Abstract
Oxidative stress is a known contributing factor in mitochondrial respiratory chain (RC) disease pathogenesis. Yet, no efficient means exists to objectively evaluate the comparative therapeutic efficacy or toxicity of different antioxidant compounds empirically used in human RC disease. We postulated that the pre-clinical comparative analysis of diverse antioxidant drugs having suggested utility in primary RC disease using animal and cellular models of RC dysfunction may improve understanding of their integrated effects and physiologic mechanisms, and enable prioritization of lead antioxidant molecules to pursue in human clinical trials. Here, lifespan effects of N-acetylcysteine (NAC), vitamin E, vitamin C, coenzyme Q10 (CoQ10), mitochondrial-targeted CoQ10 (MS010), lipoate, and orotate were evaluated as the primary outcome in a well-established, short-lived C. elegans gas-1(fc21) model of RC complex I disease. Healthspan effects were interrogated to assess potential reversal of their globally disrupted in vivo mitochondrial physiology, transcriptome profiles, and intermediary metabolic flux. NAC, vitamin E, and partially coenzyme Q rescued gas-1(fc21) lifespan toward that of wild-type N2 Bristol worms. MS010 and CoQ10 largely reversed biochemical pathway expression changes in gas-1(fc21) worms. While nearly all drugs normalized the upregulated expression of the ‘cellular antioxidant pathway’, they failed to rescue the mutant worms’ increased in vivo mitochondrial oxidant burden. NAC and vitamin E therapeutic efficacy were validated in human fibroblast and/or zebrafish complex I disease models. Remarkably, rotenone-induced zebrafish brain death was preventable partially with NAC and fully with vitamin E. Overall, these preclinical model animal data demonstrate that several classical antioxidant drugs do yield significant benefit on viability and survival in primary mitochondrial disease, where their major therapeutic benefit appears to result from targeting global cellular, rather than intramitochondria-specific, oxidative stress. Clinical trials are needed to evaluate whether the two antioxidants, NAC and vitamin E, that show greatest efficacy in translational model animals significantly improve the survival, function, and feeling of human subjects with primary mitochondrial RC disease.
Keywords: mitochondria, C. elegans, zebrafish, fibroblasts, genetic disease, therapeutic modeling, antioxidant
1. INTRODUCTION
Identification of effective therapies for primary (genetic-based) mitochondrial respiratory chain (RC) disease has been limited by an incomplete understanding of the leading pathogenic factors that underlie individual disorders [1]. Primary mitochondrial dysfunction causes a wide spectrum of clinical diseases, with features variably involving neurodevelopmental, myopathic, cardiac, renal, hepatic, gastrointestinal, endocrine, hearing and vision problems, as well as metabolic dysfunction [2]. Although the molecular mechanisms responsible for the pathogenesis of the diverse array of mitochondrial RC dysfunction-mediated diseases vary, oxidative stress is recognized to be a common element in their pathophysiology [3, 4]. Oxidative stress can be induced by a range of mechanisms, including the generation of reactive oxygen species (ROS) that damage DNA, proteins, and lipids, as well as the depletion of endogenous antioxidant systems [5]. Multiple antioxidant therapies have long been used in the clinical care of mitochondrial disease patients as an empiric means to reduce oxidative stress [6], with additional strategies under development to enhance antioxidant delivery specifically within the mitochondrial compartment [7, 8]. However, it has been unclear whether antioxidants that target oxidative stress throughout the entire cell or primarily within mitochondria are necessary or sufficient to restore cellular and organism health [9]. We postulate that more globally directed antioxidant therapies may be required in primary mitochondrial RC disease to effectively boost endogenous defenses and reduce the secondary oxidative stress that impairs many aspects of cellular function [10].
It is unknown whether antioxidants with different mechanisms of action have potential therapeutic advantanges at the level of cellular, organ, and overall individual health in primary mitochondrial RC disease [11–13]. A range of antioxidants variably used to empirically treat RC disease patients include coenzyme Q10 (CoQ10), vitamin E, vitamin C, and lipoic acid [11, 14], with several other agents proposed largely in the basic research setting such as N-acetylcysteine (NAC), orotic acid, and mitochondrial-targeted coenzyme Q10 (MitoQ) [15–19]. However, there exists no widely accepted means to clinically monitor antioxidant treatment efficacy either on oxidative stress burden in different tissues or at the more integrated outcomes level of overall patient health. Furthermore, it is not known whether there are optimal doses, or possible toxicities, that may result from antioxidant therapy use in mitochondrial disease patients, either from off-target effects or from their potentially negative impact on physiologic oxidant levels that are essential for intracellular signaling [20]. While clinical trials are the gold-standard means to test a purported therapy’s impact in human disease at the level of survival, feeling, and function, comparative analysis of all possible antioxidant therapies without some preclinical means to prioritize lead therapeutic candidates is neither a practical nor efficient approach in a rare disease cohort such as primary RC disease.
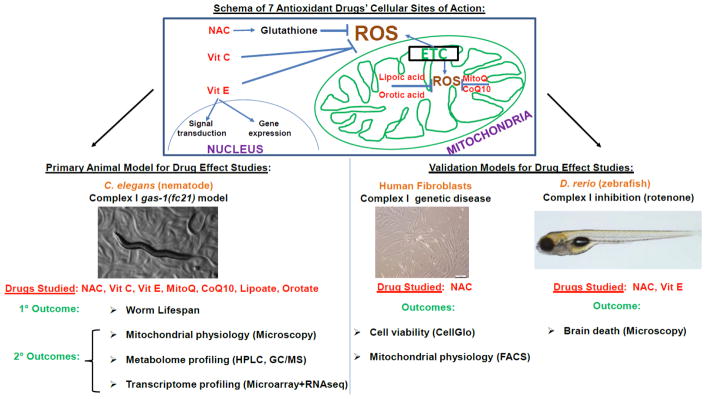
Pre-clinical modeling to prioritize therapeutic leads can enable deeper insights into these key questions [21], where antioxidants of predicted benefit in human RC disease can be objectively evaluated by mechanistic studies in simple animal and cellular models of primary RC dysfunction [10, 22, 23]. Here, C. elegans was used as the primary model animal system in which to systematically investigate the preventative (treatment from early development) and therapeutic (treatment beginning on first day of adulthood) effects of 7 antioxidant treatments that have been empirically used, or rationally proposed, for the clinical treatment of human RC disease (Fig 1). The primary model studies was the well-established C. elegans gas-1(fc21) model of RC complex I disease, which results from a homozygous p.R290K missense mutation in the nuclear-encoded complex I NDUFS2 subunit ortholog [24]. These mutant worms have been shown to have 70% decreased complex I-dependent respiratory capacity [25], significantly shortened lifespan at 20°C [25], multiple secondary alterations in intermediary metabolic pathways identifiable by genome-wide expression analysis, free amino acid profiling [26] and stable isotope based flux analysis [27], and increased mitochondrial oxidant burden [28]. Here, we systematically quantified the physiologic effects and mechanisms of N-acetylcysteine (NAC), vitamin E, vitamin C, coenzyme Q10 (CoQ10), mitochondrial-targeted CoQ10 (MitoQ, or MS010), lipoic acid (lipoate), and orotic acid (orotate) on gas-1(fc21) RC mutant animals’ lifespan as the primary outcome. Secondary mechanistic analyses of treatment effects were performed on their integrated metabolism at multiple levels to assess for potential reversal of their globally disrupted in vivo mitochondrial physiology, transcriptome, and intermediary metabolic flux. Antioxidant therapies that showed the greatest benefit in C. elegans mitochondrial complex I disease mutant worms were subsequently validated in a zebrafish RC complex I disease brain death model, and in human fibroblast cells from mitochondrial complex I disease patients.
Figure 1. Schematic experimental overview of antioxidant drug treatments in three RC complex I disease models.
Effects of 7 antioxidant drugs having different sites and/or purported modes of action were systematically studied in the C. elegans complex I deficient gas-1(fc21) mutant worm at the level of animal lifespan and physiologic mechanisms. Leading treatments showing maximal lifespan benefit were subsequently validated by assessing their effects on survival and/or mitochondrial physiology in RC complex I disease human fibroblast cells and/or a rotenone model of complex I inhibition in zebrafish. NAC, N-Acetylcysteine. ROS, reactive oxygen species. MitoQ, mitochondrial-targeted CoQ10. ETC, electron transport chain. FACS, Fluorescence-activated cell sorting. HPLC, high performance liquid chromatography. GC/MS, gas chromatography/mass spectrometry. Vit, vitamin.
2. MATERIAL AND METHODS
2.1 C. elegans lifespan analyses
Animals were maintained at 20°C throughout lifespan experiments, which were performed as previously described [22]. Briefly, synchronized nematode cultures were initiated by bleaching young adults to obtain eggs. Collected eggs were allowed to hatch overnight on 10 cm, unspread, Nematode Growth Media (NGM) plates, after which L1-arrested larvae were transferred to 10 cm NGM plates spread with OP50 E. coli. Upon reaching the first day of egg laying, synchronous young adults were moved to fresh 3.5 cm NGM plates seeded with bacteria (Lifespan experiment “Day 0”). For experiments performed without FUDR, nematodes were moved daily to fresh NGM plates throughout their egg-laying period. For experiments with FUDR (200 μg/mL or 812 μM final concentration on plate), nematodes were maintained on the same plates throughout the experiment. 60 nematodes were studied per strain, divided on three 3.5 cm NGM plates. Mortality was confirmed by stimulating nematodes lightly with a platinum wire; if the nematode did not move after stimulations it was scored as dead and removed from the plate. Worms that died of protruding/bursting vulva, bagging, or crawling off the agar were censored. Drug treatment of worms started either in early development by moving synchronized larvae on drug treated plates, or when nematodes reached the stage of young adults (YA) as discerned by visualizing eggs laid on plates. Drug treatments in gas-1(fc210) worms were performed using the highest achievable drug concentration in terms of drug solubility we experimentally confirmed to cause no developmental delay in wild-type (N2 Bristol) worms when initiated from the L1 larval stage through the first day of adulthood. 2–3 biological replicate lifespan experiments were performed per drug at the following concentrations: 2.5 mM N-acetylcysteine (NAC), 10 μM lipoic acid, 50 μM orotic acid, 100 μM, 1 mM and 10 mM Vitamin C, 650 μM CoQ10 Novasol, 10 μM decyl-TPP (decyl-triphenylphosphium, which is the control carrier compound for MS010 that lacks antioxidant activity), 10 μM MS010, 250 μM Vitamin E, and buffer control (0.1% final ethanol concentration for Vitamin E and S. basal or water for all other drugs) on NGM plates.
