Abstract
Both non-alcoholic steatohepatitis (NASH) and alcoholic hepatitis (AH) can lead to cirrhosis and hepatocellular carcinoma. However, the rate of progression to cirrhosis and tumorigenesis in AH is greater than that in NASH. We asked whether there are differences between the two conditions in the expression levels of proteins involved in the pathogenesis of hepatocellular carcinoma. The proteins tested were presented at the 2017 American Association for the Study of Liver Diseases (AASLD) Liver Meeting as overexpressed in hepatocellular carcinoma: KLF4, SCL19A1, FANCG, HRH-1, DNMT1, DNMT3B, TNFR2, DUSP4, EGFR, Integrin α6, HDACII, PDE3A, BCL-XL, and MTCO2. The expression of these proteins was measured in liver biopsy sections from NASH and AH patients using immunohistochemical staining with fluorescent antibodies and then quantifying the fluorescence intensity morphometrically. In AH patients, levels of all tested proteins except HRH-1 were elevated compared to normal patients. In NASH patients, KLF4, SCL19A1, FANCG, HDACII, BCL-XL levels were increased compared to normal controls while HRH-1, DNMT1 and PDE3A levels were decreased. The relative expression of all proteins studied except BCL-XL was significantly higher in AH compared to NASH. In conclusion, proteins involved in hepatocellular cancer development are more highly expressed in AH compared to NASH and normal liver, which corresponds with the higher rate of tumorigenesis in AH patients compared to NASH patients.
Introduction
Non-alcoholic steatohepatitis (NASH) and alcoholic hepatitis (AH) can both lead to cirrhosis and hepatocellular carcinoma, but the rate of progression to these disease stages differs. NASH leads to cirrhosis at a rate of 7–10% annually (Samala et al., 2017) whereas AH progresses to cirrhosis at a rate of 10–20% annually (Schwartz et al., 2012; Dam-Larsen et al., 2004). Once AH and NASH patients develop cirrhosis, the five-year development of hepatocellular carcinoma is approximately 10–12% (Kodama et al 2013), although hepatocellular carcinoma can develop even in the absence of cirrhosis (Cholankeril et al., 2017).
At the 2017 American Association for the Study of Liver Diseases (AASLD) liver meeting, several genes were described as overexpressed in hepatocellular carcinoma: KLF4, SCL19A1, FANCG, HRH-1, DNMT1, DNMT3B, TNFR2, DUSP4, EGFR, Integrin α6, HDACII, PDE3A. These genes have a diverse array of functions. KLF4 (Kruppel-like factor 4) is a zinc-finger containing transcription factor with functions in self-renewal, proliferation, differentiation, apoptosis and cell cycle arrest (Ding et al., 2015). It is downregulated in gastric, esophageal, prostate and lung cancers (Choi et al., 2006), consistent with its role in growth inhibition and cell cycle arrest. However, KLF4 can maintain telomerase activity in normal and cancer stem cells, suggesting a role for upregulation of this gene in tumorigenesis (Wong et al., 2010).
SCL19A1 is responsible for transporting reduced folate into all cells (Zhao et al., 2013). It is ubiquitously expressed in human tumors and is also the uptake site for antifolate chemotherapy such as methotrexate (Goldman et al., 2010).
FANCG is part of a multi-protein complex involved in recognition or repair of DNA interstrand crosslinks and other forms of DNA damage. (Matthew 2006). Germline mutations of this gene are associated with Fanconi anemia, a recessive disorder characterized by chromosomal fragility, aplastic anemia, and high risk of leukemia and solid tumors (Alter 2003). Sporadic mutations of this gene have also been found in a pancreatic cancer cell line (van der Heijden et al., 2004).
HRH-1 (Histamine Receptor H1) is expressed in a wide variety of tissues and is involved in allergic processes and autoimmune disease (Wang et al., 2014). It is also involved in cell proliferation and can stimulate proliferation of breast carcinoma, melanoma, and astrocytoma tumor cells (Davio et al., 1995).
