Abstract
Background
Hearing impairment is a common problem in patients with mucopolysaccharidosis IV (MPS IV) throughout their life. Many of the adult patients with MPS IV exhibit permanent or severe hearing loss. However, there has been no systematic review of detailed audiological test results in MPS IV.
Materials and methods
Fourteen individuals with MPS IV (13 MPS IVA and 1 MPS IVB; aged between 12 and 38 years old) participated in the current study. We obtained auditory neurophysiological responses (auditory brainstem responses and otoacoustic emissions test) in addition to pure-tone audiometry and middle ear function tests (tympanometry and acoustic reflexes).
Results
The results indicated various levels and types of hearing loss with abnormal neurophysiological responses even in those patients with MPS IVA with normal pure tone thresholds. We also found a strong relationship between height (short stature is an indicator of skeletal severity) and hearing sensitivity as well as a strong relationship between height and outer hair cell function in the inner ear (measured by otoacoustic emissions) among MPS IVA patients.
Conclusion
The strong correlation between reduced height and hearing loss indicates that patients with severe skeletal dysplasia may be at higher risk of developing more severe hearing loss. More importantly, the spectrum of hearing disorders indicates that MPS IV patients should have annual neurophysiological hearing tests in addition to audiometric testing from an early age regardless of their skeletal severity to more carefully monitor disease progression.
Keywords: mucopolysaccharidosis IV, auditory system, electrophysiology, audiological testing, hearing impairment/loss
1 Introduction
1.1 General characteristics of mucopolysaccharidosis IV
Mucopolysaccharidosis IV (MPS IV), also known as Morquio syndrome, is an autosomal recessive lysosomal storage disorder. MPS IV includes two types of disorders, MPS IVA (OMIM 253000) and MPS IVB (OMIM 253010), due to a deficiency of two different lysosomal enzymes, N-acetylgalactosamine-6-sulfate sulfatase (GALNS) and β-galactosidase, respectively. The majority of patients (95%) exhibit Type A [1]. MPS IV is characterized by a unique skeletal dysplasia caused by excessive accumulation of keratan sulfate (KS) and/or chondroitin 6-sulfate (C6S). Although most patients with MPS IV appear normal at birth, patients show skeletal abnormality within a few years of age or even at birth.
Patients with MPS IV have a wide spectrum of clinical manifestations [2–4] including growth impairment and progressive spondyloepiphyseal dysplasia frequently resulting in limited ambulation or complete wheel-chair bound condition. Patients with a severe phenotype of MPS IVA often do not survive beyond the second or third decade of life. Patients with a mild form of MPS IVA may survive until the seventies [4]. Recent advancements in medical and surgical treatment and management as well as early awareness of the disease have led to extended life expectancy [5] in the patients with MPS IVA [6]. In general, patients with MPS IVB have a milder skeletal dysplasia with taller stature.
1.2 Hearing in MPS IV
Although hearing impairment does not directly contribute to mortality, hearing impairment does have significant contribution to development of speech and language, and quality of life [2]. Recurring ear infections and hearing loss are common symptoms in MPS [7–9]. Because skeletal deformities are usually not apparent in the first couple of years of life, it is common for patients with MPS to see otolaryngologists before their diagnosis of MPS. Although commonly reported hearing loss in MPS IVA is conductive in nature [10, 11], sensorineural hearing loss has also been documented in some patients [12]. However, because MPS IVA is such a rare disorder, previous studies on hearing loss in MPS IVA were based on very small sample sizes, and detailed descriptions of the types and degree of hearing impairment are sparse. Based on a few studies, it is suggested that hearing loss in MPS IVA is bilateral and progressive in general [13, 14] similar to other MPS types [7, 15], and its severity ranges from mild to moderate [4, 16]. Riedner and Levin [13] reported that conductive hearing loss was observed in patients under eight years old, but that mixed and sensorineural hearing loss were also found in older patients. Permanent hearing loss has not been observed until adolescence [17]. Abnormal auditory brainstem response (ABR) results were also reported, but systematic chart review or study to correlate the auditory function to skeletal dysplasia in MPS IVA have not been made until now. Last, the majority of studies are based on MPS IVA. So far, two patients with MPS IVB were reported as having no hearing loss [18, 19].
The aims of this study are to examine hearing function in patients with MPS IV (mainly MPS IV A) and to correlate auditory phenotype with skeletal severity (measured as short stature) and/or activity of daily living.
2 Materials and Methods
The current study was approved by the Nemours Institutional Review Board (Local Board Reference Number: 458707 and 750932).
2.1 Participants
Fourteen participants (5 males and 9 females) aged between 12 and 38 years old, who had been diagnosed biochemically with MPS IV (13 patients for MPS IVA; one patient for MPS IVB), participated in the study. They were recruited by referrals from the Departments of Orthopaedics and Genetics at the Nemours/Alfred I. duPont Hospital for Children or fliers distributed through the Carol Ann Foundation. The study was conducted at the Nemours/Alfred I. duPont Hospital for Children, Wilmington, Delaware, after obtaining the informed consent of each participant. Demographic information about the participants is summarized in Table 1. Skeletal (clinical) severity of the disease was determined by the height as described previously [20–22]. Eight of the patients with MPS IVA had received enzyme replacement therapy (ERT) for 1 to 5 years.