2.2 Relative quantitation of mitochondrial matrix superoxide burden, mitochondrial membrane potential, and mitochondria content by fluorescence microscopy in young adult C. elegans
Mitochondrial oxidant burden (MitoSOX Red), membrane potential (tetramethylrhodamine ethyl ester, TMRE), and mitochondrial content (MitoTracker Green FM, MTG) were performed at 20°C using in vivo terminal pharyngeal bulb relative fluorescence microscopic quantitation, as previously described [22, 28]. Briefly, synchronous populations of Day 0 young adults were moved to 35 mm NGM plates spread with OP50 E. coli, a desired drug treatment (2.5 mM N-acetylcysteine, 10 μM lipoic acid, 50 μM orotic acid, 10 mM Vitamin C, 650 μM CoQ10 Novasol, 10 μM decyl-TPP, 10 μM MS010, and 250 μM Vitamin E) or buffer control (0.1% final ethanol concentration for Vitamin E and S-basal/water for all other drugs on NGM plates), and either 10 μM MitoSOX Red (matrix oxidant burden), 100 nM TMRE (mitochondrial membrane potential), or 2 μM MitoTracker Green FM (mitochondria content) for 24 h. The next day, worms were transferred with a pick onto 35 mm agar plates spread with OP50 E. coli without dye for 1 h to allow clearing of residual dye from the gut. Worms were then paralyzed in situ with 5 mg/ml levamisole. Photographs were taken in a darkened room at 160× magnification with a Cool Snap cf2 camera (Nikon, Melville, NY). A CY3 fluorescence cube set (MZFLIII, Leica, Bannockburn, IL) was used for MitoSOX and TMRE. A GFP2 filter set (Leica) was used for MitoTracker Green FM. Respective exposure times were 2 s, 320 ms, and 300 ms for each of MitoSOX, TMRE, and MitoTracker Green FM. Representative confocal images from our prior publications validating this method confirmed the high fluorescent labeling of the 1 uM size mitochondrial structures within the mitochondrial-dense, 8 cell terminal pharyngeal bulb [28, 29]. The resulting images were background subtracted, and the nematode terminal pharyngeal bulb (mitochondrial-rich 8 cell organ) was manually circled to obtain mean intensity of the region by using Fiji Is Just ImageJ [30]. Fluorescence data for each strain were normalized to its same day control to account for day-to-day variation. A minimum of 3 independent experiments of approximately 50 animals per replicate were studied per strain per dye. The significance of the difference in the mean fluorescence intensity between strains under different experimental conditions was assessed by mixed-effect ANOVA, which takes into account potential batch effect due to samples being experimentally prepared, processed, and analyzed on different days by including a batch random effect in the model. A statistical significance threshold was set at P < 0.05. All statistical analyses were performed in SAS 9.3.
2.3 Antioxidant drug treatment expression profiling by Affimetrix GeneChip C. elegans Genome Array analysis
The nematode C. elegans were maintained at 20°C by established protocol [31]. C. elegans strains studied included wild-type N2 Bristol and the mitochondrial RC complex I mutant gas-1(fc21). For pharmacologic treatments, synchronous young adult populations were obtained and treated with six different water-soluble antioxidant drugs, per previously established protocol [31] at the same concentration as described in the fluorescence microscopy method section above (2.5 mM N-acetylcysteine, 10 μM lipoic acid, 50 μM orotic acid, 650 μM CoQ10 (Novasol), 10 μM MS010, and 10 μM decyl-TPP as a control for MS010), except Vitamin C that was used at 100 mM final concentration based on it being the maximally tolerated concentration during normal worm development. After drug treatment, total RNA was isolated and prepared for gene expression study by microarray, as prior described [31]. Briefly, RNA was isolated with a modified Trizol method, total RNA concentration was measured using NanoDrop-1000, and total RNA quality was determined using the Agilent Bioanalyzer in the CHOP DNA sequencing core facility. Samples with RNA integrity number (RIN) between 8 to 10 were accepted for further analysis. Gene expression analysis was performed by Affymetrix C. elegans microarray in the Nucleic Acid Core Facility at the Children’s Hospital of Philadelphia, GEO Series ID #GSE66680 (Bioproject Accession Number PRJNA277719).
2.4 Vitamin E treatment transcriptome profiling by C. elegans RNA-Seq analysis
Sample preparation for gene expression profiling by RNA-Seq technique was performed, as previously described [10]. Briefly, wild-type (N2 Bristol) and mitochondrial RC complex I deficient gas-1(fc21) animals were maintained at 20°C by established protocol [31]. Synchronous young adult populations of approximately 1,000–2,000 nematodes were obtained and treated on the first day of egg laying for 24 hours on NGM plates spread with 250 μM Vitamin E, or ethanol buffer control, per established protocol [31]. After drug treatment, total RNA was isolated and prepared for transcriptome profiling, as previously described [31]. Briefly, total RNA was isolated using the Trizol method and RNA concentration was measured using the NanoDrop-1000. RNA quality was determined by using Agilent Bioanalyzer in the NapCore Facility at The Children’s Hospital of Philadelphia Research Institute, where RIN number between 8 and 10 as required for further sample analysis. Library preparation was performed using the Illumina TruSeq Stranded Total RNA Sample Preparation Kit (San Diego, CA), with indexing to enable 8 samples to be run per lane. Samples were submitted to the BGI @ CHOP Sequencing Core Facility at The Children’s Hospital of Philadelphia Research Institute CHOP for next generation sequencing (RNA-Seq) analysis on Illumina HiSeq 2000 instruments. Samples were run in High Throughput Mode of 100 base pair paired end reads with 8 samples per lane to generate an estimated 20 million reads per sample. Library quality was assessed by Bio A analysis to check concentration, library size, and contamination, as well as by gel analysis to assess degradation. Quantitative PCR was then performed to determine optimal sample concentrations using the Applied Biosystems Step One Plus Real Time PCR machine to enable proper sample pooling. Sequencing reads saved in FASTQ files were aligned to obtain gene-level expression data for bioinformatic analysis. Data quality issues, such as total throughput, confounding factors, and outlier samples, were fully evaluated to ensure validity of analysis results. Gene and KEGG Pathway-level analyses were performed as previously described [32]. RNA-Seq data was submitted to Sequence Read Archive (SRA Bioproject ID PRJNA284422).
2.5 Whole worm amino acid profiling and stable-isotopic intermediary metabolic flux analysis in C. elegans
Whole worm free-amino acid profiling [26] and metabolic flux analysis [33] were performed as previously described. Briefly, synchronous populations of 1000–1500 worms were grown to adulthood on NGM plates. 10 mM universal labeled 13C-glucose and appropriate drug in desired concentration were added to plates before first day adult worms were transferred to fresh plates. The same drug treatments at the same concentration were used as described above in the microarray and RNA-Seq method sections. Following 24 hours of incubation with drug, adult worms were washed clear of bacteria 5 times with S. basal. Worm number was estimated by counting. Three biological triplicate experiments were performed per condition. Metabolic reactions were stopped by the addition of 4% perchloric acid (PCA) containing 20 nmol internal standard (e-aminocaproic acid, 16.7 μM). Samples were ground using a plastic homogenizer and motorized drill until visual inspection confirmed worm disruption. Precipitated protein was removed, re-dissolved in 1 normal NaOH, and protein concentration was determined DC Protein Assay (Bio-Rad). 50 μl neutralized samples were separated for HPLC analysis. From the remaining neutralized samples amino acids and organic acids were extracted using ion exchange resin (Bio-Rad) in AG50 and AG1 columns, respectively, to measure relative enrichment in amino acids and organic acids, by isotope ratio mass spectrometry, as previously described [33]. Isotope ratio MS analyses were performed in the Metabolomics Core Facility at The Children’s Hospital of Philadelphia. Stable isotopic enrichment was calculated in Excel (Microsoft) for each species as previously described [22], according to the following formula: Atom Percent Excess, corrected (APE) = (Rsa-Rst)*100/[(Rsa-Rst)+100], where Rsa - Ratio of the sample and Rst - Ratio of the standard. Statistical comparison between groups was performed using random-effects ANOVA (JMP version 10, SAS Institute, Cary NC).
2.6 Cell viability and fluorescence-activated cell sorting (FACS) analyses of mitochondrial physiology in primary fibroblasts from RC complex I disease human subjects
Fibroblast cell lines (FCLs) were obtained from prior skin biopsies when available and/or established in the Clinical CytoGenomics Laboratory from skin biopsies performed in the Mitochondrial Disease Center at The Children’s Hospital of Philadelphia (M.J.F.). Informed consent was obtained per The Children’s Hospital of Philadelphia Institutional Review Board approved study #08-6177 (M.J.F., PI). FCLs were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 1 g/l glucose and supplemented with 10% FBS (Gibco), 1 mM sodium pyruvate (Cell- Gro), 2 mM L-glutamine and 50 μg/ml uridine (Calbiochem). Fibroblast cell viability was evaluated using the CellTiter-Glo luminescent cell viability assay (Promega, G7571). Specifically, 100 μl of 100,000 cells per well were cultured in growth medium overnight in 96 well plates, changed to experimental media conditions (10% FBS, DMEM containing 5 mM glucose or glucose-free media, with or without antioxidant drugs) and incubated at 37°C for the indicated time (1–5 days). CellTiter-Glo reagent was then added to the wells (100 μl reagent per 100 μl of medium-containing cells per well), well contents were mixed for 2 minutes on a shaker, and the plate was then incubated at room temperature for 10 minutes after which time chemiluminescence was recorded on a BioTek,Synergy HTX Multi-Mode Reader, 590/35nm. Fibroblasts for FACS analysis were grown to 80–100% confluence in T75 flasks at 37°C before treatment with varying antioxidant drug concentrations for 24 hours. Fibroblasts were then treated with trypsin, collected in cell growth media, centrifuged, and the cell pellets were resuspended in HBSS to obtain 1–5 × 105 cells per sample, as previously reported [34, 35]. Samples were loaded with either 50 nM MitoTracker Green (MTG) at 37°C for 30 minutes to evaluate mitochondrial content, or 5μM MitoSOX 37°C for 10 minutes to evaluate mitochondrial matrix oxidant burden. Cells were then washed twice with phosphate buffered saline (PBS) and resuspended in 400 μl PBS. FACS analysis was performed in the CHOP Cell Sorting Core Facility using an Accuri C6 flow cytometer (BD Biosciences) equipped with a 488 nm laser with 530/30 nm emission (FL-1) for MTG and 647/52 nm emission (FL-3) for MitoSOX. A total of 10,000 events were recorded per condition. Gating was set to capture approximately 95% of events, and measured fluorescence data were normalized by multiplying the mean fluorescence of gated cells by the number of cells in the gated region. Three technical replicates were performed per experiment, with a minimum of 3 biological replicate experiments performed per condition. The relative quantitation of mitochondrial content (MTG, MitoTracker Green) and matrix oxidant burden (MitoSOX) in cell was indicted by mean and standard deviation of fluorescence intensity. Statistical analysis was performed by two-way Student’s T-test to compare relative fluorescence intensity in the complex I disease proband versus healthy control cells, and in treated versus untreated complex I disease proband cells.
2.7 Zebrafish larvae antioxidant treatment studies
All procedures and husbandry using zebrafish (Danio rerio) were approved by the Institutional Animal Care and Use Committee (IACUC) of the Children’s Hospital of Philadelphia (IAC15-1154) and were performed in accordance with Office of Animal Welfare (OLAW) regulations. Zebrafish larvae were bred and raised using standard practices [36]. Larvae were raised at 28°C in E3 medium [36] and were staged by age post-fertilization and morphological criteria (e.g., size, shape of yolk, formation of swim bladder). If applicable, 0.03 mg/ml 1-Phenyl-2-thiourea (PTU) from 1 dpf [36] was added to prevent pigment formation. Larvae were pre-treated from 3.5 days post fertilization (dpf) with 1 mM N-acetyl-cysteine (NAC) or from 6 dpf with 25 μM tocopherol (Vitamin E) in 10 mM tris pH 7.2, 0.1% DMSO, with solutions refreshed every 24 h. Control larvae were treated with 10 mM tris pH 7.2, 0.1% DMSO. On 7 dpf, larvae were treated with rotenone (mitochondrial RC complex I inhibitor) for 4 h in the presence of NAC or tocopherol and their brain phenotype was scored as either positive (grey, dead, necrotic tissue in brain) or negative (WT). Three independent, biological replicate experiments were performed with NAC and Tocopherol. Statistical analysis was performed using Student’s t-test. Control larvae were treated with equal amount of ethanol (vehicle). Rotenone concentration was batch dependent (30–100 nM) and adjusted to cause brain death in approximately 80% of larvae after 4 h of treatment at 7 dpf. Rotenone stock solutions (100 mM) were prepared with ethanol. NAC stock solution was prepared at 100 mM in E3. Final solutions contained 0.1% DMSO and were adjusted to pH 7.2 using 10 mM Tris/HCl.
