values for baseline and tumor characteristics; Education - 56, Current Health Care Provider - 61, Mammography - 224, BMI - 69, Waist Circumference - 15, High Cholesterol - 561, Diabetes - 3, Blood pressure - 6, Tumor Size - 259, Lymph Node Involvement - 122, Smoking status - 108, Alcohol intake - 65, Exercise - 462, HRT use - 7
p value calculated from Mantel-Haenszel chi-square test for ordinal characteristics and Cochran-Armitage trend test for dichotomous characteristics; and unknown/other categories were not included in p value calculations
Elevated blood pressure defined as systolic > 130 and/or Diastolic Blood Pressure > 85
After a median follow-up of 11.3 years post diagnosis there were a total of 2,181 deaths. Causes of death included 619 deaths due to breast cancer (28%), 459 due to CVD (21%), 401 due to other cancer deaths (18.4%), 196 due to unknown causes (9.0%), and 506 due to other causes (23.2%).. The most prevalent other causes of death were dementia or Alzheimer’s (n=114), chronic obstructive pulmonary disease (n=52), and pneumonia (n=46) (Table 2).
Table 2.
All Causes of Death
| Cause of Death | N | % |
|---|---|---|
| Breast Cancer | 619 | 28.4 |
| Cardiovascular Disease | 459 | 21.0 |
| Other Cancer Death | 401 | 18.4 |
| Unknown Cause | 196 | 9.0 |
| Known Other Cause | 153 | 7.0 |
| Dementia, NOS | 67 | 3.1 |
| Chronic Obstructive Pulmonary Disease | 52 | 2.4 |
| Alzheimer’s Disease | 47 | 2.2 |
| Pneumonia | 46 | 2.1 |
| Sepsis | 41 | 1.9 |
| Accident | 25 | 1.1 |
| Renal Failure | 17 | 0.8 |
| Parkinson’s Disease | 15 | 0.7 |
| Pulmonary Fibrosis | 13 | 0.6 |
| Hepatic Cirrhosis | 11 | 0.5 |
| Connective Tissue Cancer | <11 | <0.5 |
| ALS | <11 | <0.5 |
| Suicide | <11 | <0.5 |
| Other Injury | <11 | <0.5 |
| Homicide | <11 | <0.5 |
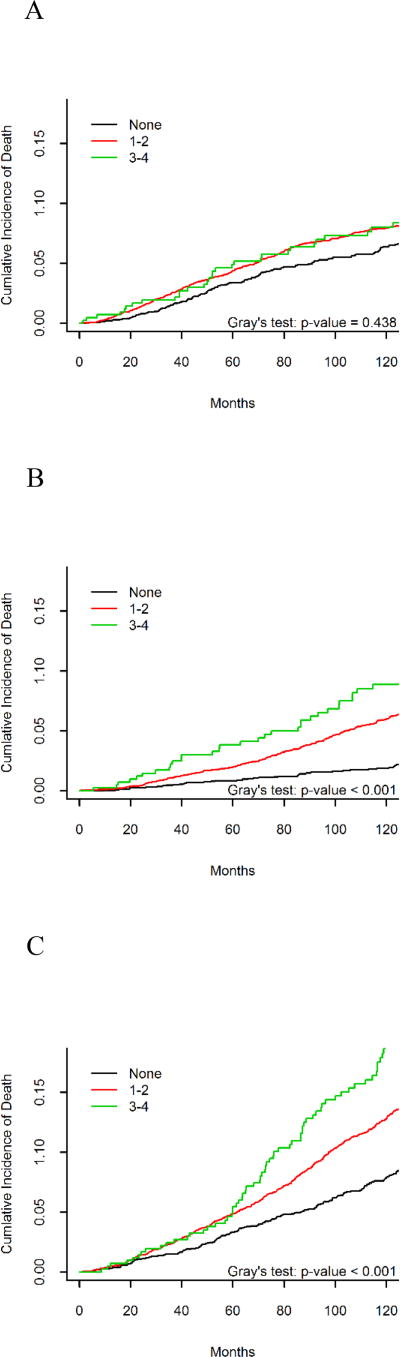
Figures 1A–1C illustrate the unadjusted relationship between number of cardiometabolic risk factors and death due to breast cancer, CVD or other causes. There was a higher death risk due to breast cancer associated with 1–2 or 3–4 risk factors compared to none (Figure 1A), although this association was not statistically significant (p=0.44). A greater number of cardiometabolic risk factors was associated with a higher risk of death due to CVD (Figure 1B) and death due to other causes (Figure 1C) (p<0.001 for both).