Table 1.
Summary of audiological test results and participant demographic and disease information. The key at the bottom shows the color codes used for PTA values, DPOAE, and ABR results.
| Left Ear | Right Ear | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age (years) |
Morquio Type |
Height (cm) |
BMI | ERT | ADL score | Hearing Loss Type |
PTA | DPOAE (% present) |
ABR | Hearing Loss Type |
PTA | DPOAE (% present) |
ABR |
| S1 | 12 | A | 94 | 16 | Y | 36.0 | Conductive | 35 | 17 | Abnormal | Conductive | 25 | 0 | Normal |
| S2 | 15 | B | 135 | 19 | N | 36.0 | Normal | 10 | 100 | Normal | Normal | 5 | 100 | Normal |
| S3 | 17 | A | 115 | 25 | Y | 51.0 | Sensorineural | 50 | 0 | Normal | Sensorineural | 45 | 8 | Normal |
| S4 | 17 | A | 122 | 23 | Y | 45.0 | Sensorineural | 10 | 75 | Abnormal | Normal | 0 | 83 | Normal |
| S5 | 17 | A | 99 | 24 | Y | 52.0 | Mixed | 35 | 0 | Normal | Mixed | 30 | 17 | Normal |
| S6 | 18 | A | 99 | 45 | Y | 50.0 | Mixed | 55 | 0 | Abnormal | Mixed | 50 | 0 | Abnormal |
| S7 | 19 | A | 137 | 24 | N | 47.0 | Sensorineural | 5 | 100 | Abnormal | Sensorineural | 5 | 92 | Abnormal |
| S8 | 21 | A | 114 | 27 | Y | 56.0 | Sensorineural | 15 | DNT | Abnormal | Normal | 20 | DNT | Normal |
| S9 | 22 | A | 122 | 23 | N | 49.0 | Normal | 5 | 92 | Normal | Normal | 5 | 100 | Normal |
| S10 | 24 | A | 97 | 27 | N | 37.0 | Conductive | 65 | 0 | Normal | Conductive | 70 | 0 | Normal |
| S11 | 28 | A | 122 | 22 | Y | 46.5 | Sensorineural | 15 | 50 | Abnormal | Normal | 10 | 75 | Abnormal |
| S12 | 32 | A | 99 | 25 | Y | 55.0 | Mixed | 75 | 0 | Abnormal | Mixed | 90 | 0 | Abnormal |
| S13 | 36 | A | 109 | 27 | N | 55.0 | Mixed | 20 | 33 | Abnormal | Mixed | 15 | 42 | Abnormal |
| S14 | 38 | A | 97 | 25 | N | 55.0 | Mixed | 25 | 8 | Abnormal | Conductive | 15 | 58 | Normal |
ERT: enzyme replacement therapy ADL: Activity of daily living DNT: The test was not administrated.
|
| ||
| PTA | DPOAE | ABR |
|
| ||
| Normal | Present (76-100%) | Normal |
|
Slight to Mild | ||
| Moderateto Moderately Severe | Partially present (26-75%) | – |
|
| ||
| Severe | Absent (0-25%) | Abnormal |
2.2 Procedure
Following otoscopic inspection, middle ear function was assessed by tympanometry and middle ear muscle reflexes (MEMR) (Titan, Interacoustics, Inc). MEMR thresholds were recorded at 0.5, 1, 2, and 4 kHz for those who did not have pressure equalization tubes. Distortion products otoacoustic emissions (DPOAE) were collected from each ear to obtain an objective measure of the function of the cochlear outer hair cells. DPOAE were recorded at 12 different f2 frequencies varying from 1 to 10 kHz (Titan, Interacoustics, Inc). To assess the brainstem nervous pathways, auditory brainstem responses (ABRs) were obtained using 100 μsec air-conduction clicks at a rate of 27.7/s and at levels ranging 40 to 90 dBHL (SmartEP, Intelligent Hearing System, Inc). The responses to the clicks at 80 dBHL or 90 dBHL were reported in this study. To obtain behavioral hearing data, we conducted audiometry and speech perception test. Pure tone air conduction thresholds at 125, 250, 500, 1, 2, 3, 4, 6, 8 kHz were obtained in each ear using insert earphones. All air conduction audiometry was conducted without masking because none of our participants indicated more than 40 dB threshold difference between the ears. Pure tone bone conduction thresholds were obtained for some participants with suspected conductive hearing loss with and without masking. Speech reception thresholds were obtained using spondee words, and word recognition scores were obtained using the Central Institute for the Deaf (CID) W22 word list. These two speech perception tests were conducted using insert earphones without hearing aids. All the audiological data were obtained in a sound proof booth. The participant’s demographic information, medical/health history including frequency of ear infection, and functional activity of daily living (ADL) [23, 24] were obtained with a questionnaire.
2.3 Data analysis
The types of hearing loss (conductive, sensorineural, and mixed) were categorized by an audiologist based on all the tests results. The severity of hearing loss was based on the pure tone average (PTA) values of 0.5, 1, and 2 kHz (per ear) [25].