DNMT1 and DNMT3B are both DNA methyltransferases. Loss of DNMT1 in murine hepatic progenitor cells leads to severe DNA damage, cell cycle arrest, senescence and death (Kaji et al, 2016). Loss of DNMT1 also promotes tumorigenesis and metastasis in prostate cancer cells (Lee et al., 2016). DNMT3B loss inhibits proliferation, migration, and invasion in hepatocellular carcinoma cell lines (Wang et al., 2015). It has been previously noted that livers of patients with alcoholic hepatitis have increased DNA methylation (Shen et al., 2015).
TNFR2 is a receptor for tumor necrosis factor (TNF) expressed mainly in hematopoietic and endothelial cells. It has anti-inflammatory and immunoregulatory functions, and is also expressed in many tumors such as colorectal carcinoma (Zhao et al., 2017; Vanamee and Faustman, 2017).
DUSP4 (dual specificity protein phosphatase 4) is a negative regulator of the MAP kinase pathway. Expression of DUSP4 is higher in colorectal cancer tissue compared to normal mucosa, and decreased levels of DUSP4 expression are associated with advanced disease and metastasis (Saigusa et al., 2013).
EGFR (epidermal growth factor receptor) promotes cell growth and division; hepatocellular carcinoma proliferation and metastasis were increased by stimulation with epidermal growth factor (Huang et al, 2013). EGFR-targeted doxorubicin can suppress growth of hepatocellular carcinoma transplanted into mice (Fang et al., 2017).
Integrin α6 (CD49f) is a receptor for laminin on platelets and epithelial cells and is a component of the hemidesmosome. It is overexpressed in a variety of cancers including breast (Cariata et al., 2007), esophageal (Kwon et al., 2013) and hepatocellular carcinoma (Begum et al., 1995).
HDACII (histone deacetylase II) removes acetyl groups from histones, leading to tighter winding of DNA around histones and transcriptional suppression (Chen et al., 2015). HDACII is overexpressed in hepatocellular carcinoma and is associated with poor survival in low-grade and early-grade tumors (Quint et al., 2011).
PDE3A (phosphodiesterase 3A) hydrolyzes both cAMP and cGMP and regulates the signaling activity of these molecules. It is overexpressed in several cancer cell lines including lung adenocarcinoma, cervical carcinoma and melanoma (de Waal et al., 2016).
BCL-XL is an inhibitor of the apoptotic pathway whose overexpression can promote cell survival. It is overexpressed in hepatocellular carcinoma (Watanabe et al., 2002) and a higher expression level of BCL-XL correlates with poorer prognosis (Watanabe et al., 2004).
MTCO2 (mitochondrially encoded cytochrome c oxidase II) is a critical component of the electron transport chain for aerobic respiration. It is downregulated in many tumors and may play a role in the transition of these cancers from aerobic to glycolytic pathways (Masri et al., 2015).
Methods
Formalin-fixed paraffin-embedded biopsies of 5 AH liver, 4 NASH liver, and 3 normal liver were obtained from Harbor-UCLA Medical Center and from the Long Beach Veterans Affairs’ clinical trial in treatment of alcoholic hepatitis. The study was designated as exempt by our institutional review board and data was analyzed anonymously. For each protein studied, the biopsies were stained first with protein-specific antibody followed by a secondary fluorescence antibody. Either donkey-anti mouse or anti rabbit Alex Fluor (Jackson Labs, West Grove, PA) were used as the second antibody (Table 1). The slides were also stained for ubiquitin using Texas Red (Millipore, Temecula, CA) and nuclear stained by DAPI. The staining of all the samples was done at the same time to provide accurate comparison between groups.
Table 1.
Sources of antibodies used including the host animal and supplier.