3. RESULTS
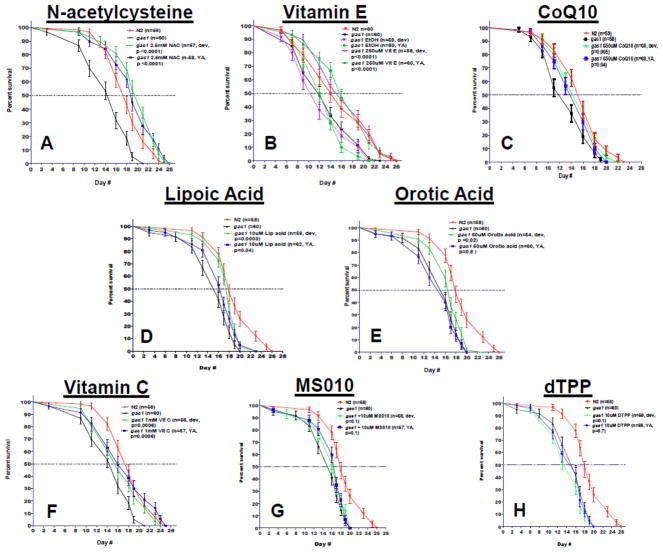
3.1 gas-1(fc21) worm short lifespan was rescued completely by N-acetylcysteine (NAC) or vitamin E, and partially by CoQ10 or lipoic acid
N-acetylcysteine (NAC, 2.5 mM) significantly rescued gas-1(fc21) median and maximal lifespan whether treatment was begun in early development or upon reaching adulthood (Fig 2A, 31% improvement in median lifespan over untreated gas-1(fc21) during development and 19% at young adulthood, p<0.0001). Vitamin E (250 μM) also completely rescued gas-1(fc21) median and maximal lifespan (Fig 2B, 33% improvement in median lifespan over untreated gas-1(fc21) when begun during development and 23% at adult stages, p<0.0001), despite the ethanol buffer alone in which vitamin E needed to be dissolved itself caused a modest reduction in gas-1(fc21) lifespan. Interestingly, the median lifespan of NAC and Vitamin E treated gas-1(fc21) even exceeded that of wild-type N2 Bristol worms. CoQ10 (650 μM, Novasol) partially rescued gas-1(fc21) median lifespan over untreated gas-1(fc21) at both developmental and young adult stages (Fig 2C 14% improvement in median lifespan over untreated gas-1(fc21) when begun at both developmental (p=0.005) and adult (p=0.04) stages). Similarly but only when started in early development, statistically different improvement in gas-1(fc21) median lifespan was seen with lipoic acid (10 μM) (Fig 2D) 13% improvement in median lifespan over untreated gas-1(fc21) worms, (p=0.0003) and 6% increase at adult stage (p=0.04). Orotic acid at 50 μM concentration significantly increased lifespan during developmental stage by 6% (p=0.02), but there was no significant change at young adult hood (Fig 2E). Vitamin C effects on mitochondrial mutant worm longevity was concentration dependent: 1 mM mildly rescued maximum lifespan (Fig 2F, and Fig S1, 14% improvement in maximum lifespan over untreated gas-1(fc21) worms, p=0.0006) and had no effect on median lifespan, whereas at either stage 100 μuM was ineffective and 10 mM was mildly toxic with 15% reduction in median lifespan shown in two independent lifespan replicates (Fig S1, p<0.001). Neither MS010 (MitoQ) (Fig 2G) nor its control carrier compound, decyl-triphenylphosphium (dTPP, Fig 2H) at 10 uM concentration significantly effected gas-1(fc21) lifespan. Overall, of the 7 antioxidant compounds tested on gas-1(fc21) complex I mutant worm lifespan, partial rescue was achieved with CoQ10, lipoic acid, or mid-dose vitamin C and full restoration back to that of wild-type animals was achieved with NAC or vitamin E.
Figure 2. Antioxidant drugs variably rescue short lifespan of complex I mutant gas-1(fc21) C. elegans.
All drug studies were performed at 20°C compared to buffer-only treated gas-1(fc21) and wild-type N2 Bristol worms, with effects of exposure tested from initiation in development at L1 stage (‘Dev’) or at initiation upon reaching adulthood (‘young adult, YA’). The complex I mutant strain, gas-1(fc21) has significantly shortened median and maximal lifespan compare to N2 (see panels A–H). (A) N-acetylcysteine [NAC, 2.5 mM] significantly rescued median lifespan (31% increase relative to untreated gas-1(fc21) worms) when treatment was started during early development, and 19% when started at the young adult stage (p<0.0001 and p<0.0001, respectively). (B) Vitamin E [250 μM] increased both maximal and median lifespan by up to 33% when started at the developmental stage and 23% when started at the young adult stage (p<0.0001 and p<0.0001, respectively). (C) CoQ10 [650 uM] also rescued both maximal and median lifespan (14%) in both developmental and young adult stage treatment (p<0.005 and p<0.03 respectively). (D) Lipoic acid [10 μM] significantly increased lifespan (p<0.0003) when it was administered during development, there was a milder effect when worms were treated in young adult hood (p<0.04). (E) Orotic acid increased both median and maximal lifespan administered in 50 μm concentration during development (p<0.02). (F) Vitamin C in 1mM concentration has a mild beneficial effect both in developmental and young adult treatment on maximal lifespan (p<0.04 and p<0.006 respectively). (G) MS010 [‘MitoQ’, 10 uM] failed to significantly increase the median lifespan in both developmental and young adult age (p>0.05). (H) dTTP [10 μM] was used as a negative control for MS010.
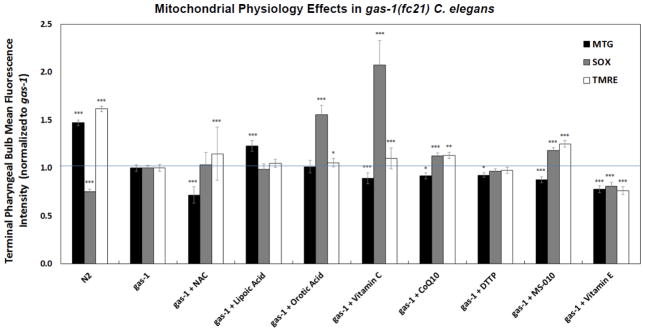
3.2 Antioxidant drugs variably affected mitochondrial physiology in gas-1(fc21) adult worms
Treatment effects on in vivo mitochondrial physiology after 24 hours of drug exposure on plates of young adult worms of each of the 7 antioxidant drugs and dTPP (MS010 control) at the same concentrations that had been used for gas-1(fc21) lifespan analyses, with the exception of Vitamin C that was evaluated only at 10 mM concentration. Effects were systematically evaluated to assess each drug’s relative ability to normalize three well-characterized alterations in key aspects of their mitochondrial physiology [28], including reduced mitochondrial content (Mitotracker Green, MTG), increased mitochondrial oxidant burden (MitoSOX), and reduced mitochondrial membrane potential (TMRE) (Fig 3). The only antioxidant drug that even partially increased mitochondrial content in gas-1(fc21) was lipoic acid (10 μM), which increased MTG relative fluorescence by 23% (p<0.0001) compared to buffer-only treated gas-1(fc21) worms. In contrast, significant further reductions from wild-type N2 Bristol in mitochondrial content were seen with treatment in gas-1(fc21) worms, in decreasing order by degree, with NAC (2.5 mM, 28% decrease), vitamin E (250 μM, 22% decrease), MS010 (10 μM, 12% decrease), vitamin C (10 mM, 11% decrease), CoQ10 (650 μM, 8% decrease), and dTPP control for MS010 (10 μM, 8% decrease). Despite general presumptions that each of these drugs has antioxidant function, the increased mitochondrial matrix oxidant burden of gas-1(fc21) worms was only significantly reduced with vitamin E (250 μM) treatment (by 19%, p < 0.0001), although this may represent an experimental artifact considering its concurrent similar magnitude reduction in mitochondrial content (22%, p < 0.0001), since quantitation of MitoSOX fluorescence depends on both mitochondrial content and oxidant burden per mitochondrion. Surprisingly, both vitamin C (10 mM) and orotic acid (50 μM) substantially increased mitochondrial superoxide burden in gas-1(fc21) worms by 107% and 55%, respectively, with less pronounced increases with CoQ10 (13%) and MS010 (18%) treatments. Considering that none of these treatments are predicted to restore mitochondrial electron transport (with the exception of CoQ or MS010, given their function in electron transport) or proton gradient flux, partial but significant rescue of mitochondrial membrane potential as assessed by increased TMRE fluorescence when gas-1(fc21) adult worms were treated not only with MS010 (10 μM, 25%) or CoQ10 (650 μM, 13%) but also with NAC (2.5 mM 15%), vitamin C (10 mM, 10%), and marginally with orotic acid (50 μM, 5%). Thus, our studies revealed that the altered mitochondrial physiology of gas-1(fc21) RC complex I mutant worms can be partially rescued at the level of mitochondrial content by lipoic acid, potentially at the level of mitochondrial oxidant burden by vitamin E but not by any of the other 6 ‘antioxidant’ therapies, and surprisingly, at the level of mitochondrial membrane potential by MS010, CoQ10, NAC, vitamin C, and orotic acid.
Figure 3. Antioxidant drugs variably rescue mitochondrial pathophysiology of complex I mutant gas-1(fc21) C. elegans.
In vivo fluorescence analysis of relative mitochondrial oxidant burden, membrane potential, and mitochondrial content in gas-1(fc21) mutants treated with antioxidant drugs was performed relative to buffer-only treated gas-1(fc21) and wild-type worms. Specifically, mitochondrial content, mitochondrial oxidant burden, and mitochondrial membrane potential were microscopically assessed by in vivo terminal pharyngeal bulb (PB) relative fluorescence quantitation using MitoTracker Green FM (MTG), MitoSOX (SOX), or TMRE, respectively. Effects of each drug were compared to buffer control (i.e., gas-1(fc21) with either 0.1% ethanol for Vitamin E, or S. basal for other six water-soluble drugs), with control data pooled as effects of 0.1% ethanol were negligible compared to S. basal solvent. Significant differences in the mean fluorescence intensity between strains under different experimental conditions was assessed by mixed-effect ANOVA, which accounts for potential batch effect due to samples being experimentally prepared, processed, and analyzed on different days by including a batch random effect in the model. Statistical significance threshold was set at P < 0.05 and all statistical analyses were performed in SAS 9.3. P-value conveys the significance of the difference between untreated N2 and untreated gas-1(fc21) (strain effect) or the difference between gas-1(fc21) plus drug and untreated gas-1(fc21) (treatment effect). For each parameter, each drug treatment assay was repeated in 3 to 10 independent trials, with n=50 worms per trial. Bars and error bars convey mean +/− SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus concurrent gas-1(fc21) buffer control.
3.3 Altered intermediary metabolism of gas-1(fc21) animals was not affected by antioxidant treatments
Intermediary metabolism was evaluated in gas-1(fc21) worm both at the level of steady state metabolism (whole worm population amino acid and organic acid quantitation) and flux through key intermediary pathways after feeding synchronized worm populations 13C-glucose for 24 hours beginning on the first day of adult life (Figs S2–S7). Similar as we previously observed [22, 26], many amino acid levels were significantly altered in gas-1(fc21) relative to wild-type N2 worms, with gas-1(fc21) having decreased concentrations of GLU, GLN, THR, ARG and PHE and increased concentrations of ALA and LEU (Fig S2). However, none of the antioxidant drug treatments significantly rescued nor exacerbated these steady state metabolic changes; trends were seen for some conditions that did not reach statistical significance, possibly due to inherent biochemical variance and/or small sample size. Isotopic enrichment in relative (Fig S3) and absolute amino acid (Fig S4) and organic acid molecular species (Figs S5–S7) also showed no significant effect of any antioxidant drug.