For each of the 12 frequencies, DPOAE was considered present when the DPOAE amplitude level was −10 dB SPL or above, and the signal to noise ratio at least of 6 dB SPL. The percentage of present DPOAE (per 12 frequencies) was calculated for each ear. Responses were classified as abnormal when only less than 25% of the DPOAE frequencies were present and partially abnormal when the percent was between 26-75%.
The ABR was categorized as normal or abnormal by two experienced clinicians. Waveforms were judged as abnormal when waves I, III, and V were absent, delayed, of low amplitude or with an atypical morphology.
Pearson’s correlation coefficients were obtained to analyze relationships between hearing test results and other measures only with the MPS IVA data. We excluded the MPS IVB data from correlation analysis because the mild skeletal severity associated with MPS IVB could have biased the results.
3 Results
3.1 Ear Infection
All but one participant reported that they had multiple ear infections in the past. Five participants had chronic ear infections. Half of the participants in this study had one or more sets of ear pressure equalization tubes, and two patients (both adults) had pressure equalization tubes at the time of our data collection. Despite the history of frequent ear infections in most patients, only two participants reported speech/language delays when they were young. The results are consistent with previous descriptions of normal speech/language skills in MPS IV. We should point out that recurring middle ear infection in patients with MPS IV persists even after young childhood.
3.2 Audiological test results
Table 1 lists the audiometry results in all participants. All participant exhibited normal tympanometry. All but two participants (S10 and S12) exhibited presence of middle ear reflexes to at least one of the stimuli in both ears. S10 did not exhibit middle ear reflex (at the maximum tested level of 100 dB) in the right ear and S12 exhibited reflexes in the left ear, but we could not obtain conclusive results in her right ear. The degree of hearing loss is indicated by the degree of darkness in the PTA columns in Table 1. Except for one subject (S9) who exhibited normal hearing in both ears, all other subjects with MPS IVA in this study presented with some type of hearing loss. The hearing thresholds varied from normal to severe according to pure-tone audiometry. The type of hearing loss was not uniform among patients, as some presented with conductive hearing loss, some with sensorineural hearing loss, and some with mixed hearing loss. Within each patient, their hearing loss level and type are quite similar in both ears. The patient with MPS IVB (S2) had normal hearing in both ears.
3.3 Speech recognition results
Speech reception thresholds obtained from twelve participants were comparable to the pure tone average thresholds. Mean differences of two thresholds were 5 dB for the left ear and 2 dB for the right ear. Correlations between speech reception thresholds and PTA values were also very strong, rs(10) = 0.96 and 0.91, p < 0.01 for the left and right ears respectively.
3.4 OAE and ABR results
Table 1 also summarises the severity of DPOAE and ABR abnormality in all participants. Over half of the patients tested in this study had abnormal DPOAEs in both ears. Figure 1 shows DPOAE responses of three subjects. The top panel showed normal DPOAE responses to all frequencies tested in subject S7. In the middle panel, DPOAE responses are partially absent, as the subject S13 did not exhibit DPOAE responses to low and high frequency stimuli although the pure tone thresholds were within the normal range. The bottom panel showed almost no DPOAE response to the tested frequencies in the subject S3. Distribution of abnormal DPOAE is quite even among the tested ears although slightly more ears showed absent DPOAE at higher frequencies as seen in Figure 2.
Figure 1.
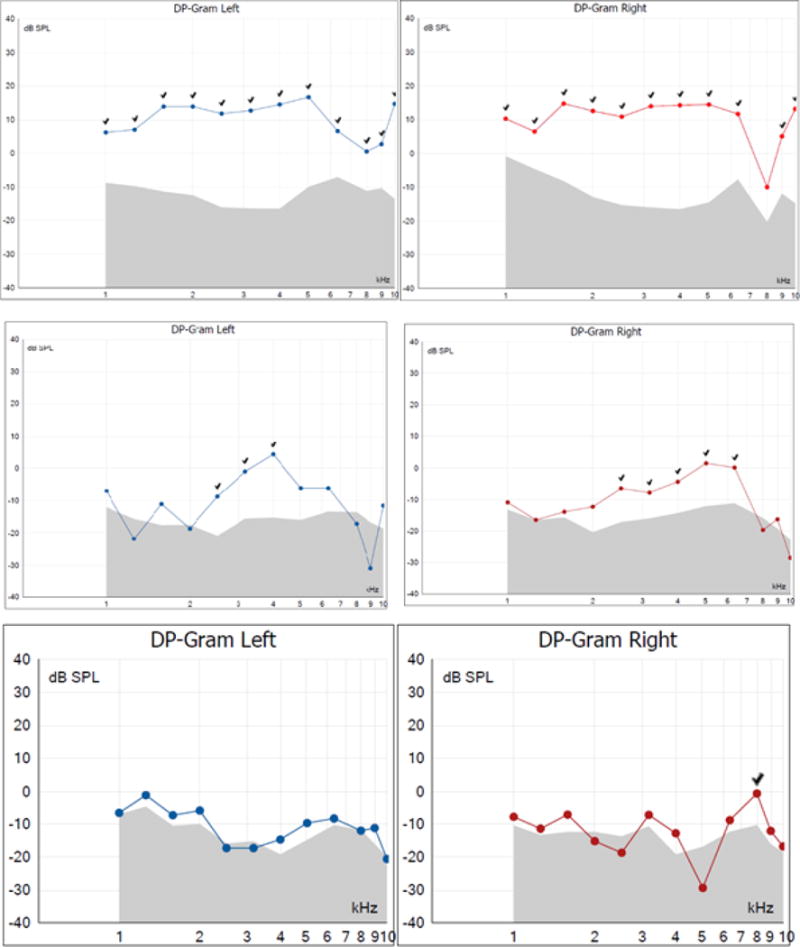
Examples of normal (top) and abnormal (middle and bottom) DPOAE results of the subjects S7, S13, S3. Present DPOAE responses were indicated by check marks in the graphs.