Figure 1.
Unadjusted relationship between number of cardiometabolic features and death due to (A) breast cancer, (B) cardiovascular disease, and (C) other causes.
Table 3 shows the unadjusted and adjusted HRs and 95% CIs for the association of cardiometabolic risk factors and mortality due to breast cancer, CVD and other causes. In our unadjusted models, high WC was associated with higher risk of death from breast cancer (HR 1.23, 95% CI, 1.05–1.44); however, after multivariable adjustment the confidence interval included the null (HR 1.19, 95% CI, 0.93–1.52). After multivariable adjustment all cardiometabolic risk factors were individually associated with death from CVD: high WC - HR 1.28, 95% CI, 1.05–1.57, diabetes - HR 1.52, 95% CI, 1.07–2.16, high cholesterol - HR 1.30, 95% CI 1.02–1.65 and high BP - HR 1.67, 95% CI, 1.37–2.04. Similarly, high WC – HR 1.32, 95% CI 1.16–1.49, diabetes – HR 1.58, 95% CI 1.25–1.98, and high BP – HR 1.15, 95% CI 1.02–1.31 were associated with mortality from other causes (Table 3). Lastly, having 1–2 or 3–4 cardiometabolic risk factors vs. none was significantly associated with an increased risk of CVD mortality (p-trend < 0.001) and death due to other causes (p < 0.001).
Table 3.
Adjusted hazard ratios and 95% confidence intervals for breast cancer, cardiovascular disease, and other cause mortality by the presence of cardiometabolicrisk factors at WHI baseline enrollment
| Breast Cancer Mortality | CVD Mortality | Other Cause Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths | HR | 95 % CI | Deaths | HR | 95 % CI | Deaths | HR | 95% CI | |
| Unadjusted | |||||||||
|
| |||||||||
| High Waist Circumference | |||||||||
|
| |||||||||
| No | 339 | Ref | 234 | Ref | 575 | Ref | |||
|
| |||||||||
| Yes | 279 | 1.23 | 1.05–1.44 | 224 | 1.46 | 1.22–1.76 | 528 | 1.41 | 1.25–1.59 |
|
| |||||||||
| History of High Cholesterol | |||||||||
|
| |||||||||
| No | 496 | Ref | 342 | Ref | 883 | Ref | |||
|
| |||||||||
| Yes | 75 | 1.01 | 0.79–1.28 | 90 | 1.77 | 1.40–2.23 | 149 | 1.14 | 0.96–1.36 |
|
| |||||||||
| History of Diabetes | |||||||||
|
| |||||||||
| No | 589 | Ref | 419 | Ref | 1,015 | Ref | |||
|
| |||||||||
| Yes | 30 | 1.04 | 0.72–1.50 | 39 | 1.92 | 1.38–2.66 | 88 | 1.80 | 1.44–2.23 |
|
| |||||||||
| High Blood Pressure | |||||||||
|
| |||||||||
| No | 360 | Ref | 178 | Ref | 559 | Ref | |||
|
| |||||||||
| Yes | 259 | 1.09 | 0.93–1.27 | 279 | 2.40 | 1.99–2.90 | 543 | 1.49 | 1.33–1.68 |
|
| |||||||||
| Cardiometabolic Abnormalities | |||||||||
|
| |||||||||
| None | 181 | Ref | 68 | Ref | 254 | Ref | |||
|
| |||||||||
| 1 – 2 Abnormalities | 357 | 1.18 | 0.99–1.41 | 313 | 2.83 | 2.17–3.67 | 693 | 1.68 | 1.45–1.94 |
|
| |||||||||
| 3 – 4 Abnormalities | 32 | 1.23 | 0.84–1.79 | 48 | 5.13 | 3.54–7.42 | 84 | 2.42 | 1.89–3.09 |
|
| |||||||||
| p-trend | 0.068 | <.001 | <.001 | ||||||
|
| |||||||||
| Adjusted* | |||||||||
|
| |||||||||
| High Waist Circumference | |||||||||
|
| |||||||||