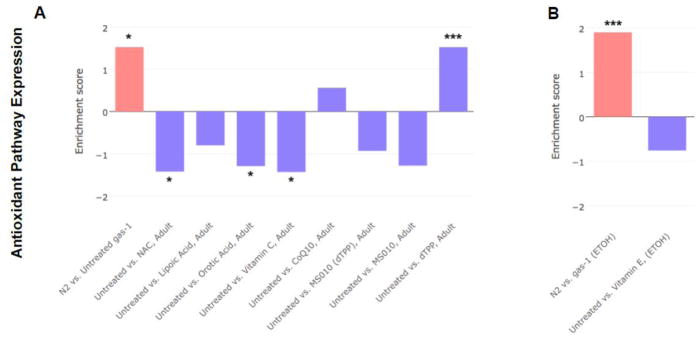
3.4 Antioxidant treatments variably rescued upregulated antioxidant pathway expression in gas-1(fc21) complex I mutant C. elegans
Global gene expression profiling was performed by C. elegans microarray analysis for the 6 water-soluble antioxidant drugs, and due to newer technology that became available as the study progressed, by RNA-Seq profiling for the lipophilic vitamin E. Both microarray and RNA-Seq analyses yielded similar gene-level and gene-set level changes in the gas-1(fc21) mutant relative to wild-type N2 worms. To specifically evaluate whether the 7 antioxidant drugs changed core antioxidant signaling pathways, we compared expression changes in the gene ontology (GO) defined antioxidant (GO:0016209) pathway in nematodes treated during adulthood (Fig 4) or for the 6 water-soluble antioxidants also beginning at the L1 early larval stage through early adulthood (Fig S8). Similar level reductions in this GO-defined pathway were seen in the young adult treated worms with 5 of the 6 antioxidant therapies (all except CoQ) (Fig 4a), as well as with vitamin E treatment (Fig 4b). Treatment from the early larval stage with dTPP also normalized expression in this GO-defined pathway (Fig S8), which is surprising given it is the inert carrier that transports compounds into the mitochondria but itself is not thought to have activity and no similar effect was seen when treatment was begun at the adult stage (Fig 4a). Also surprising was that NAC normalized these antioxidant pathways when fed to adult stage worms, but had no effect when initiated in early development (Fig S8). Thus, although none of the antioxidant drugs fully rescued the mitochondrial-matrix oxidant burden of gas-1(fc21) worms, most did normalize gene expression of the upregulated antioxidant pathway in these animals.
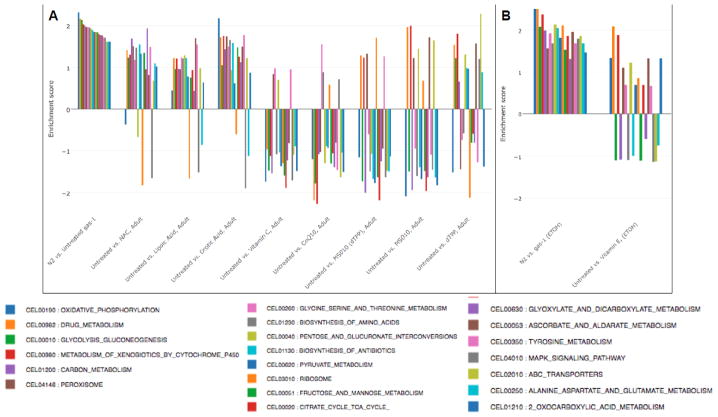
Figure 4. Antioxidant drug effects on upregulated antioxidant pathway in gas-1(fc21) adult worms.
Transcriptome expression changes in the gene ontology (GO) defined antioxidant (GO:0016209)) pathway were evaluated in gas-1(fc21) mitochondrial complex I mutant nematodes treated for 24 hours during early adulthood with one of (A) six water-soluble drugs relative to S. basal control or (B) lipophilic vitamin E relative to ethanol control. Wild-type worms (N2 Bristol) were treated only with buffer control. Each bar indicates comparative pathway changes in second group listed as compared to first. Similar level reduction in the GO-defined defense pathway was seen in the young adult treated worms with each antioxidant therapy tested except CoQ10. Enrichment score conveys degree to which upregulated antioxidant pathway in gas-1(fc21) relative to N2 control were normalized by each treatment in gas(fc21) worms.
We further selected 89 key antioxidant genes to evaluate gene-level expression changes within the transcriptome data in gas-1(fc21) versus N2 wild-type controls (both in S. basal and ethanol solvents), and in response to each of the antioxidant treatments started either in early development (“larval” tabs) or limited to 24 hours in early adulthood (“adult” tabs) (see Table S1), where significantly changed genes within each group comparison are highlighted in red for p < 0.01 and in purple for p < 0.05). As expected, antioxidant genes showed increased expression in gas-1(fc21) relative to wild-type, particularly involving glutathione transferases (GSTs), alcohol dehydrogenase, and both manganese (mitochondrial, SOD2 homologue) superoxide dismutase and copper-zinc (cytosolic, SOD1 homologue) superoxide dismutase genes. Interestingly, Vitamin E partially reversed changes in SOD2 and some GST genes but further upregulated many other GSTs. NAC begun in adults only exacerbated expression of these antioxidant genes, while starting NAC in early larvae exacerbated expression of SOD1 but largely downregulated GST gene expression. MS010 started in either development or adult worms caused a mix of increased and decreased GST gene expression in gas-1(fc21) when compared to buffer-treated gas-1(fc21), with significant downregulation of alcohol dehydrogenase expression. Surprisingly, dTPP alone also significantly changed expression of many antioxidant genes compared to buffer-treated gas-1(fc21) including induction of several glutathione peroxidase gene. However, when MS010 treatment was compared to its dTPP-treated gas-1(fc21) control, MSO10 significantly lowered many types of antioxidant genes including SOD2 in gas-1(fc21) worms, particularly when begun in the larval period. CoQ10 largely downregulated antioxidant gene expression in gas-1(fc21), including multiple GSTs and alcohol dehydrogenases, in both larval and adult conditions. Lipoic acid had minimal effect on antioxidant genes when started in adult gas-1(fc21), but when started in development largely induced GSTs, glutathione peroxidases, and SOD1 while reducing alcohol dehydrogenase expression. Vitamin C largely further induced antioxidant gene expression in gas-1(fc21) regardless of stage initiated; however, vitamin C initiated in larval stages showed the most significant reversal of any treatment studied at the level of global gene expression changes (Fig S9a) and glutathione pathway signaling (Fig S9b). Orotic acid initiated in adult worms yielded similar effect, although orotic acid begun in development significantly decreased expression of several GST genes. Overall, these individual antioxidant gene level analyses demonstrated the oxidative stress response is upregulated in the gas-1(fc21) mitochondrial complex I mutant worms, quite variably rescued versus further exacerbated by the different purported antioxidant drugs, and largely normalized with MS010 and CoQ10 treatments.
3.5 Antioxidant treatments variably rescued global expression alterations at the level of KEGG biochemical pathways in gas-1(fc21) complex mutant C. elegans
Similarly as we have previously observed, gas-1(fc21) mutant worms had extensive alterations relative to wild-type N2 Bristol control worms in their global metabolism, as were most clearly delineated at the level of KEGG biochemical pathways (Figs 5, 6, S10, and S11). Of the 21 significantly upregulated KEGG biochemical pathways as ranked in gas-1(fc21) relative to wild-type N2 (p < 0.05), only partial normalization toward wild-type levels were seen with 24 hour treatments in young adults (Fig 5). Interestingly, the single most upregulated pathway in gas-1(fc21), oxidative phosphorylation, showed significant normalization with nearly all of the treatments regardless of treatment initiation, with the exception in adults of lipoic acid, orotic acid, and vitamin E, and in development-treated worms of vitamin C (Figs 5 and S9). The most extensive rescue of upregulated pathway expression from treating adult worms occurred with CoQ, MS010, dTPP, and vitamin C, again suggesting a biologic effect of dTPP alone occurs in these animals, while expression of only 4 or less of these 21 pathways normalized with lipoic acid, orotic acid, or NAC (Fig 5). When treatment was initiated for the water-soluble antioxidants in the early larval period, however, CoQ rescued nearly all upregulated pathways (20 of 21), with substantial improvement across the majority of pathways with MS010 (more so than with dTPP), lipoic acid, orotic acid, or NAC (Fig S10); interestingly, vitamin C had nearly no effect when begun in the early larval period. These data suggest that prolonged treatment from the early developmental period may allow for improved rescue of the global transcriptome changes that occur across cell metabolism in the complex I mutant worms.
Figure 5. Antioxidant drug effects on upregulated KEGG pathways in gas-1(fc21) adult worms.
Transcriptome expression changes in the KEGG biochemical pathway that were significantly upregulated in gas-1(fc21) relative to wild-type (N2 Bristol) worms (first comparison, p<0.05) were evaluated in gas-1(fc21) mitochondrial complex I mutant nematodes treated for 24 hours during early adulthood with one of (A) six water-soluble drugs relative to S. basal control or (B) lipophilic vitamin E relative to ethanol control. Wild-type worms (N2 Bristol) were treated only with buffer control. Each bar indicates comparative pathway changes in second group listed as compared to first. Enrichment score conveys degree to which upregulated KEGG biochemical pathways in gas-1(fc21) relative to N2 control were normalized by each treatment in gas(fc21) worms.
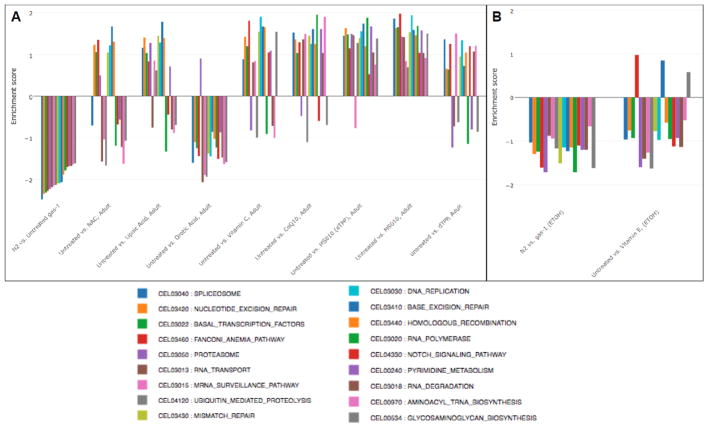
Figure 6. Antioxidant drug effects on downregulated KEGG pathways in gas-1(fc21) adult worms.
Transcriptome expression changes in the KEGG biochemical pathway that were significantly downregulated in gas-1(fc21) relative to wild-type (N2 Bristol) worms (first comparison, p<0.05) were evaluated in gas-1(fc21) mitochondrial complex I mutant nematodes treated for 24 hours during early adulthood with one of (A) six water-soluble drugs relative to S. basal control or (B) lipophilic vitamin E relative to ethanol control. Wild-type worms (N2 Bristol) were treated only with buffer control. Each bar indicates comparative pathway changes in second group listed as compared to first. Enrichment score conveys degree to which upregulated KEGG biochemical pathways in gas-1(fc21) relative to N2 control were normalized by each treatment in gas-1(fc21) worms.
Similar analysis of the 18 most down-regulated KEGG pathways in gas-1(fc21) relative to wild-type worms revealed the spliceosome to be the most decreased but at least partially normalized by CoQ, MS010 (and by dTPP), lipoic acid, and vitamin C (Fig 6). MS010 had the most extensive effect at reversing downregulated pathways in gas-1(fc21) when given to young adult worms for 24 hours, with substantial improvement also seen with CoQ, dTPP, lipoic acid, and vitamin C, minimal normalization with NAC, and nearly no rescue seen with orotic acid or vitamin E (Fig 6). Surprisingly, water-soluble antioxidant treatment initiation in early development suggested different effects at the level of normalizing expression pathways that are down-regulated in gas-1(fc21) worms, with near complete rescue seen with vitamin C, substantial normalization seen MS010, dTPP, and CoQ, and lipoic acid, and less than 50% of the downregulated pathways showing reversal with orotic acid or NAC (Fig S11). Overall, orotic acid, NAC, and vitamin E showed similar poor ability to normalize global transcriptome alterations regardless of their direction or treatment initiation timing, while MS010 (and largely dTPP as well), CoQ, and frequently vitamin C and lipoic acid, each had significant benefit at the level of transcriptional normalization in gas-1(fc21) complex I deficient worms.