Figure 2.
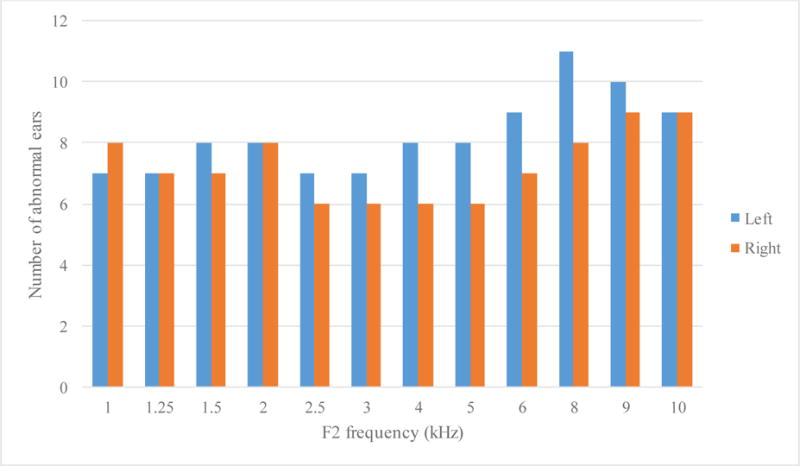
Number of abnormal DPOAE among the 26 tested ears (13 left and 13 right) for each tested tone pair (frequency of the second tone is indicated in the x-axis).
Out of fourteen patients, five patients showed normal ABRs in both ears. Four patients showed normal ABRs in their right ear but abnormal ABRs in their left ear. All others had abnormal ABRs bilaterally. Figure 3 shows examples of abnormal and normal ABR waveforms exhibited from subject S4. His audiometric results indicated normal hearing sensitivity in both ears, but the electrophysiological results suggested some abnormality in the retrocochlear regions of the left ear. His left ear ABR waveform did not exhibit identifiable wave I and wave III. A range of ABR abnormalities were found among the participants as listed in Table 2. Although abnormal ABR results were not consistent among the patients, delayed or absent wave III was found in eleven ears (six patients). Wave V showed delayed responses (six ears in four participants), but was identified bilaterally in all but one patient (S12). The results indicated that despite having normal pure tone thresholds, some patients had abnormal DPOAEs, ABR, or both.
Figure 3.
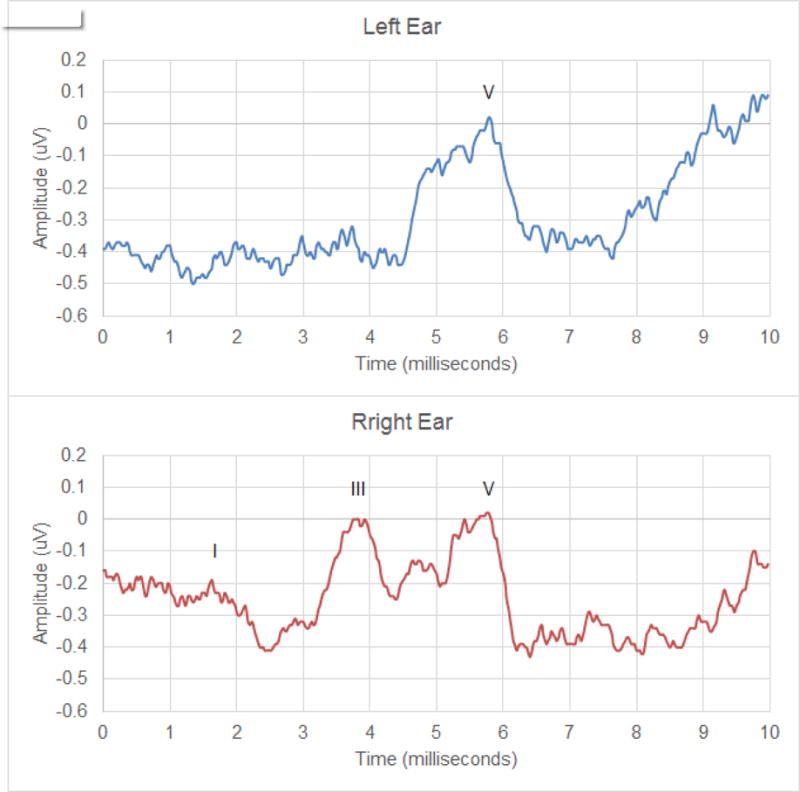
ABRs of Participant S4. Note poor waveform morphology in the left ear (top) compared to the right ear (bottom) despite of the participant’s normal results from audiometry and DPOAE results.
Table 2.