| Antibody | Host animal | Company supplier | |
|---|---|---|---|
| KLF4 | Rabbit | Abcam | Cambridge, MA |
| SCL19A1 | Rabbit | Abcam | Cambridge, MA |
| FANCG | Rabbit | Abcam | Cambridge, MA |
| HRH-1 | Rabbit | Abcam | Cambridge, MA |
| DNMT1 | Rabbit | Abcam | Cambridge, MA |
| DNMT3B | Rabbit | Abcam | Cambridge, MA |
| TNFR2 | Rabbit | LS Bio | Seattle, WA |
| DUSP4 | Rabbit | Abcam | Cambridge, MA |
| EGFR | Rabbit | Abcam | Cambridge, MA |
| Integrin | Rabbit | Abcam | Cambridge, MA |
| HDACII | Rabbit | LS Bio | Seattle, WA |
| PDE3A | Rabbit | Abcam | Cambridge, MA |
| BCL-XL | Rabbit | Abcam | Cambridge, MA |
| MTCO2 | Rabbit | Abcam | Cambridge, MA |
For each protein, we measured the intensity of the fluorescent staining in three different areas on each slide with 40x magnifications and 800ms standard exposure time by using a Nikon 400 fluorescent microscope. On each slide area, 10 peak fluorescence intensities were measured. The Nikon morphometric system was used to quantitate the florescent intensity. The mean, standard error, and statistical differences of data achieved from the Nokia were analyzed by Graph pad statistical software. Controls vs AH, controls vs NASH, and AH vs NASH were compared by unpaired t -test with a p-value of <0.05 considered statistically significant.