Additional bioinformatics analyses of transcript data were performed to evaluate the potential mechanism and toxicity of the 7 antioxidant drugs at the level of biochemical pathway expression in gas-1(fc21) (Table S2). Complete analysis results are available through a custom online app that supports interactive data exploration and visualization of selected group comparisons, such as bar plots of enrichment scores and color-coded pathway maps (http://projectmito1.awsomics.org).
3.6 Validation of lead compound efficacy in mitochondrial complex I disease human fibroblasts
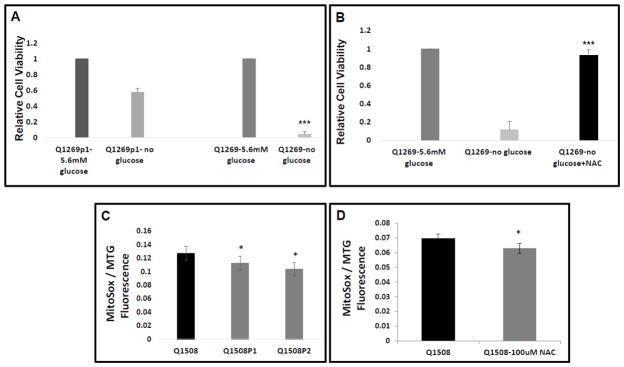
To evaluate the effects the lead antioxidant that rescued C. elegans models of mitochondrial complex I disease, we evaluated whether NAC treatment rescued the reduced viability and increased mitochondrial oxidant burden that occurs in primary fibroblast cell lines from mitochondrial complex I disease pediatric subjects. Specifically, we studied the primary fibroblast cell line (ID Q1269) obtained from a young girl with Leigh syndrome due to high-level heteroplasmy for the m.13513G>A mutation in the mitochondrial DNA-encoded complex I ND5 subunit gene, as well as a primary fibroblast cell line (ID 1508) derived from an infant girl with complex I disease caused by compound heterozygous mutations (c.160C>G;P.R54W; c.58G>C:p.G20R) in the nuclear-encoded complex I NDUFS8 subunit gene. Both cell lines showed significantly reduced viability when grown for prolonged periods in glucose-free media, given their reliance on glycolysis to maintain energy production in the setting of severe complex I deficiency. No rescue of viability under these extreme conditions was seen in the NDUFS8 line (data not shown). However, a therapeutic effect was seen in the ND5 mutant line, which had reduced viability relative to control fibroblasts from her unaffected mother (ID Q1269p1) in whom next generating sequencing confirmed the ND5 mutation was not present (Fig 7A, >90% death in Q1269 versus 40% death in Q1269p1 relative to respective growth in glucose-containing media, n=3, p < 0.001). When Q1269p1 were co-treated with 1 mM NAC while in glucose-free media for 5 days, significant rescue of their viability resulted (Fig 7B, n=3, improvement to 93% viability, p < 0.05).
Figure 7. N-acetylcysteine rescues the decreased cellular viability and increased mitochondrial oxidant burden that occur in RC complex I-deficient human fibroblasts from Leigh syndrome subjects.
(A) ND5 (m.13513G>A) heteroplasmic mutant complex I deficient human fibroblasts (Q1269 line) have greater than 95% death when grown for 5 days in glucose-free media, whereas the control line from her mother who does not carry the mtDNA pathogenic mutation in ND5 has 43% death under the same growth conditions. p < 0.001, n=3 biological replicates. (B) Remarkably, NAC [1 mM] co-treatment fully restored viability in the ND5 line grown in glucose-free conditions for 5 days. (C) NDUFS8 compound heterozygous mutant (c.160C>G;P.R54W; c.58G>C:p.G20R) complex I deficient human fibroblasts (Q1508 line) have increased mitochondrial oxidant burden relative to either heterozygous, unaffected parental control fibroblast line (Q1508p1 and Q1508p2). Relative fluorescence quantitation of Mitotracker Green (MTG) and MitoSOX (SOX) were measured by fluorescence-activated cell sorting (FACS) analysis. n=3 per condition. *, p<0.05. (D) NDUFS8 compound heterozygous mutant cells had reduced mitochondrial oxidant burden when treated with 100 μM NAC. Cells were cultured in 10% FBS DMEM media containing 5.6 mM glucose for 24 hour, p<0.05s.
When grown in normal glucose (5.6 mM) media for 24 hours, the NDUFS8 compound heterozygous mutant line (Q1508) survived, but had significantly increased mitochondrial oxidant burden relative to either healthy heterozygoous carrier parental control (Q1508p1 and Q1508p2), as determined by comparing relative mitochondrial oxidant burden (as measured with MitoSOX fluorescence) normalized to relative mitochondrial content (as measured with Mitotracker Green) (Fig 7C, n=3, p < 0.05). The increased mitochondrial oxidant burden of the NDUFS8 compound heterozygous mutant cells was significantly reduced by 24 hour treatment with 100 uM NAC (9% reduction, n=3, p < 0.05 Fig 7D). Similarly, beneficial effects of NAC treatment in diverse mitochondrial disease subject human fibroblasts were recently reported [37]. Thus, these studies in fibroblasts from two different pediatric Leigh syndrome subjects with genetically-confirmed mitochondrial complex I disease demonstrate that a lead antioxidant therapy identified in the C. elegans complex I subunit NDUFS2-orthologue mutant gas-1(fc21) model does yield significant benefit in cells from a human RC disease subject. However, the inherent variability between disease subjects seen here that likely results from differences in the relative contribution to oxidative stress among other pathogenic factors causing disease (e.g., NADH/NAD+ redox imbalance, ATP deficienct, nucleotide deficiency, etc), demonstrates the utility of developing in vitro tests that are predictive of a given disorder’s likely response to a particular therapeutic agent.
3.7 Validation of lead compound efficacy in zebrafish model of complex I disease
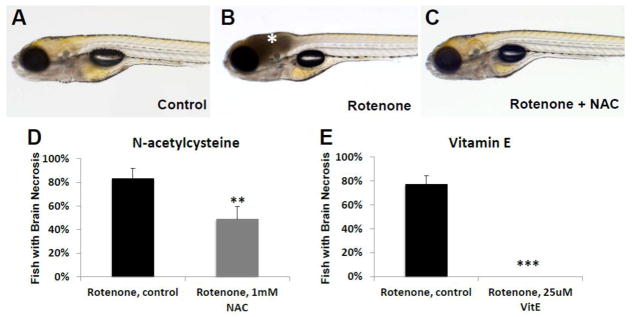
To evaluate animal and organ-level effects of antioxidant treatment in a vertebrate model for RC complex I mitochondrial disease, we treated zebrafish larvae with varying concentrations of the pharmacologic complex I inhibitor, rotenone, at different developmental stages [38]. While rotenone has been shown to induce abnormal development and death when administered early (up to 80 hpf) during development [39], we sought to induce an organ-specific defect relevant to human mitochondrial RC complex I disease. We found that rotenone (30–100 nM range, depending on batch) consistently causes cell death (presumably necrosis) in the brain when administered for 4 h at 7 dpf, as is apparent by graying of the brain tissue (Fig 8A–B), reduced larval response to external stimuli, and an absence of spontaneous swimming [38]. This phenotype is similar to the Leigh-syndrome brain phenotype that occurs in children with severe RC complex I dysfunction, often on exposure to acute stressors [40]. Remarkably, pre-treating zebrafish larvae for 3 days priors to rotenone-exposure with either 1 mM NAC or 25 uM Vitamin E significantly reduced or prevented rotenone-induced brain death. Specifically, we found that 1 mM NAC pre-treatment reduced brain death from 83% of control buffer treated (n=53) to 49% of NAC treated larvae (n=55, p=0.006) (Fig 8C–D). More dramatically, vitamin E pre-treatment nearly completely prevented the rotenone-induced brain necrosis zebrafish phenotype as compared to the 77% induction of necrosis in rotenone exposed buffer-only control larvae (n=42, p=0.00003) (Fig 8E). Thus, zebrafish organ-level experimental studies suggest both vitamin E and NAC have therapeutic value in the setting of adding resiliency and reducing or preventing brain death that occurs in the setting of RC complex I inhibition.
Figure 8. N-Acetylcysteine (NAC) and Vitamin E treatments prevented rotenone-induced cell death in zebrafish larvae brain.
(A) Control (EtOH/DMSO) larvae at 7 dpf have normal brain morphology, development, and swimming behavior. (B) Larval treatment with rotenone (30–100 nM) for 4 hours on 7 dpf causes cellular death in their brains as evidenced by grey tissue (asterisk). (C) Pre-treatment with NAC prevented brain necrosis. (D) While acute rotenone exposure on 7 dpf induced brain death in 83% of buffer-only treated larvae (n=53), brain death was significantly reduced to 49% of animals who received pre-treatment from 3.5 dpf with 1 mM NAC (n=55, p=0.006). (E) Vitamin E (25 μM) pre-treatment from 6 dpf led to near-complete rescue of brain death when zebrafish larvae were later exposed to rotenone on 7 dpf (p < 0.001). **, p < 0.01; ***, p < 0.001.
4. DISCUSSION
Preclinical analysis of 7 antioxidants empirically used or postulated to have clinical benefit in primary mitochondrial disease revealed great variability in their therapeutic efficacy across diverse lifespan and metabolic healthspan endpoints in a C. elegans well-established model of primary mitochondrial disease (Table 1). Overall, NAC and vitamin E showed the greatest promise among the class of treatments evaluated in diverse complex I disease models in restoring animal lifespan and several aspects of animal health span in C. elegans gas-1(fc21) complex I disease worms. Indeed, both therapies consistently improved viability and/or stress resiliency across three species studied. These models included the C. elegans genetic model of mitochondrial complex I disease due to homozygous mutation in the NDUFS2 complex I subunit homologue, human fibroblast cell viability or mitochondrial oxidant burden from Leigh disease subjects with mutations in the mtDNA-encoded ND5 and nuclear-encoded NDUFS8 complex I subunits, and a zebrafish larval brain death model of mitochondrial complex I dysfunction from rotenone-based complex I inhibition. Among the other 5 antioxidant drugs whose effects were systematically studied in C. elegans, partial improvement in the short lifespan of gas-1(fc21) complex I mutant worms was achieved with CoQ10, lipoic acid, and vitamin C (1 mM), while no significant effect occurred with either MS010 (MitoQ) or orotic acid. As only the maximal tolerated concentration of each drug with the exception of vitamin C was tested (e.g., the highest dose found to be non-lethal and causing delay in development of N2 Bristol wild-type worms), it is conceivable that lower concentrations might have different effects. Further, while a water-soluble Novasol CoQ10 formulation of ubiquinone was tested as a bioavailable and dissolvable coenzyme formulation that is of prime relevance to human mitochondrial disease [11], the predominant coenzyme species in C. elegans is CoQ8 [41]; thus, the alternative isoprenyl tail length in CoQ10 vs CoQ8 may potentially have contributed to the only partial improvement in animal lifespan observed with CoQ10 treatment in gas-1(fc21) worms.
Table 1. Compiled results of 7 antioxidant treatment effects on survival and altered mitochondrial physiology in 3 evolutionarily distinct complex I disease models.