ABR abnormalities in the participants.
| ID | Left ear | Right ear |
|---|---|---|
| S1 | Slightly low amplitude | Normal |
| S2 | Normal | Normal |
| S3 | Normal | Normal |
| S4 | Absent Wave I and III | Normal |
| S5 | Normal | Normal |
| S6 | Delayed Wave III | Delayed Wave III |
| S7 | Delayed Wave I, III, and V | Delayed Wave I, III, and V |
| S8 | Poor wave morphology; low amplitude Delayed Wave V (Borderline) |
Normal |
| S9 | Normal | Normal |
| S10 | Normal | Normal |
| S11 | Delayed Wave III and V | Delayed Wave III and V |
| Poor wave morphology | Poor wave morphology | |
| S12 | Poor wave morphology | Poor wave morphology |
| Absent Wave I, III, and V | Absent Wave I, III, and V | |
| S13 | Delayed Wave III | Delayed Wave III |
| S14 | Delayed Wave V | Normal |
3.5 Relationship between hearing function and skeletal severity
To examine the relationship between hearing function and skeletal severity (height), partial correlations were computed between the current height of the patient and pure tone threshold average (a measure of hearing sensitivity) or DPOAE (a measure of inner ear function). Because height is generally correlated well with age in children, we computed partial correlations while controlling for the effect of age. The skeletal severity strongly correlated with pure tone threshold average for both ears (Right ear: r(10) = −0.59, p < 0.005; Left ear: r(10) = −0.71, p = 0.01). Strong correlations were also found between DPOAE and skeletal severity, rs(9) = −0.84 and −0.87, p < 0.0001 for the right and left ears respectively. Zero-order correlations showed significant correlation results between hearing functions and skeletal severity, indicating that in this study group the age had very little influence in controlling for the relationship between skeletal severity and hearing sensitivity or DPOAE.
3.6 Relationship between hearing function and other measures (body weight, BMI, and ADL)
Both body weight and body mass index (BMI) had no significant correlations with hearing functions, rs = −0.33 to 0.51. To examine the relationship between hearing functions and functional ability to perform ADL, Pearson’s correlation tests were performed on ADL total scores against hearing function. The results showed no correlation between ADL scores and each hearing measure (PTA and DPOAE per ear), rs = −0.11 to 0.12.
3.7 Age-dependency
Some of the younger participants had hearing loss in terms of pure-tone audiometry and abnormal ABR results. Because the current study did not include participants younger than 11 years old, it is not clear if this trend also exists in younger children. Similarly, although partial correlation test results indicated that age is not an influencing factor of the relationship between hearing function and skeletal severity, it is still possible that the hearing-severity relation among younger children with MPS IVA might be not as strong as shown in the current study as permanent hearing loss is usually not identified until adolescence [17].
4 Discussion
In this study, we have demonstrated that 1) patients with MPS IV tend to have chronic ear infections, 2) patients with MPS IVA (except one patient with an attenuated form) exhibit abnormal hearing function bilaterally based on DPOAE and ABR results despite normal audiometric results, 3) there is a strong relationship between skeletal severity (height) and hearing function (based on audiometry and DPOAE) in patients with MPS IVA, indicating that more severely affected patients in skeleton exhibit more severe level of hearing dysfunction, 4) the patient with MPS IVB exhibit normal hearing.
Although we observed that younger participants exhibit some degree of hearing loss, we did not obtain data to determine whether patients younger than 11 years old behave similarly. Since the initial sign and symptom of MPS IVA do not appear at birth except for minor skeletal abnormality, patients are very likely to pass their newborn hearing test. Normal findings of pure tone audiometry during early childhood may hinder the discovery of some abnormal auditory function. Although pure tone audiometry is necessary to assess behavioral hearing thresholds and is commonly used for hearing screening at school, audiometric results alone cannot reveal some inner ear or auditory pathway anomalies such as auditory neuropathy [26]. Some of the consequences of such anomalies might be increased difficulty understanding speech, especially in noisy environment. Although apparent hearing problems may not surface before adolescence [13, 17], it is likely that hearing progressively worsens as the disease progresses. Since in this study we observe normal development of speech and language skills in patients with MPS IV, it is likely that auditory function remains normal during a critical time of language acquisition in their early life. However, patients may start having difficulty in speech perception in certain conditions (such as noisy environment) in early childhood. The finding of a strong correlation between skeletal dysplasia (height) and hearing loss indicates that height may be a good indicator for prognosis of hearing loss. However, it is important to emphasize that patients with MPS IVA should monitor their hearing status regularly, regardless of their skeletal severity, since the cause of sensorineural hearing loss is unknown.
Hearing loss is most likely due to multiple factors [4]. Chronic middle ear infections [7, 27], deformity of the ossicles, and/or abnormalities of the inner ear [4] could lead to hearing loss. Conductive hearing loss is generally attributed to chronic or recurring middle ear dysfunction, which can be associated with frequent upper airway infections. As the disease progresses, depository of glycosaminoglycans (GAGs) in the tympanic membrane, auditory ossicles may cause the ossicular dysfunction. Significant high accumulation of GAGs was found in the middle ear fluid and adenoid tissues among patients with MPS [28, 29].