Results
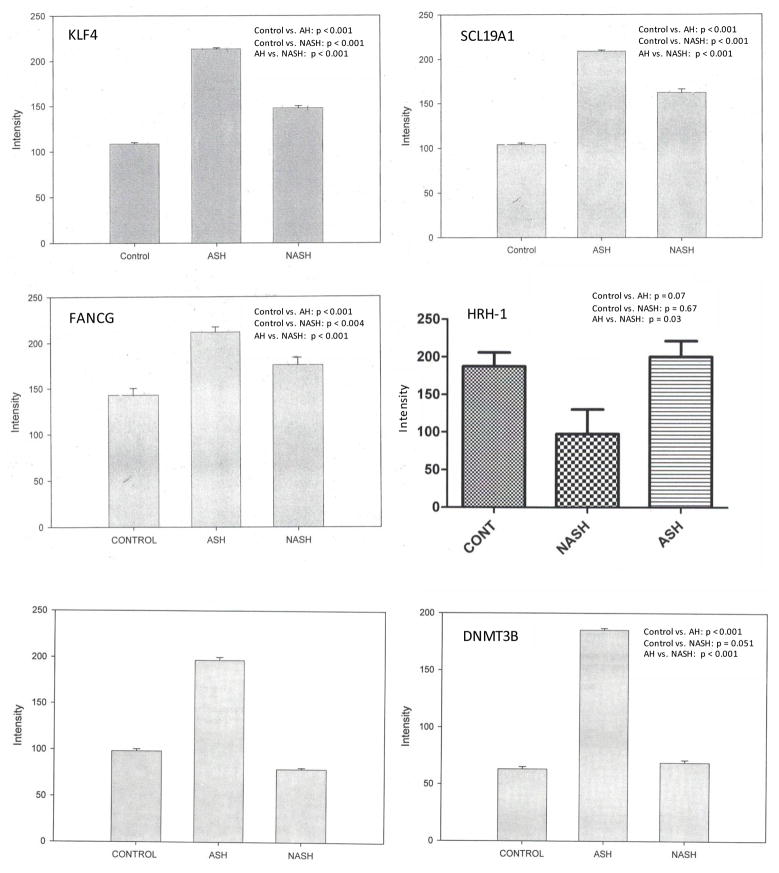
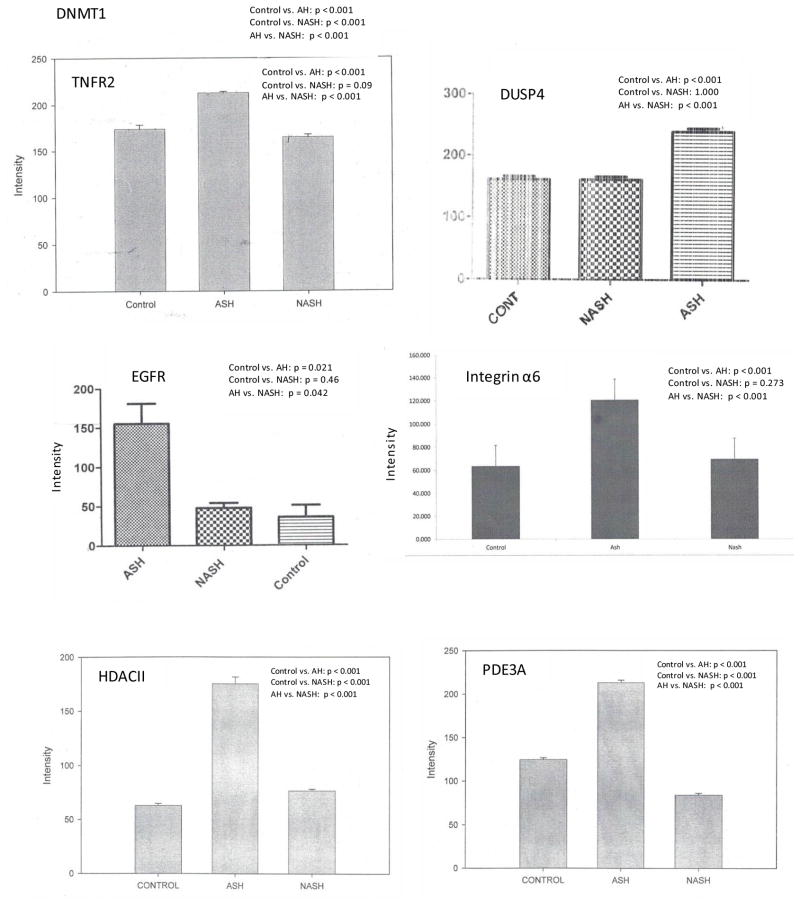
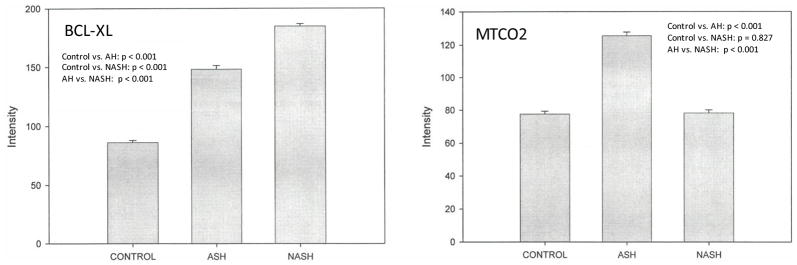
We compared expression of several genes in patients with AH, NASH, and normal controls. Representative data are shown in Figure 1. In AH patients, levels of all tested proteins except HRH-1 were elevated compared to normal patients (Figure 2, Table 2). In NASH patients, KLF4, SCL19A1, FANCG, DNMT1, DNMT3B, and HDACII levels were increased compared to normal controls while HRH-1 and PDE3A levels were decreased (Figure 2). The relative expression of all proteins studied except DNMT-1 was higher in AH compared to NASH (Table 2).
Figure 1.
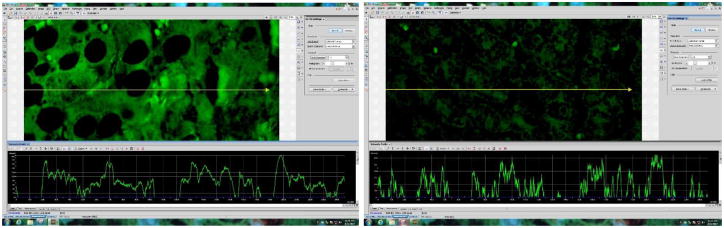
Representative images of fluorescence intensity to measure integrin alpha-6 expression in AH liver (A) and normal control (B). A line is drawn through the image to yield a fluorescence intensity graph; the intensity of the ten highest peaks are measured, excluding nuc lear regions which are highlighted by DAPI (not shown). Three areas per slide are measured in this manner.
Figure 2.
Level of expression of proteins upregulated in hepatocellular carcinoma in AH, NASH or normal controls. Expression is measured as fluorescence intensity and displayed as mean +/− standard deviation.
Table 2.