| No | 339 | Ref | 234 | Ref | 575 | Ref | |||
|
| |||||||||
| Yes | 279 | 1.19 | 0.93–1.52 | 224 | 1.28 | 1.05–1.57 | 528 | 1.32 | 1.16–1.49 |
|
| |||||||||
| History of High Cholesterol | |||||||||
|
| |||||||||
| No | 496 | Ref | 342 | Ref | 883 | Ref | |||
|
| |||||||||
| Yes | 75 | 0.96 | 0.75–1.22 | 90 | 1.30 | 1.02–1.65 | 149 | 0.94 | 0.79–1.12 |
|
| |||||||||
| History of Diabetes | |||||||||
|
| |||||||||
| No | 589 | Ref | 419 | Ref | 1,015 | Ref | |||
|
| |||||||||
| Yes | 30 | 0.81 | 0.54–1.21 | 39 | 1.52 | 1.07–2.16 | 88 | 1.58 | 1.25–1.98 |
|
| |||||||||
| High Blood Pressure | |||||||||
|
| |||||||||
| No | 360 | Ref | 178 | Ref | 559 | Ref | |||
|
| |||||||||
| Yes | 259 | 0.95 | 0.80–1.13 | 279 | 1.67 | 1.37–2.04 | 543 | 1.15 | 1.02–1.31 |
|
| |||||||||
| Cardiometabolic Abnormalities | |||||||||
|
| |||||||||
| None | 181 | Ref | 68 | Ref | 254 | Ref | |||
|
| |||||||||
| 1 – 2 Abnormalities | 357 | 1.05 | 0.86–1.29 | 313 | 2.06 | 1.58–2.69 | 693 | 1.39 | 1.20–1.61 |
|
| |||||||||
| 3 – 4 Abnormalities | 32 | 0.97 | 0.65–1.46 | 48 | 3.29 | 2.25–4.82 | 84 | 1.90 | 1.49–2.44 |
|
| |||||||||
| p-trend | 0.857 | <.001 | <.001 | ||||||
Models adjusted for age group, WHI trial (OS vs CT), and covariates that modified the HR for 3–4 cardiometabolic abnormalities by 5% or more. In addition, individual cardiometabolic abnormalities were adjusted for one another. For breast cancer mortality, models adjusted for age group, WHI trial, race, alcohol intake, episodes of moderate to strenuous physical activity, and BMI. For CVD mortality, models adjusted for age group, WHI trial, alcohol intake, episodes of moderate to strenuous physical activity, and hormone therapy use. For other cause mortality, models adjusted for age group and WHI trial.
Additional analyses tested whether the association between cardiometabolic risk factors and death due to breast cancer, CVD, and other causes differed by race, obesity, and ER status and no significant effect modification was observed. Separate sensitivity analyses excluding women diagnosed with breast cancer five or more years after baseline and controlling for first course breast cancer treatment also did not change our results.
Discussion
Among postmenopausal women with early stage breast cancer in the WHI, a higher number of cardiometabolic risk factors was associated with death due to CVD and other causes, but was not significantly associated with death due to breast cancer. Only elevated WC was significantly associated with higher breast cancer mortality in unadjusted analysis; after adjustments for BMI and physical activity this relationship was no longer significant. Additional sensitivity analyses evaluating time since diagnosis, receipt of treatment and exclusion of participants in the treatment arms of the DM and HT had no significant impact on the reported outcomes. African-American participants and those with higher grade tumors had a greater number of cardiometabolic abnormalities.