The current results also indicate that the disease affects the brainstem as well. Since hearing loss progressively worsens and changes from conductive to sensorineural loss as the disease progresses, there may be other factors affecting the inner ear and the brainstem in a rather slow pace in addition to recurring ear infections. Similar progressive sensorineural hearing loss is found in patients with MPS I and II [27], and histopathology results indicated that the number of cochlear hair cells (both inner and outer hair cells) is reduced in the patients with MPS I [30]. Absence of DPOAEs indicates hearing loss resulting from dysfunction of outer hair cells, and decrease and disappearance of wave I of ABRs can indicate less inner hair cells. Our findings of abnormal DPOAEs and ABRs suggest that the loss of inner ear hair cells may play a role of sensorineural hearing loss in the patients with MPS IVA. It is also possible that the vestibular system is also affected by GAG accumulation. Some of the participants reported that they experience migraine headache and tinnitus.
To establish the etiology of hearing loss in in MPS IVA, patients need to be examined longitudinally from the age at MPS IVA diagnosis in childhood until and during adulthood. Imaging of the temporal bones may also refine the hearing loss diagnosis. Our data clearly show that pure tone audiometry should not be the sole test used to assess and manage hearing loss in these patients.
Growth impairment due to accumulation of C6S and KS in cartilage and bone is primary cause of MPS IVA, leading to various skeletal-related manifestations including disproportionate short stature, tracheal obstruction, pectus carinatum, spinal cord compression, deformity of the bones, and conductive hearing loss. Growth (height) correlates or provides an impact with ADL [24], severity of tracheal obstruction [24], KS level in blood and urine [31, 32], and bone mineral density [33]. Therefore, it is likely that the severity of hearing loss and height correlates as well although it would require to collect longitudinal data from childhood to adulthood to demonstrate. Accumulation of more data and successive accurate correlation will define severity and prognosis of hearing loss.
5 Conclusions
Hearing loss is a major concern in patients with MPS IV. Some patients with MPS IVA may have sensorineural hearing impairment despite normal audiometric results. Pure tone audiometry does not identify sensorineural hearing dysfunction when the impairment is mild, leading to a delay of awareness of hearing loss. Therefore, it is the best to conduct regular hearing evaluations involving behavioral and electrophysiological testing from the early stage.
Highlights.
Patients with MPS IVA exhibit abnormal hearing function at the cochlea and auditory brainstem levels despite normal audiometric results
A strong relation was found between hearing function and skeletal severity (height) in MPS IVA
The results indicate various levels and types of hearing loss in patients with MPS IVA
More severely affected patients in skeleton exhibit more severe level of hearing dysfunction
Neurophysiological hearing tests should be conducted regardless of audiometric results to ensure optimal outcome of patients with MPS IV
Acknowledgments
We thank the Carol Ann Foundation and the patients with Morquio and their family members for their contribution to this study. We also thank Dr. William Mackenzie and Coleen Ditro at Nemours/Alfred I. duPont Hospital for Children, Department of Orthopaedics, and Dr. Michael Bober and Angela Duker at Nemours/Alfred I. duPont Hospital for Children, Department of Pediatrics for their support on subject recruitment; Nemours Clinical Research Services Core (NIH Grant #: 2P20RR020173-06A1 and P30GM114736), Kimberly Klipner and Stacey Price in particular, for scheduling and coordinating the research participants, and the Nemours Bioinformatics Core (NIH Grant #: 2P20RR020173-06A1 and P30GM114736), Dr. Suzanne McCahan in particular, for their support on our online database management. We also thank Kimberly Fiorentino and Rachele Sklar for their assistance during data collection. The authors would like to thank the anonymous reviewers for their valuable comments and suggestions to improve the quality of the paper.
Funding: This work was partially supported by the Carol Ann Foundation.
Abbreviation
- MPS
mucopolysaccharidosis
- ABR
auditory brainstem response
- DPOAE
distortion products of otoacoustic emission
- MEMR
middle ear muscle reflex
- PTA
pure tone average
- C6S
chondroitin-6-sulfate
- ERT
enzyme replacement therapy
- GALNS
N-acetylgalactosamine-6-sulfate sulfatase
- KS
keratan sulfate
- GAG
glycosaminoglycan
- ADL
activity of daily living
- CNS
central nervous system
- CT
Computed tomography
- MR images
Magnetic resonance images
Footnotes
Contributions to the project:
Kyoko Nagao is a principal investigator of this project and an expert in pediatric audiology. She has contributed to the concept and planning of the article, collection of previous articles and data, and reporting of the work described.
Thierry Morlet is an expert in auditory neuroscience. He has contributed to the concept of the project, planning, analysis of data, and reporting of the work described in the article.
Elizabeth Haley is an audiologist who has contributed to the data collection, data analysis, and reporting of the work described.
Julianne Nemith has contributed to the planning, data collection, data analysis, and reporting of the work described.
Jennifer Padilla has contributed to the planning, data collection, data analysis, and reporting of the work described.
Robert W. Mason has contributed to the data collection and reporting of the work described. He also contributed to administrative support coordinating the current and other Morquio related projects.