Relative expression of genes upregulated in hepatocellular carcinoma in AH, NASH, and normal controls. Relative expression is either increased (↑), decreased (↓) or unchanged (no arrow).
| ASH/normal liver | NASH/normal liver | ASH/NASH | |
|---|---|---|---|
| KLF4 | ↑ | ↑ | ↑ |
| SCL19A1 | ↑ | ↑ | ↑ |
| FANCG | ↑ | ↑ | ↑ |
| HRH-1 | ↓ | ↑ | |
| DNMT1 | ↑ | ↓ | ↑ |
| DNMT3B | ↑ | ↑ | |
| TNFR2 | ↑ | ↑ | |
| DUSP4 | ↑ | ↑ | |
| EGFR | ↑ | ↑ | |
| Integrin | ↑ | ↑ | |
| HDACII | ↑ | ↑ | ↑ |
| PDE3A | ↑ | ↓ | ↑ |
| BCL-XL | ↑ | ↑ | ↓ |
| MTCO2 | ↑ | ↑ |
Discussion and Conclusion
In the seminal paper “The Hallmarks of Cancer,” Hanahan and Weinberg described six common characteristics of malignant cells: sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis (Hanahan and Weinberg, 2000). More recently they have added additional characteristics including tumor-promoting inflammation, avoiding immune destruction, and deregulating cellular energetics (Hanahan and Weinberg, 2011).
The gene products studied all correspond to one of these characteristics. KLF4, EGFR, and HRH-1 have roles in proliferative signaling, whereas DUSP4 has tumor suppressor function. FANCG, DNMT1, DNMT3B, and HDACII are involved in genome stability and epigenetic regulation. KLF4 also can promote replicative immortality while PDE3A is involved in angiogenesis. SCL19A1 is a folate transporter whose increased expression suggests an increased replicative requirement. MTCO2 overexpression suggests metabolic derangements, while BCL-XL protects against apoptosis.
Interestingly, nearly all of these proteins are overexpressed in alcoholic hepatitis compared to normal controls. The increased expression of genes promoting proliferation such as EGFR can lead to increased cellular replication. Increased expression of tumor suppressors and epigenetic regulators may correspond to loss-of-function mutations in these protective genes. In contrast, only some of the genes studied are overexpressed in non-alcoholic steatohepatitis patients, which suggests a lower rate of carcinogenesis in these patients compared to alcoholic hepatitis. The increased relative expression of nearly all genes studied in AH versus NASH supports this observation. This is also in keeping with previous work showing differential expression of genes in the Molecular Mechanisms of Cancer Pathway in NASH and AH patients (Nguyen et al., 2018).
In conclusion, proteins overexpressed in hepatocellular carcinoma are more highly expressed in AH compared to NASH and normal liver, which corresponds with the higher rate of progression to cirrhosis and tumorigenesis in AH patients compared to NASH patients. Further research is needed to fully elucidate the mechanisms for progression from AH and NASH to cirrhosis and hepatocellular carcinoma.
Acknowledgments
This study was funded by NIH/AAA grant # UO-21898-05.
Part of the study has been submitted for a poster presentation at Experimental Biology in San Diego in 2018.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter BP. Cancer in Fanconi anemia. Cancer. 2003;97:425–440. doi: 10.1002/cncr.11046. [DOI] [PubMed] [Google Scholar]
- Begum NA, Mori M, Matsumata T, Takenaka K, Sugimachi K, et al. Differential display and integrin alpha 6 messenger RNA overexpression in hepatocellular carcinoma. Hepatology. 1995;22:1447–1455. doi: 10.1002/hep.1840220518. [DOI] [PubMed] [Google Scholar]
- Cariati M, Naderi A, Bron JP, Smalley MJ, Pinder SE, et al. Alpha-6 integrin is necessary for tumourigenicity of a stem-cell like subpopulation within the MCF7 breast cancer line. Int J Cancer. 2007;122:298–304. doi: 10.1002/ijc.23103. [DOI] [PubMed] [Google Scholar]