Within each major outcome shown, red and green highlights convey increase and decrease, respectively in treated relative to untreated mutant model. Percent changes within each assay are indicated. ‘No treatment’ column indicates baseline alterations in untreated mutant relative to healthy control within each model system. n.d., not determined.
| ANALYSIS | MODEL SYSTEM | OUTCOME | SPECIFIC CONDITION | No treatment | NAC | VitE | VitC | MS010 | CoQ10 | Lipoic Acid | Orotic Acid |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SURVIVAL | C. elegans (worm) | Lifespan | gas-1(fc21)treated from early development | −23% | 31% | 39% | 0% | 0% | 14% | 13% | 6% |
| gas-1(fc21)treated from young adulthood | 43% | 23% | 0% | 0% | 14% | 6% | 0% | ||||
| Human Fibroblast Cells | Viability | No glucose | 90% | 10% | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| D. rerio (zebrafish) | Brain Death | Rotenone | 83% | 49% | 0% | n.d. | n.d. | n.d. | n.d. | n.d. | |
| PHYSIOLOGY | C. elegans (worm) | Mitochondrial Physiology | MTG in gas-1(fc21) adults treated for 24 h | −47% | −28% | −22% | −11% | −12% | −8% | 23% | 0% |
| TMRE in gas-1(fc21) adults treated for 24 h | −62% | 15% | −24% | 10% | 25% | 13% | 0% | 5% | |||
| MitoSOX in gas-1(fc21) adults treated for 24 h | 25% | 0% | −19% | 107% | 18% | 13% | 0% | 55% | |||
| Human Fibroblast cells | Mitochondrial Physiology | MitoSOX:MTG ratio | 19% | −9% | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| C. elegans (worm) | Antioxidant Defense Pathway Expression | gas-1(fc21)adults treated for 24 h | 1.5 | −1.4 | −0.7 | −1.4 | −1.3 | 0.5 | −0.8 | −1.3 |
To gain insight into potential therapeutic mechanisms and toxicity of antioxidant drugs in the setting of RC complex I dysfunction, animal healthspan effects across key aspects of intermediary metabolism of all 7 antioxidant drugs were systematically evaluated in gas-1(fc21) worms, Severe mitochondrial physiology defects in these complex I mutant worms were variably ameliorated by antioxidant treatments, where lipoic acid partially rescued their reduced mitochondrial content and vitamin E lowered their increased mitochondrial oxidant burden (i.e., the balance of mitochondrial matrix oxidant production and scavenging). MS010, CoQ10, NAC, vitamin C, and orotic acid all significantly improved the animals reduced mitochondrial membrane potential, which is suggestive some improvements occur in overall RC chain electron transport integrated function. Of course, none are postulated to directly ameliorate the inherent severe complex I deficiency known to result from the genetic NDUFS2 homozygous missense mutation in gas-1(fc21) worms. While most of these purported ‘antioxidant’ treatments did not reduce the complex I mutant worms’ increased mitochondrial matrix oxidant burden, nearly all normalized gene expression at the level of the combined ‘antioxidant pathway’ after the same short-term (24 hour) adult treatment (Table 1). Considering these results together with the finding that complete lifespan rescue occurred only with NAC and vitamin E that work to reverse cellular oxidant stress but not with mitochondrial-targeted antioxidants (Fig 1), total cellular oxidative stress appears to be a more relevant treatment target in primary RC disease than the mitochondria-localized oxidant burden.
As anticipated, no major effects were apparent on the altered intermediary metabolic profiles or intermediary metabolite flux of complex I mutant worms by any antioxidant treatment. We recognize that the inherent variability between biological replicate analyses characteristic of this nematode mutant strain [22] may have limited our ability to identify modest effects, although clear alterations of the mutant worm relative to wild-type worms were apparent. Indeed, biochemical pathway expression analysis performed at the transcriptome level to evaluate effects of antioxidant treatments on cellular adaptations well-known to occur in RC disease [26, 32, 42] showed that many of the antioxidants significantly improved or normalized the differentially-regulated KEGG biochemical pathways in gas-1(fc21). Of these pathways, 21 were increased and 16 were decreased in gas-1(fc21) relative to wild-type N2 worms, reflecting these mutants’ global biochemical adaptation to primary mitochondrial complex I dysfunction. Most pronounced rescue effects at the biochemical pathway transcriptome level were seen with CoQ10 or MS010 (although the inactive dTPP control molecule for MS010 unexpectedly also had significant effects), as well as when C. elegans worm treatments were begun starting in the early development larval period rather than at the post-mitotic, young adult stage. We postulate that these improvements in secondary biochemical alterations at the transcriptome level likely relate to improved electron transport flux that occurs from CoQ10 and MS010 roles downstream of complexes I and II in electron transport, which does not occur with the other antioxidant treatments studied.
The efficacy of two antioxidant therapies, NAC and vitamin E, that were most effective at rescuing the short lifespan of the invertebrate C. elegans mitochondrial mutant gas-1(fc21) worm model were further validated in fibroblasts from two RC complex I disease human subjects and/or in a pharmacologic inhibitor (rotenone)-based zebrafish vertebrate animal model of acute mitochondrial complex I dysfunction. NAC rescued cell viability in the mitochondrial DNA-based ND5 Leigh syndrome patient cell line, and modestly reduced mitochondrial oxidant burden in the nuclear gene-based NDUFS8 Leigh syndrome patient fibroblast line. Most impressively, rotenone-based complex I inhibition brain death in a zebrafish larval model was partially prevented by NAC and fully prevented with vitamin E. These data suggest that NAC and vitamin E are antioxidants with particular promise to provide central nervous system resiliency in the setting of acute mitochondrial dysfunction, a finding of direct relevance to the mitochondrial Leigh syndrome population that often develops acute neurodevelopmental regression with intervening metabolic or catabolic stressors such as fever, illness, and fasting.
While NAC is a well-known antioxidant in the oxidative stress field, NAC has not been a commonly utilized antioxidant therapy for human primary mitochondrial diseases [11]. However, other groups have recently reported that NAC attenuates rotenone toxicity in a neuroblastoma cell model [43] as well as in rotenone-induced Parkinson’s disease substantia nigra and striatum [44], similarly as we report here in the rotenone-inhibited zebrafish model. NAC has also previously been shown to inhibit the cytotoxic and apoptotic effects of another mitochondrial toxin, paraquat [45]. Further, NAC has been shown to have strong physiologic effects in diverse organs and models of secondary mitochondrial dysfunction. Specifically, NAC has been shown to attenuate ischemia-reperfusion induced apoptosis and autophagy in mouse liver via regulation of the ROS/JNK/Bcl-2 pathway [15], protect mitochondrial function in isoproterenol-treated myocardial infarcted rat [46], improve insulin resistance and type 2 diabetes mellitus development [45], and attenuate hexavalent chromium-induced hypersensitivity through inhibition of cell death and cytokine expression, inhibiting or altering IL-alpha and TNF-alpha production, and decreasing apoptosis and autophagy [47]. In neurologic disorders, NAC has been shown to act as a precursor for the endogenous antioxidant glutathione [48], block apoptosis induced by LPS in endothelial cells and by TNFα in neuronal cells [45], as well as to modulate glutamergic, neurotrophic, and inflammatory pathways including NF-kB whose in vitro activation is suppressed by NAC in cultured pancreatic cells [49]. However, NAC’s cytoprotective effect against ROS-induced cytotoxicity has been shown to depend on its ability to enhance glutathione synthesis [50]. Overall, our findings of beneficial viability, lifespan, health span, and systemic resiliency effects of NAC in C. elegans, human cell, and zebrafish models of mitochondrial complex I disease extend a rich literature that show diverse physiologic benefits of NAC. These data suggest the need for robust clinical trial evaluation of NAC in human mitochondrial RC disease subjects.
Vitamin E is commonly used as an empiric antioxidant in mitochondrial RC disease [11, 12], although no human clinical trials have been conducted to robustly evaluate its efficacy in this population. A fat-soluble antioxidant comprised of eight fat-soluble compounds including α-tocopherol, vitamin E is also an essential micronutrient for humans that has been showed to modulate signal transduction and gene expression [51], including modulating expression of key enzymes involved in signal transduction such as protein kinase C, protein kinase B, protein tyrosine kinases, phospholipase A2, phosphatidylinositol-3-kinase and cyclooxygenase-2 [51]. Vitamin E inhibition of protein kinase C inhibits the assembly of NADPH-oxidase, which reduces oxidative species and is thought to contribute to the prevention of chronic inflammatory processes [51]. Vitamin E, combined with lipoic acid and CoQ10, was previously shown to improve running performance and mitochondrial function in an untrained wild-type female mouse model [52]. Interestingly, the beneficial effect of vitamin E was reported in zebrafish (Danio rerio) exposed to the fat-soluble insecticide endosulfan (ESF) that impairs embryo development, morphology, locomotor activity and antioxidant gene expression and causes uncontrolled excitation and brain damage [53]. While vitamin E (α-tocopherol) decreased the morphological aberrations and larval locomotor hyperactivity, it did not prevent antioxidant gene expression dysregulation in the ESF model [53]. Similarly, we report here that vitamin E has a strong beneficial effect in the prevention of rotenone-induced brain death in zebrafish. Meta-analyses investigating vitamin E effects on cardiovascular events have yielded conflicting results, where vitamin E dietary supplements in humans decreased the incidence of myocardial infarction and risk of ischemic stroke, but increased risk for hemorrhagic stroke and in higher doses may increase all-cause mortality [54]. Reports of vitamin E effects in neurodegenerative diseases have also been conflicting, including in Alzheimer disease (AD) and Parkinson disease (PD) in which oxidative stress has a pathophysiologic role [55, 56]. While clinical cases of cardiovascular and neurodegenerative disease in which vitamin E was studied may plausibly involve secondary mitochondrial impairment as part of their complex pathophysiology, they are not primary mitochondrial RC disorders. Therefore, our results demonstrating that vitamin E leads to complete rescue of C. elegans lifespan in a genetic-based chronic mitochondrial complex I disease, and provides protection from brain death in zebrafish exposed to acute mitochondrial RC inhibition, suggest that further evaluation of vitamin E in properly-designed clinical trials is warranted within the human primary mitochondrial RC disease population.
Given our aim to harness translational model animals of mitochondrial complex I disease as a pre-clinical means to efficiently identify therapeutic compounds to improve survival and function in primary mitochondrial disease, antioxidant compounds were only tested in primary mitochondrial disease models. Wild-type (non-mutated) animals were included solely to serve as the genetic baseline relative to the mitochondrial disease strain, for purposes of aiding in the determination whether candidate therapies exacerbate or rescue (and if so, to what degree) the disease phenotype toward that of wild-type, healthy animals. While a possible limitation of this work could be viewed that these antioxidant compounds’ general health effects and mechanisms were not comparatively tested in a wild-type population without any inherent mitochondrial dysfunction or overt disease state, this study design was intentional. Firstly, the basic mechanisms of action of the antioxidants studied here are well-known. More importantly, potential health improvement effects that may be observed in a healthy (wild-type) individual taking a ‘dietary supplement’ is irrelevant to whether objective efficacy or toxicity at the level of major disease phenotypic outcomes results from candidate ‘drugs’ given in a genetic disease model [57]. In other words, whether or not any antioxidant(s) have aging benefits in the general population carries no clear guidance for whether a therapy should or should not be given in a rare disease state. In contrast, this work establishes a means by which efficient and relatively inexpensive pre-clincial testing can be performed to prioritize drug candidates for further clinical development that demonstrate significant improvement on major survival and functional outcomes of direct relevance to metabolic disease human patients. Such model systems can further be used to objectively evaluate for possible synergistic or adverse interaction effects of multi-drug treatment combinations (“cocktail”), a common practice in mitochondrial medicine [12] whose utility has not been rigorously demonstrated.