Shunji Tomatsu has 30 years of clinical and research experience in MPS, publishing over 180 articles in this field. He has contributed to the concept of the project, planning, analysis of data, and reporting of the work described in the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
All the authors contributed to the original article and had no conflict of interest with any other party. Kyoko Nagao, Thierry Morlet, Elizabeth Haley, Julianne Nemith, Jennifer Padilla, Robert W. Mason, and Shunji Tomatsu declare that they have no conflict of interests.
References
- 1.Tomatsu S, Montaño AM, Nishioka T, Orii T. Mucopolysaccharidosis IV (Morquio Syndrome; MPS IV) In: Barranger JA, Cabrera-Salazar M, editors. Lysosomal Storage Disorders. Springer US; Boston, MA: 2007. pp. 433–445. [Google Scholar]
- 2.Hendriksz CJ, Harmatz P, Beck M, Jones S, Wood T, Lachman R, Gravance CG, Orii T, Tomatsu S. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol Genet Metab. 2013;110:54–64. doi: 10.1016/j.ymgme.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hecht JT, Scott CI, Jr, Smith TK, Williams JC. Mild manifestations of the Morquio syndrome. Am J Med Genet. 1984;18:369–371. doi: 10.1002/ajmg.1320180222. [DOI] [PubMed] [Google Scholar]
- 4.Tomatsu S, Montaño AM, Oikawa H, Smith M, Barrera L, Chinen Y, Thacker MM, Mackenzie WG, Suzuki Y, Orii T. Mucopolysaccharidosis type IVA (Morquio A disease): Clinical review and current treatment: A special review. Curr Pharm Biotechnol. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- 5.Lavery C, Hendriksz C. Mortality in Patients with Morquio Syndrome A. In: Zschocke J, Gibson KM, Brown G, Morava E, Peters V, editors. JIMD Reports. Vol. 15. Springer Berlin Heidelberg; 2015. pp. 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizarro C, Davies RR, Theroux M, Spurrier EA, Averill LW, Tomatsu S. Surgical Reconstruction for Severe Tracheal Obstruction in Morquio A Syndrome. Ann Thorac Surg. 2016;102:e329–331. doi: 10.1016/j.athoracsur.2016.02.113. [DOI] [PubMed] [Google Scholar]
- 7.Wold SM, Derkay CS, Darrow DH, Proud V. Role of the pediatric otolaryngologist in diagnosis and management of children with mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol. 2010;74:27–31. doi: 10.1016/j.ijporl.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Mesolella M, Cimmino M, Cantone E, Marino A, Cozzolino M, Della Casa R, Parenti G, Iengo M. Management of otolaryngological manifestations in mucopolysaccharidoses: our experience. Acta Otorhinolaryngol Ital. 2013;33:267–272. [PMC free article] [PubMed] [Google Scholar]
- 9.Montano A, Tomatsu S, Gottesman G, Smith M, Orii T. International Morquio A Registry: Clinical manifestation and natural course of Morquio A disease. Journal of Inherited Metabolic Disease. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 10.Giugliani R, Harmatz P, Wraith JE. Management guidelines for mucopolysaccharidosis VI. Pediatrics. 2007;120:405–418. doi: 10.1542/peds.2006-2184. [DOI] [PubMed] [Google Scholar]
- 11.Lin HY, Shih SC, Chuang CK, Lee KS, Chen MR, Lin HC, Chiu PC, Niu DM, Lin SP. Assessment of hearing loss by pure-tone audiometry in patients with mucopolysaccharidoses. Mol Genet Metab. 2014;111:533–538. doi: 10.1016/j.ymgme.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Ruckenstein MJ, Macdonald RE, Clarke JT, Forte V. The management of otolaryngological problems in the mucopolysaccharidoses: a retrospective review. J Otolaryngol. 1991;20:177–183. [PubMed] [Google Scholar]
- 13.Riedner ED, Levin LS. Hearing patterns in Morquio’s syndrome (mucopolysaccharidosis IV) Archives of otolaryngology. 1977;103:518–520. doi: 10.1001/archotol.1977.00780260048003. [DOI] [PubMed] [Google Scholar]
- 14.Kasmann-Kellner B, Weindler J, Pfau B, Ruprecht KW. Ocular changes in mucopolysaccharidosis IV A (Morquio A syndrome) and long-term results of perforating keratoplasty Ophthalmologica. Journal international d’ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde. 1999;213:200–205. doi: 10.1159/000027420. [DOI] [PubMed] [Google Scholar]
- 15.Motamed M, Thorne S, Narula A. Treatment of otitis media with effusion in children with mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol. 2000;53:121–124. doi: 10.1016/s0165-5876(00)00320-7. [DOI] [PubMed] [Google Scholar]