Clustering of cardiovascular risk factors recognized as MetS centers on the premise of insulin resistance that could influence breast cancer pathogenesis and progession through several proposed mechanisms (28). Adipose tissue has an active role in endocrine signaling through leptin and other adipokines, which may initiate carcinogenesis and progression through their function as growth factors (36). This process may be in part through activation of downstream signaling of the extracellular signal regulated kinase (ERK) and signal reducer and activator of transcription 3 (STAT3) pathways by the long form of the leptin receptor (37). Another mechanism could include the stimulation of aromatase activity and consequent increased synthesis of estrogen and transactivation of ER alpha in breast cancer cells which could also influence progression of disease (38). Risk associated with insulin resistance and chronic inflammation is increased by elevated levels of tumor necrosis factor alpha, interleukin-6 (IL-6), and plasminogen activator inhibitor 1 (PAI1), all of which are thought to promote angiogenesis and metastases (39–41). Obesity and hyperglycemia are associated with hyperinsulinemia, resulting in a reduction in insulin-like growth factor (IGF) binding proteins and an increase in free IGF-1 (42), which in turn creates changes in the cellular environment that promotes tumor development and progression via mitogenic, anti-apoptotic, pro-angiogenic and induction of tumor-related lymphangiogenesis (43).
While the relationship between obesity, MetS and breast cancer incidence has been largely established (5;12;13;17;44) the relationship between cardiometabolic risk factors and breast cancer specific-mortality is less clear (18–22;45). The majority of previous studies assessed individual risk factors including diabetes (18;19;45), and obesity (20;21;27) with only a few analyzing the relationship between groups of risk factors and breast cancer outcomes (23–25). To our knowledge, the current analysis is the only study of breast cancer survivors that, in addition to assessment of breast cancer mortality, evaluated the relationship between cardiometabolic risk factors and death due to CVD. The unique features of the WHI include validated measurements of WC and BP, long follow-up and medical record review with adjudication of causes of death with linkage to the National Death Index (32). As shown by others (46–48), our results suggest that following breast cancer death, CVD represents the leading competing cause of death in survivors of early breast cancer, and that the presence of multiple cardiometabolic risk factors markedly increases CVD specific mortality.
In a previous cohort including participants sampled from a population based registry and including a median of 6.3 years of follow-up, history of 3 or more MetS components was associated with worse breast cancer specific (HR 1.65, 95% CI 1.02–2.69) and overall mortality (HR 1.81 95% CI 1.44, 2.29) among women with stage I and II disease (24). Only weight was associated with higher breast cancer specific mortality (HR 1.36, 95% CI 1.04–1.78) while history of hypertension and diabetes were significantly associated with overall mortality (24). In the Berrino study of participants from 11 institutions, after an average of 2.8 years follow-up, MetS was associated with an increased risk of breast cancer recurrences and distant metastases. However as individual components only low HDL (HR 1.83, 95% CI 1.24–2.70) and high triglycerides (HR 1.58, 95% CI 1.01–2.46) were significant (23). Lastly, in the Fan study, data from a single institution were stratified by triple negative vs. non-triple negative status (25) and after a median of 6.6 years, MetS was not associated with either relapse free or overall survival. However among women with triple negative breast cancer, low HDL cholesterol was associated with both worse relapse-free (HR 3.27, 95% CI 2.08–5.11) and overall-survival (HR 3.07, 95% CI 1.73–5.44).
Our results showing a relationship between WC and breast cancer mortality are similar to the Calip study and consistent with other reports (20;21;24;26). Differences in strength of the associations may be in part due to different definitions and populations, for example, we used ≥88 cm as high waist circumference (11)) while the Calip study used BMI ≥27.7kg/m2 as a proxy for central obesity. We did not observe a significant relationship between history of hypercholesterolemia and breast cancer specific mortality as reported by others (23;25) however this could be due to our use of self-report for history of high cholesterol, or use of cholesterol-lowering medications as a proxy for hypercholesterolemia.. In addition, self-report of high cholesterol is more likely representative of high LDL-C as opposed to low HDL and elevated triglycerides that are the actual components of the MetS definition, and which were cited to have a significant influence on breast cancer outcomes in other studies (23;25)..
Our results demonstrating an association between cardiometabolic risk factors and death due to CVD are consistent with other studies in the general population (49–52). Our findings highlight the high prevalence of CVD risk factors in postmenopausal breast cancer survivors, with particularly high prevalence of overweight/obesity and hypertension in the WHI. Since straightforward testing of WC and blood pressure can identify a subpopulation of breast cancer survivors at high risk of death due to CVD, these results focus attention on the need to address CVD risk factors in long term survivors of breast cancer. Multimodality breast cancer treatment, including radiation and chemotherapy, may also act in concert with cardiometabolic risk factors and contribute to mortality. This has been recently highlighted in the American Society of Clinical Oncology (ASCO) guidelines for cancer survivors (53), with the addition of guidelines that specifically address the breast cancer population (54).