5. CONCLUSION
Preclinical modeling to objectively evaluate the potential efficacy, mechanism, and toxicity of antioxidants drugs empirically used or postulated to have benefit in human mitochondrial disease revealed great variability in their therapeutic efficacy across diverse lifespan and health span endpoints in a C. elegans model of primary mitochondrial complex I disease. None were notably toxic in the mitochondrial disease C. elegans complex I disease model at the concentrations tested, with the exception of high-dose (10 mM) vitamin C that significantly reduced median gas-1(fc21) lifespan (Fig S1). Both NAC and vitamin E consistently provided significant benefit across the divergent evolutionary species tested, with full restoration of animal lifespan in C. elegans, improvement in some aspects of animal health span in C. elegans, and/or improvement in organ-level resiliency (brain death) in the setting of acute mitochondrial dysfunction in a zebrafish model of RC complex I inhibition. These translational research findings suggest that antioxidant therapies may indeed have objective therapeutic value and low toxicity in individuals with primary mitochondrial RC complex I disease. Randomized, double-blind, placebo-controlled clinical treatment trials are needed to the prioritized, lead candidates identified from this work, NAC and vitamin E, ideally alone and potentially in combination [13, 58], to assess their comparative efficacy on patient-important health outcomes, overall well-being, and survival in mitochondrial RC complex I disease [59].
Supplementary Material
Figure S1. Vitamin C effect on lifespan. Vitamin C effect on the mitochondrial mutant gas-1(fc21) worms showed concentration dependence. 100 μM was ineffective, 10 mM was mildly toxic, but 1 mM had therapeutic effect on the lifespan of gas-1(fc21) mutant worm.
Figures S2–S7. Biochemical analysis of intermediary metabolism. We evaluated the intermediary metabolism effects gas-1(fc21) worms by quantitating amino acid and organic acid changes during the different antioxidant treatments using mass spectrometry and HPLC. Many amino acids (Fig S2) and organic acid (Fig S5) levels were altered. None of the antioxidants used rescued the altered steady state metabolic changes. Figs S3–S4 show the isotopic enrichment in relative and absolute level of amino acids. Figs S6–S7 show the isotopic enrichment in relative and absolute level in organic acids.
Figure S8. Transcriptome analysis of gas-1(fc21) worms treated with antioxidant drugs during development at the antioxidant level. Most of the drugs rescued the worms’ altered antioxidant pathway expression, with the exception of NAC.
Figure S9. Vitamin C effect on the antioxidant pathways at the transcriptome level. Vitamin C was found to be the most potent drug to rescue changes in (A) global gene expression and (B) glutathione pathway expression.
Figure S10. Antioxidant drugs effect on the upregulated KEGG pathways. When gas-1(fc21) worms were treated during development with 7 antioxidant drugs, CoQ10 was the most benefical to rescue the upregulated pathway expression, followed by MS010, lipoic acid, orotic acid and NAC. Vitamin C did not rescue the upregulated pathways in gas-1(fc21) worms when exposed during development.
Figure S11. Antioxidant drug treatment effect on the downregulated KEGG pathways. Of the 7 antioxidant drugs studied, Vitamin C showed the greatest reversal of downregulated pathway expression in gas-1(fc21) worms when exposed during development, followed by MS010, CoQ10, lipoic acid, orotic acid and NAC.
We evaluated 89 key antioxidant genes expression changes in gas-1(fc21) versus N2 wild-type worms that resulted from drug treatments during developmental stages and in early adulthood. Genes which were significantly changed are highlighted in red (p<0.01) or purple (p<0.05).
This table summarizes the different biochemical pathway expression changes that occurred from different antioxidant drug treatments in both the larval stage and upon treatment in young adult gas-1(fc21) worms.
Highlights.
Antioxidants have varying efficacy in C. elegans model of mitochondrial disease
NAC and vitamin E rescue short lifespan in gas-1(fc21) complex I mutant C. elegans
CoQ10 & MitoQ partially rescue lifespan but improve healthspan in gas-1(fc21) worms
Vitamin E, and partially NAC, prevents brain death in complex I inhibited D. Rerio
NAC improves cell viability in fibroblasts from complex I ND5 disease human subject
Cellular antioxidant stress is a highly relevant treatment target in mitochondrial disease
Acknowledgments
Funding
This work was funded by in part by the National Institutes of Health (R01-HD065858 and R01-GM120762 to M.J.F.; and 5-K12-DK094723 to S.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We are grateful to Michael Murphy, PhD, for providing MitoQ; Qinwen Tang and Crystal Yan for assistance with RNA extraction and handling; Judith Preston, M.S. for assistance with worm handling and sample preparation; Ilana Nissim, Evgueni Daikhin, and Itzhak Nissim in the CHOP Metabolomic Core Facility; and Sujay Guha, PhD, for providing a C. elegans image.
Abbreviations
- C. elegans
Caenorhabditis elegans
- CI
complex I
- D. rerio
Danio rerio
- FCL
fibroblast cell line
- MTG
mitotracker green
- RC
respiratory chain
- NAC
N-acetylcysteine
- TMRE
tetramethylrhodamine ethyl ester
Footnotes
Conflict of Interest
The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, Suomalainen A, Thorburn DR, Zeviani M, Turnbull DM. Mitochondrial diseases Nature reviews. Disease primers. 2016;2:16080. doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- 2.Mitochondrial Medicine Society’s Committee on D. Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, Wong LJ, Cohen BH, Naviaux RK. The in-depth evaluation of suspected mitochondrial disease. Molecular genetics and metabolism. 2008;94:16–37. doi: 10.1016/j.ymgme.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkuri KR, Cowan TM, Kwan T, Ng A, Herzenberg LA, Herzenberg LA, Enns GM. Inherited disorders affecting mitochondrial function are associated with glutathione deficiency and hypocitrullinemia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3941–3945. doi: 10.1073/pnas.0813409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi G, Cortopassi G. Oxidative stress in inherited mitochondrial diseases. Free radical biology & medicine. 2015;88:10–17. doi: 10.1016/j.freeradbiomed.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-biological interactions. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Karaa A, Kriger J, Grier J, Holbert A, Thompson JL, Parikh S, Hirano M. Mitochondrial disease patients’ perception of dietary supplements’ use Molecular genetics and metabolism. 2016;119:100–108. doi: 10.1016/j.ymgme.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochimica et biophysica acta. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Annals of the New York Academy of Sciences. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 9.Feniouk BA, Skulachev VP. Cellular and molecular mechanisms of action of mitochondria-targeted antioxidants. Current aging science. 2016 doi: 10.2174/1874609809666160921113706. [DOI] [PubMed] [Google Scholar]
- 10.Peng M, Ostrovsky J, Kwon YJ, Polyak E, Licata J, Tsukikawa M, Marty E, Thomas J, Felix CA, Xiao R, Zhang Z, Gasser DL, Argon Y, Falk MJ. Inhibiting cytosolic translation and autophagy improves health in mitochondrial disease. Human molecular genetics. 2015;24:4829–4847. doi: 10.1093/hmg/ddv207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh S, Saneto R, Falk MJ, Anselm I, Cohen BH, Haas R Medicine Society TM. A modern approach to the treatment of mitochondrial disease. Current treatment options in neurology. 2009;11:414–430. doi: 10.1007/s11940-009-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh S, Goldstein A, Koenig MK, Scaglia F, Enns GM, Saneto R, Anselm I, Cohen BH, Falk MJ, Greene C, Gropman AL, Haas R, Hirano M, Morgan P, Sims K, Tarnopolsky M, Van Hove JL, Wolfe L, DiMauro S. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial. Medicine Society Genetics in medicine: official journal of the American College of Medical Genetics. 2015;17:689–701. doi: 10.1038/gim.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagano G, Aiello Talamanca A, Castello G, Cordero MD, d’Ischia M, Gadaleta MN, Pallardo FV, Petrovic S, Tiano L, Zatterale A. Current experience in testing mitochondrial nutrients in disorders featuring oxidative stress and mitochondrial dysfunction: rational design of chemoprevention trials. International journal of molecular sciences. 2014;15:20169–20208. doi: 10.3390/ijms151120169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas RH. The evidence basis for coenzyme Q therapy in oxidative phosphorylation disease. Mitochondrion. 2007;7(Suppl):S136–145. doi: 10.1016/j.mito.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Chen K, Xia Y, Dai W, Wang F, Shen M, Cheng P, Wang J, Lu J, Zhang Y, Yang J, Zhu R, Zhang H, Li J, Zheng Y, Zhou Y, Guo C. N-acetylcysteine attenuates ischemia-reperfusion-induced apoptosis and autophagy in mouse liver via regulation of the ROS/JNK/Bcl-2 pathway. PloS one. 2014;9:e108855. doi: 10.1371/journal.pone.0108855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hey-Mogensen M, Goncalves RL, Orr AL, Brand MD. Production of superoxide/H2O2 by dihydroorotate dehydrogenase in rat skeletal muscle mitochondria. Free radical biology & medicine. 2014;72:149–155. doi: 10.1016/j.freeradbiomed.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Beuneu C, Auger R, Loffler M, Guissani A, Lemaire G, Lepoivre M. Indirect inhibition of mitochondrial dihydroorotate dehydrogenase activity by nitric oxide. Free radical biology & medicine. 2000;28:1206–1213. doi: 10.1016/s0891-5849(00)00239-2. [DOI] [PubMed] [Google Scholar]
- 18.Loffler M, Jockel J, Schuster G, Becker C. Dihydroorotat-ubiquinone oxidoreductase links mitochondria in the biosynthesis of pyrimidine nucleotides. Molecular and cellular biochemistry. 1997;174:125–129. [PubMed] [Google Scholar]
- 19.Oyewole AO, Birch-Machin MA. Mitochondria-targeted antioxidants. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2015;29:4766–4771. doi: 10.1096/fj.15-275404. [DOI] [PubMed] [Google Scholar]
- 20.Linnane AW, Eastwood H. Cellular redox regulation and prooxidant signaling systems: a new perspective on the free radical theory of aging. Annals of the New York Academy of Sciences. 2006;1067:47–55. doi: 10.1196/annals.1354.008. [DOI] [PubMed] [Google Scholar]
- 21.Kuszak AJ, Espey MG, Falk MJ, Holmbeck MA, Manfredi G, Shadel GS, Vernon HJ, Zolkipli-Cunningham Z. Nutritional Interventions for Mitochondrial OXPHOS Deficiencies: Mechanisms and Model Systems. Annual review of pathology. 2017 doi: 10.1146/annurev-pathol-020117-043644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormack S, Polyak E, Ostrovsky J, Dingley SD, Rao M, Kwon YJ, Xiao R, Zhang Z, Nakamaru-Ogiso E, Falk MJ. Pharmacologic targeting of sirtuin and PPAR signaling improves longevity and mitochondrial physiology in respiratory chain complex I mutant Caenorhabditis elegans. Mitochondrion. 2015;22:45–59. doi: 10.1016/j.mito.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon YJ, Falk MJ, Bennett MJ. Flunarizine rescues reduced lifespan in CLN3 triple knock-out Caenorhabditis elegans model of batten disease. Journal of inherited metabolic disease. 2016 doi: 10.1007/s10545-016-9986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayser EB, Morgan PG, Hoppel CL, Sedensky MM. Mitochondrial expression and function of GAS-1 in Caenorhabditis elegans. The Journal of biological chemistry. 2001;276:20551–20558. doi: 10.1074/jbc.M011066200. [DOI] [PubMed] [Google Scholar]