- 16.Gokdogan C, Altinyay S, Gokdogan O, Tutar H, Gunduz B, Okur I, Turner L, Kemaloglu YK. Audiologic evaluations of children with mucopolysaccharidosis. Braz J Otorhinolaryngol. 2016;82:281–284. doi: 10.1016/j.bjorl.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendriksz CJ, Al-Jawad M, Berger KI, Hawley SM, Lawrence R, Mc Ardle C, Summers CG, Wright E, Braunlin E. Clinical overview and treatment options for non-skeletal manifestations of mucopolysaccharidosis type IVA. J Inherit Metab Dis. 2013;36:309–322. doi: 10.1007/s10545-012-9459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien JS, Gugler E, Giedion A, Wiessmann U, Herschkowitz N, Meier C, Leroy J. Spondyloepiphyseal dysplasia, corneal clouding, normal intelligence and acid beta-galactosidase deficiency. Clin Genet. 1976;9:495–504. doi: 10.1111/j.1399-0004.1976.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 19.Arbisser AI, Donnelly KA, Scott CI, Jr, DiFerrante N, Singh J, Stevenson RE, Aylesworth AS, Howell RR. Morquio-like syndrome with beta galactosidase deficiency and normal hexosamine sulfatase activity: mucopolysacchariodosis IVB. Am J Med Genet. 1977;1:195–205. doi: 10.1002/ajmg.1320010205. [DOI] [PubMed] [Google Scholar]
- 20.Montano AM, Tomatsu S, Brusius A, Smith M, Orii T. Growth charts for patients affected with Morquio A disease. Am J Med Genet A. 2008;146A:1286–1295. doi: 10.1002/ajmg.a.32281. [DOI] [PubMed] [Google Scholar]
- 21.Tomatsu S, Montaño AM, Oikawa H, Giugliani R, Harmatz P, Smith M, Suzuki Y, Orii T. Impairment of Body Growth in Mucopolysaccharidoses. In: Preedy VR, editor. Handbook of Growth and Growth Monitoring in Health and Disease. Springer New York; New York, NY: 2012. pp. 2091–2117. [Google Scholar]
- 22.Tomatsu S, Montaño AM, Lopez P, Trandafirescu G, Gutierrez MA, Oikawa H, Nishioka T, Vieira MB, Orii T, Noguchi A. Determinant factors of spectrum of missense variants in mucopolysaccharidosis IVA gene. Mol Genet Metab. 2006;89:139–149. doi: 10.1016/j.ymgme.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Tanjuakio J, Suzuki Y, Patel P, Yasuda E, Kubaski F, Tanaka A, Yabe H, Mason RW, Montano AM, Orii KE, Orii KO, Fukao T, Orii T, Tomatsu S. Activities of Daily Living in patients with Hunter syndrome: Impact of enzyme replacement therapy and hematopoietic stem cell transplantation. Mol Genet Metab. 2015;114:161–169. doi: 10.1016/j.ymgme.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasuda E, Suzuki Y, Shimada T, Sawamoto K, Mackenzie WG, Theroux MC, Pizarro C, Xie L, Miller F, Rahman T, Kecskemethy HH, Nagao K, Morlet T, Shaffer TH, Chinen Y, Yabe H, Tanaka A, Shintaku H, Orii KE, Orii KO, Mason RW, Montaño AM, Fukao T, Orii T, Tomatsu S. Activity of daily living for Morquio A syndrome. Mol Genet Metab. 2016;118:111–122. doi: 10.1016/j.ymgme.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark JG. Uses and abuses of hearing loss classification. Asha. 1981;23:493–500. [PubMed] [Google Scholar]
- 26.Berlin CI. Auditory neuropathy. Current Opinion in Otolaryngology & Head and Neck Surgery. 1998;6:325–329. [Google Scholar]
- 27.Simmons MA, Bruce IA, Penney S, Wraith E, Rothera MP. Otorhinolaryngological manifestations of the mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol. 2005;69:589–595. doi: 10.1016/j.ijporl.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Gonuldas B, Yilmaz T, Sivri HS, Gucer KS, Kilinc K, Genc GA, Kilic M, Coskun T. Mucopolysaccharidosis: Otolaryngologic findings, obstructive sleep apnea and accumulation of glucosaminoglycans in lymphatic tissue of the upper airway. Int J Pediatr Otorhinolaryngol. 2014;78:944–949. doi: 10.1016/j.ijporl.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Fujitani T, Kimura A, Inoue K, Okada S. Pathological and biochemical study in the adenoid of mucopolysaccharidosis II. Int J Pediatr Otorhinolaryngol. 1985;10:205–212. doi: 10.1016/s0165-5876(85)80066-5. [DOI] [PubMed] [Google Scholar]
- 30.Kariya S, Schachern PA, Nishizaki K, Paparella MM, Cureoglu S. Inner ear changes in mucopolysaccharidosis type I/Hurler syndrome. Otol Neurotol. 2012;33:1323–1327. doi: 10.1097/MAO.0b013e3182659cc3. [DOI] [PubMed] [Google Scholar]
- 31.Tomatsu S, Montaño AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Kida K, Kubota M, Barrera L, Orii T. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J Inherit Metab Dis. 2010;33(Suppl 3):S35–42. doi: 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]
- 32.Tomatsu S, Okamura K, Taketani T, Orii KO, Nishioka T, Gutierrez MA, Velez-Castrillon S, Fachel AA, Grubb JH, Cooper A, Thornley M, Wraith E, Barrera LA, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Noguchi A. Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA. Pediatr Res. 2004;55:592–597. doi: 10.1203/01.PDR.0000113767.60140.E9. [DOI] [PubMed] [Google Scholar]
- 33.Kecskemethy HH, Kubaski F, Harcke HT, Tomatsu S. Bone mineral density in MPS IV A (Morquio syndrome type A) Mol Genet Metab. 2016;117:144–149. doi: 10.1016/j.ymgme.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