Study strengths include the large sample size and long follow-up, resulting in a robust number of outcomes, comprehensive data collection and physician-adjudicated cases and causes of death. Moreover, we examined the impact of each individual feature on mortality in addition to an ordinal analysis focused on number of features. Generally, mortality risk has been shown to increase with an increasing number of features; however analysis of each feature individually has the potential to provide more information about the underlying biology of drivers of progression. Limitations include a reliance on indirect measures of laboratory variables and diabetes and high cholesterol defined by medical history and medication use. In a prior WHI analysis, Luo and colleagues demonstrated that medical record data on diabetes matched the reliability of Medicare data (55), however the possibility for under-detection of diabetes and high cholesterol is an important limitation. In a comparison to other similar analyses (23–25) the WHI cohort had comparable levels of obesity and high BP, but lower prevalence of diabetes and high cholesterol, suggesting that our results may be less generalizable and more relevant for populations similar to the WHI. Also for 16% of our included deaths the cause was classified as “unknown or other cause”, which could have resulted in misclassification especially if some of the unclassified deaths were actually related to breast cancer or CVD. It should be noted that in the WHI, agreement between local and central adjudication was 94.4% for death due to cancer and 73% for death due to CVD or other causes (32). Lastly, we evaluated the presence of cardiometabolic risk factors at baseline and information on these factors at time of diagnosis was not available. To address this concern, we conducted an analysis excluding women diagnosed 5 or more years after baseline and our results were unchanged. The variability in the time between assessment of cardiovascular risk factors and breast cancer diagnosis (median 7.1 years, range 0.003–20.6 years) could have potentially weakened the associations between cardiovascular risk factors and breast cancer specific mortality in particular. It is also possible that the prevalence of the measured cardiovascular risk factors could have changed over time, which could have resulted in a stronger relationship with breast cancer mortality. however we were not able to assess this with our data set..
Conclusions
Cardiometabolic risk factors are positively associated with higher mortality from CVD and other causes, but not with higher risk of death from breast cancer in postmenopausal women with breast cancer. Identifying and managing cardiometabolic risk factors should be standard of care for all postmenopausal women diagnosed with breast cancer.
Supplementary Material
Acknowledgments
Funding: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221”, the Cancer Center Support Grant NIH:NCI P30CA022453 and the Blue Cross Blue Shield Foundation of Michigan.
We acknowledge the dedicated efforts of investigators and staff at the Women’s Health Initiative (WHI) clinical centers, the WHI Clinical Coordinating Center, the WHI Life and Longevity After Cancer Study (LILAC) funded by NCI grant 1UM1CA173642, and the National Heart, Lung and Blood program office (listing available at http://www.whi.org). We also recognize the WHI participants for their extraordinary commitment to the WHI program.
Footnotes
Conflict of Interest: Conflicts of interest – Rowan Chlebowski is a consultant for Novartis, Pfizer, Genentech, and AstraZeneca.
For a list of all the investigators who have contributed to WHI science, please visit: http://www.whiscience.org/publications/WHI_investigators_longlist.pdf
This work was presented as a poster discussion at the American Society of Clinical Oncology Meeting in Chicago, June 2015
Author’s contributions
Michael S. Simon: Conceptualization, methodology, writing – original draft and writing- review and editing…
Jennifer L Beebe Dimmer: Conceptualization, methodology, writing – original draft and writing- review and editing.
Theresa A Hastert: Conceptualization, methodology and writing-review and editing.
JoAnn E Manson: Conceptualization, methodology and writing-review and editing.
Elizabeth M Cespedes Feliciano: Conceptualization, methodology and writing-review and editing..
Marian L Neuhouser: Conceptualization, methodology and writing-review and editing..
Gloria Ho: Conceptualization, methodology and writing-review and editing
Jo Freudenheim: Conceptualization, methodology and writing-review and editing
Howard Strickler: Conceptualization, methodology and writing-review and editing
Julie Ruterbusch: Formal analysis, methodology and writing-review and editing.
Ana Barac: Conceptualization, methodology and writing-review and editing.
Rowan Chlebowski: Conceptualization, methodology and writing-review and editing.