- 25.Kayser EB, Sedensky MM, Morgan PG. The effects of complex I function and oxidative damage on lifespan and anesthetic sensitivity in Caenorhabditis elegans. Mechanisms of ageing and development. 2004;125:455–464. doi: 10.1016/j.mad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Falk MJ, Zhang Z, Rosenjack JR, Nissim I, Daikhin E, Nissim I, Sedensky MM, Yudkoff M, Morgan PG. Metabolic pathway profiling of mitochondrial respiratory chain mutants in C. elegans. Molecular genetics and metabolism. 2008;93:388–397. doi: 10.1016/j.ymgme.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vergano SS, Rao M, McCormack S, Ostrovsky J, Clarke C, Preston J, Bennett MJ, Yudkoff M, Xiao R, Falk MJ. In vivo metabolic flux profiling with stable isotopes discriminates sites and quantifies effects of mitochondrial dysfunction in C. elegans. Molecular genetics and metabolism. 2014;111:331–341. doi: 10.1016/j.ymgme.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dingley S, Polyak E, Lightfoot R, Ostrovsky J, Rao M, Greco T, Ischiropoulos H, Falk MJ. Mitochondrial respiratory chain dysfunction variably increases oxidant stress in Caenorhabditis elegans. Mitochondrion. 2010;10:125–136. doi: 10.1016/j.mito.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon YJ, Guha S, Tuluc F, Falk MJ. High-throughput BioSorter quantification of relative mitochondrial content and membrane potential in living Caenorhabditis elegans. Mitochondrion. 2017 doi: 10.1016/j.mito.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological- image analysis. Nature methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polyak E, Zhang Z, Falk MJ. Molecular profiling of mitochondrial dysfunction in Caenorhabditis elegans. Methods in molecular biology. 2012;837:241–255. doi: 10.1007/978-1-61779-504-6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Tsukikawa M, Peng M, Polyak E, Nakamaru-Ogiso E, Ostrovsky J, McCormack S, Place E, Clarke C, Reiner G, McCormick E, Rappaport E, Haas R, Baur JA, Falk MJ. Primary respiratory chain disease causes tissue-specific dysregulation of the global transcriptome and nutrient-sensing signaling network. PloS one. 2013;8:e69282. doi: 10.1371/journal.pone.0069282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falk MJ, Rao M, Ostrovsky J, Daikhin E, Nissim I, Yudkoff M. Stable isotopic profiling of intermediary metabolic flux in developing and adult stage Caenorhabditis elegans. Journal of visualized experiments: JoVE. 2011 doi: 10.3791/2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dingley S, Chapman KA, Falk MJ. Fluorescence-activated cell sorting analysis of mitochondrial content, membrane potential, and matrix oxidant burden in human lymphoblastoid cell lines. Methods in molecular biology. 2012;837:231–239. doi: 10.1007/978-1-61779-504-6_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gai X, Ghezzi D, Johnson MA, Biagosch CA, Shamseldin HE, Haack TB, Reyes A, Tsukikawa M, Sheldon CA, Srinivasan S, Gorza M, Kremer LS, Wieland T, Strom TM, Polyak E, Place E, Consugar M, Ostrovsky J, Vidoni S, Robinson AJ, Wong LJ, Sondheimer N, Salih MA, Al-Jishi E, Raab CP, Bean C, Furlan F, Parini R, Lamperti C, Mayr JA, Konstantopoulou V, Huemer M, Pierce EA, Meitinger T, Freisinger P, Sperl W, Prokisch H, Alkuraya FS, Falk MJ, Zeviani M. Mutations in FBXL4, encoding a mitochondrial protein, cause early-onset mitochondrial encephalomyopathy. American journal of human genetics. 2013;93:482–495. doi: 10.1016/j.ajhg.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westerfield M. The Zebrafish Book: A Guide for the Laboratory use of Zebrafish (Danio rerio) University of Oregon Press; 1995. [Google Scholar]
- 37.Douiev L, Soiferman D, Alban C, Saada A. The Effects of Ascorbate, N-Acetylcysteine, and Resveratrol on Fibroblasts from Patients with Mitochondrial Disorders. Journal of clinical medicine. 2016;6 doi: 10.3390/jcm6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrnes J, Ganetzky R, Lightfoot R, Tzeng M, Nakamaru-Ogiso E, Seiler C, Falk MJ. Pharmacologic modeling of primary mitochondrial respiratory chain dysfunction in zebrafish. Neurochemistry international. 2017 doi: 10.1016/j.neuint.2017.07.008. pii: S0197-0186(17)30062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinho BR, Santos MM, Fonseca-Silva A, Valentao P, Andrade PB, Oliveira JM. How mitochondrial dysfunction affects zebrafish development and cardiovascular function: an in vivo model for testing mitochondria-targeted drugs. British journal of pharmacology. 2013;169:1072–1090. doi: 10.1111/bph.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lake NJ, Compton AG, Rahman S, Thorburn DR. Leigh syndrome: One disorder, more than 75 monogenic causes. Annals of neurology. 2016;79:190–203. doi: 10.1002/ana.24551. [DOI] [PubMed] [Google Scholar]
- 41.Yang YY, Gangoiti JA, Sedensky MM, Morgan PG. The effect of different ubiquinones on lifespan in Caenorhabditis elegans. Mechanisms of ageing and development. 2009;130:370–376. doi: 10.1016/j.mad.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z, Falk MJ. Integrated transcriptome analysis across mitochondrial disease etiologies and tissues improves understanding of common cellular adaptations to respiratory chain dysfunction. The international journal of biochemistry & cell biology. 2014;50:106–111. doi: 10.1016/j.biocel.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alkholifi FK, Albers DS. Attenuation of rotenone toxicity in SY5Y cells by taurine and N-acetyl cysteine alone or in combination. Brain research. 2015;1622:409–413. doi: 10.1016/j.brainres.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 44.Rahimmi A, Khosrobakhsh F, Izadpanah E, Moloudi MR, Hassanzadeh K. N-acetylcysteine prevents rotenone-induced Parkinson’s disease in rat: An investigation into the interaction of parkin and Drp1 proteins. Brain research bulletin. 2015;113:34–40. doi: 10.1016/j.brainresbull.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Lasram MM, Dhouib IB, Annabi A, El Fazaa S, Gharbi N. A review on the possible molecular mechanism of action of N-acetylcysteine against insulin resistance and type-2 diabetes development. Clinical biochemistry. 2015;48:1200–1208. doi: 10.1016/j.clinbiochem.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Basha RH, Priscilla DH. An in vivo and in vitro study on the protective effects of N-acetylcysteine on mitochondrial dysfunction in isoproterenol treated myocardial infarcted rats. Experimental and toxicologic pathology: official journal of the Gesellschaft fur Toxikologische Pathologie. 2013;65:7–14. doi: 10.1016/j.etp.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Lee YH, Su SB, Huang CC, Sheu HM, Tsai JC, Lin CH, Wang YJ, Wang BJ. N-acetylcysteine attenuates hexavalent chromium-induced hypersensitivity through inhibition of cell death, ROS-related signaling and cytokine expression. PloS one. 2014;9:e108317. doi: 10.1371/journal.pone.0108317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacology & therapeutics. 2014;141:150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Bavarsad Shahripour R, Harrigan MR, Alexandrov AV. N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain and behavior. 2014;4:108–122. doi: 10.1002/brb3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang F, Lau SS, Monks TJ. The cytoprotective effect of N-acetyl-L-cysteine against ROS-induced cytotoxicity is independent of its ability to enhance glutathione synthesis. Toxicological sciences: an official journal of the Society of Toxicology. 2011;120:87–97. doi: 10.1093/toxsci/kfq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zingg JM. Modulation of signal transduction by vitamin E. Molecular aspects of medicine. 2007;28:481–506. doi: 10.1016/j.mam.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Abadi A, Crane JD, Ogborn D, Hettinga B, Akhtar M, Stokl A, Macneil L, Safdar A, Tarnopolsky M. Supplementation with alpha-lipoic acid, CoQ10, and vitamin E augments running performance and mitochondrial function in female mice. PloS one. 2013;8:e60722. doi: 10.1371/journal.pone.0060722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dale K, Rasinger JD, Thorstensen KL, Penglase S, Ellingsen S. Vitamin E reduces endosulfan-induced toxic effects on morphology and behavior in early development of zebrafish (Danio rerio) Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2017;101:84–93. doi: 10.1016/j.fct.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Galli F, Azzi A, Birringer M, Cook-Mills JM, Eggersdorfer M, Frank J, Cruciani G, Lorkowski S, Ozer NK. Vitamin E: Emerging aspects and new directions. Free radical biology & medicine. 2017;102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 55.Ricciarelli R, Argellati F, Pronzato MA, Domenicotti C. Vitamin E and neurodegenerative diseases. Molecular aspects of medicine. 2007;28:591–606. doi: 10.1016/j.mam.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Fariss MW, Zhang JG. Vitamin E therapy in Parkinson’s disease. Toxicology. 2003;189:129–146. doi: 10.1016/s0300-483x(03)00158-6. [DOI] [PubMed] [Google Scholar]
- 57.Camp KM, Krotoski D, Parisi MA, Gwinn KA, Cohen BH, Cox CS, Enns GM, Falk MJ, Goldstein AC, Gopal-Srivastava R, Gorman GS, Hersh SP, Hirano M, Hoffman FA, Karaa A, MacLeod EL, McFarland R, Mohan C, Mulberg AE, Odenkirchen JC, Parikh S, Rutherford PJ, Suggs-Anderson SK, Tang WH, Vockley J, Wolfe LA, Yannicelli S, Yeske PE, Coates PM. Nutritional interventions in primary mitochondrial disorders: Developing an evidence base. Molecular genetics and metabolism. 2016;119:187–206. doi: 10.1016/j.ymgme.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle & nerve. 2007;35:235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- 59.National Academies of Sciences, and Medicine. Enabling precision medicine: The role of genetics in clinical drug development. Proceedings of a workshop; Washington, D.C: The National Academies Press; 2017. pp. 37–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Vitamin C effect on lifespan. Vitamin C effect on the mitochondrial mutant gas-1(fc21) worms showed concentration dependence. 100 μM was ineffective, 10 mM was mildly toxic, but 1 mM had therapeutic effect on the lifespan of gas-1(fc21) mutant worm.
Figures S2–S7. Biochemical analysis of intermediary metabolism. We evaluated the intermediary metabolism effects gas-1(fc21) worms by quantitating amino acid and organic acid changes during the different antioxidant treatments using mass spectrometry and HPLC. Many amino acids (Fig S2) and organic acid (Fig S5) levels were altered. None of the antioxidants used rescued the altered steady state metabolic changes. Figs S3–S4 show the isotopic enrichment in relative and absolute level of amino acids. Figs S6–S7 show the isotopic enrichment in relative and absolute level in organic acids.
Figure S8. Transcriptome analysis of gas-1(fc21) worms treated with antioxidant drugs during development at the antioxidant level. Most of the drugs rescued the worms’ altered antioxidant pathway expression, with the exception of NAC.
Figure S9. Vitamin C effect on the antioxidant pathways at the transcriptome level. Vitamin C was found to be the most potent drug to rescue changes in (A) global gene expression and (B) glutathione pathway expression.
Figure S10. Antioxidant drugs effect on the upregulated KEGG pathways. When gas-1(fc21) worms were treated during development with 7 antioxidant drugs, CoQ10 was the most benefical to rescue the upregulated pathway expression, followed by MS010, lipoic acid, orotic acid and NAC. Vitamin C did not rescue the upregulated pathways in gas-1(fc21) worms when exposed during development.
Figure S11. Antioxidant drug treatment effect on the downregulated KEGG pathways. Of the 7 antioxidant drugs studied, Vitamin C showed the greatest reversal of downregulated pathway expression in gas-1(fc21) worms when exposed during development, followed by MS010, CoQ10, lipoic acid, orotic acid and NAC.
We evaluated 89 key antioxidant genes expression changes in gas-1(fc21) versus N2 wild-type worms that resulted from drug treatments during developmental stages and in early adulthood. Genes which were significantly changed are highlighted in red (p<0.01) or purple (p<0.05).
This table summarizes the different biochemical pathway expression changes that occurred from different antioxidant drug treatments in both the larval stage and upon treatment in young adult gas-1(fc21) worms.