Bette Caan: Conceptualization, methodology and writing-review and editing.
References
- 1.McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–90. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 2.Lin CC, Liu CS, Li CI, et al. The relation of metabolic syndrome according to five definitions to cardiovascular risk factors--a population-based study. BMC. Public Health. 2009;9:484. doi: 10.1186/1471-2458-9-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 4.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–11. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito K, Chiodini P, Capuano A, et al. Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause. 2013;20:1301–9. doi: 10.1097/GME.0b013e31828ce95d. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet. Med. 1999;16:442–3. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 8.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 9.Einhorn D, Reaven GM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr. Pract. 2003;9:237–52. [PubMed] [Google Scholar]
- 10.Alberti KG, Zimmet P, Shaw J. Metabolic Syndrome—a new worldwide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 11.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 12.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 13.Cheraghi Z, Poorolajal J, Hashem T, Esmailnasab N, Doosti IA. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PLoS. One. 2012;7:e51446. doi: 10.1371/journal.pone.0051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capasso I, Esposito E, de LM, et al. Metabolic syndrome-breast cancer link varies by intrinsic molecular subtype. Diabetol. Metab Syndr. 2014;6:105. doi: 10.1186/1758-5996-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Cheng N, Zheng S, et al. Metabolic syndrome and the risk of breast cancer among postmenopausal women in North-West China. Climacteric. 2015;18:852–8. doi: 10.3109/13697137.2015.1071346. [DOI] [PubMed] [Google Scholar]
- 16.Bitzur R, Brenner R, Maor E, et al. Metabolic syndrome, obesity, and the risk of cancer development. Eur. J. Intern. Med. 2016;34:89–93. doi: 10.1016/j.ejim.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Lee JA, Yoo JE, Park HS. Metabolic syndrome and incidence of breast cancer in middle-aged Korean women: a nationwide cohort study. Breast Cancer Res. Treat. 2017;162:389–93. doi: 10.1007/s10549-017-4131-x. [DOI] [PubMed] [Google Scholar]
- 18.Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J. Clin. Oncol. 2011;29:40–6. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjorge T, Lukanova A, Jonsson H, et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol. Biomarkers Prev. 2010;19:1737–45. doi: 10.1158/1055-9965.EPI-10-0230. [DOI] [PubMed] [Google Scholar]
- 20.Ryu SY, Kim CB, Nam CM, et al. Is body mass index the prognostic factor in breast cancer?: a meta-analysis. J. Korean Med. Sci. 2001;16:610–4. doi: 10.3346/jkms.2001.16.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res. Treat. 2010;123:627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 22.Muniz J, Kidwell KM, Henry NL. Associations between metabolic syndrome, breast cancer recurrence, and the 21-gene recurrence score assay. Breast Cancer Res. Treat. 2016;157:597–603. doi: 10.1007/s10549-016-3846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res. Treat. 2014;147:159–65. doi: 10.1007/s10549-014-3076-6. [DOI] [PubMed] [Google Scholar]
- 24.Calip GS, Malone KE, Gralow JR, Stergachis A, Hubbard RA, Boudreau DM. Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Res. Treat. 2014;148:363–77. doi: 10.1007/s10549-014-3157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan Y, Ding X, Wang J, et al. Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast cancer but not non-TNBCs. Int. J. Biol. Markers. 2015;30:e200–e207. doi: 10.5301/jbm.5000143. [DOI] [PubMed] [Google Scholar]
- 26.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014;25:1901–14. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George SM, Bernstein L, Smith AW, et al. Central adiposity after breast cancer diagnosis is related to mortality in the Health, Eating, Activity, and Lifestyle study. Breast Cancer Res. Treat. 2014;146:647–55. doi: 10.1007/s10549-014-3048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohmann AE, Goodwin PJ, Chlebowski RT, Pan K, Stambolic V, Dowling RJ. Association of Obesity-Related Metabolic Disruptions With Cancer Risk and Outcome. J. Clin. Oncol. 2016;34:4249–55. doi: 10.1200/JCO.2016.69.6187. [DOI] [PubMed] [Google Scholar]
- 29.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann. Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 30.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann. Epidemiol. 2003;13:S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 31.Waist circumference and waist-hip ratio. Report of a WHO expert consultation. Geneva: World Health Organization; 2008. Ref Type: Report. [Google Scholar]
- 32.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann. Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 33.Young JL, Roffers SD, Ries LA, Fritz AG, Hurlbut AA, editors. SEER Summary Staging Manual-2000: Codes and Coding instructions. Bethesda, MD: National Cancer Institute; 2001. IH Pub. No. 01-4969. Ref Type: Report. [Google Scholar]
- 34.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999:496–509. [Google Scholar]
- 35.Gray B. cmprsk: subdistribution analysis of competing risks. R package version 2.2-7. 2014 http://CRAN.R-project.org/package=cmprsk.
- 36.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer. 2011;11:886–95. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 37.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 2000;275:14563–72. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 38.Catalano S, Mauro L, Marsico S, et al. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J. Biol. Chem. 2004;279:19908–15. doi: 10.1074/jbc.M313191200. [DOI] [PubMed] [Google Scholar]
- 39.Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol. Biol. Cell. 2010;21:687–90. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foekens JA, Schmitt M, van Putten WL, et al. Plasminogen activator inhibitor-1 and prognosis in primary breast cancer. J. Clin. Oncol. 1994;12:1648–58. doi: 10.1200/JCO.1994.12.8.1648. [DOI] [PubMed] [Google Scholar]
- 41.Pelton K, Coticchia CM, Curatolo AS, et al. Hypercholesterolemia induces angiogenesis and accelerates growth of breast tumors in vivo. Am. J. Pathol. 2014;184:2099–110. doi: 10.1016/j.ajpath.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–6. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 43.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch. Physiol Biochem. 2008;114:71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 44.Goodwin PJ, Boyd NF, Hanna W, et al. Elevated levels of plasma triglycerides are associated with histologically defined premenopausal breast cancer risk. Nutr. Cancer. 1997;27:284–92. doi: 10.1080/01635589709514539. [DOI] [PubMed] [Google Scholar]
- 45.Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin-like growth factor binding proteins 1 and 3 and breast cancer outcomes. Breast Cancer Res. Treat. 2002;74:65–76. doi: 10.1023/a:1016075709022. [DOI] [PubMed] [Google Scholar]
- 46.Park NJ, Chang Y, Bender C, et al. Cardiovascular disease and mortality after breast cancer in postmenopausal women: Results from the Women's Health Initiative. PLoS. One. 2017;12:e0184174. doi: 10.1371/journal.pone.0184174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdel-Qadir H, Austin PC, Lee DS, et al. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol. 2017;2:88–93. doi: 10.1001/jamacardio.2016.3841. [DOI] [PubMed] [Google Scholar]
- 48.Gernaat SAM, Ho PJ, Rijnberg N, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res. Treat. 2017;164:537–55. doi: 10.1007/s10549-017-4282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin CY, Yun KE, Park HS. Blood pressure has a greater impact on cardiovascular mortality than other components of metabolic syndrome in Koreans. Atherosclerosis. 2009;205:614–9. doi: 10.1016/j.atherosclerosis.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 51.Marroquin OC, Kip KE, Kelley DE, et al. Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women: a report from the Women's Ischemia Syndrome Evaluation. Circulation. 2004;109:714–21. doi: 10.1161/01.CIR.0000115517.26897.A7. [DOI] [PubMed] [Google Scholar]
- 52.Sung KC, Rhee EJ, Ryu S, et al. Increased Cardiovascular Mortality in Subjects With Metabolic Syndrome Is Largely Attributable to Diabetes and Hypertension in 159,971 Korean Adults. J. Clin. Endocrinol. Metab. 2015;100:2606–12. doi: 10.1210/jc.2014-4031. [DOI] [PubMed] [Google Scholar]
- 53.Armenian SH, Lacchetti C, Lenihan D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. Journal of Oncology Practice. 2017;13 doi: 10.1200/JOP.2016.018770. [DOI] [PubMed] [Google Scholar]
- 54.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J. Clin. Oncol. 2016;34:611–35. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 55.Luo J, Hendryx M, Virnig B, et al. Pre-existing diabetes and breast cancer prognosis among elderly women. Br. J. Cancer. 2015;113:827–32. doi: 10.1038/bjc.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.