- Chen HP, Zhao YT, Zhao TC. Histone deacetylases and mechanisms of regulation of gene expression. Crit Rev Oncog. 2015;20:35–47. doi: 10.1615/critrevoncog.2015012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BJ, Cho YG, Song JW, et al. Altered expression of the KLF4 in colorectal cancers. Pathol Res Pract. 2006;202:585–9. doi: 10.1016/j.prp.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Cholankeril G, Patel R, Khurana S, Satapathy SK. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Current knowledge and implications for management. World J Hepatol. 2017;9:533–543. doi: 10.4254/wjh.v9.i11.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750–755. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davio C, Baldi A, Mladovan A, Cricco G, Fitzsimons C, et al. Expression of histamine receptors in different cell lines derived from mammary gland and human breast carcinomas. Inflamm Res. 1995;44:S70–S71. doi: 10.1007/BF01674401. [DOI] [PubMed] [Google Scholar]
- De Waal L, Lewis TA, Rees MG, Tsherniak A, Wu X, et al. Identification of cancer cytotoxic modulators of PDE3A by predictive chemogenomics. Nat Chem Biol. 2016;12:102–108. doi: 10.1038/nchembio.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Liu P, Liu W, Sun P, Wang CL. Emerging roles of Kruppel-like factor 4 in cancer and cancer stem cells. Asian Pac J Cancer Prev. 2015;16:3629–3633. doi: 10.7314/apjcp.2015.16.9.3629. [DOI] [PubMed] [Google Scholar]
- Fang Y, Yang W, Cheng L, Meng F, Zhang J, et al. EGFR-targeted multifunctional polymersomal doxorubicin induces selective and potent suppression of orthotopic human liver cancer in vivo. Acta Biomater. 2017;64:323–333. doi: 10.1016/j.actbio.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Goldman ID, Chattopadhyay S, Zhao R, Moran RG. The Antifolates: Evolution, New Agents in the Clinic, and How Targeting Delivery Via Specific Membrane Transporters Is Driving the Development of a Next Generation of Folate Analogs. Curr Opin Investig Drugs. 2010;11:1409–1423. [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Huang P, Xu X, Wang L, Zhu B, Wang X, Xia J. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J Cell Mol Med. 2014;18:218–30. doi: 10.1111/jcmm.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K, Factor VM, Andersen JB, Durkin ME, Tomokuni A, et al. DNMT1 is a required genomic regulator for murine liver histogenesis and regeneration. Hepatology. 2016;64:582–98. doi: 10.1002/hep.28563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama K, Tokushige K, Hashimoto E, Taniai M, Shiratori K. Hepatic and extrahepatic malignancies in cirrhosis caused by nonalcoholic steatohepatitis and alcoholic liver disease. Alcohol Clin Exp. 2013;37(Suppl 1):E247–52. doi: 10.1111/j.1530-0277.2012.01900.x. [DOI] [PubMed] [Google Scholar]
- Kwon J, Lee TS, Lee HW, Kang MC, Yoon HY, et al. Integrin alpha 6: a novel therapeutic target in esophageal squamous cell carcinoma. Int J Oncol. 2013;43:1523–1530. doi: 10.3892/ijo.2013.2097. [DOI] [PubMed] [Google Scholar]
- Lee E, Wang J, Yumoto K, Jung Y, Cackowski FC, et al. DNMT1 regulates epithelial-mesenchymal transition and cancer stem cells, which promotes prostate cancer metastasis. Neoplasia. 2016;18:553–566. doi: 10.1016/j.neo.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri MA, Elhassan NM, Mohammed HS, Wasunna C, Abukashawa S, et al. In-vitro “depletion” of mitochondrial cytochrome C oxidase subunit II affects the patterns of gene expression across multiple cancer pathways. J Mol Biomark Diagn. 2015;S2:011. [Google Scholar]
- Matthew CG. Fanconi anaemia genes and susceptibility to cancer. Oncogene. 2006;25:5875–84. doi: 10.1038/sj.onc.1209878. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Masouminia M, Menodza A, Samadzadeh S, Tillman B, et al. Alcoholic hepatitis versus non-alcoholic steatohepatitis: levels of expression of some proteins involved in tumorigenesis. Exp Mol Path. 2018;104:45–49. doi: 10.1016/j.yexmp.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint K, Agaimy A, Di Fazio P, Montalbano R, Steindorf C, et al. Clinical Significance of Histone Deacetylases 1, 2, 3, and 7: HDAC2 Is an Independent Predictor of Survival in HCC. Virchows Archiv. 2011;459:129–139. doi: 10.1007/s00428-011-1103-0. [DOI] [PubMed] [Google Scholar]
- Saigusa S, Inoue Y, Tanaka K, Toiyama Y, Okugawa Y, et al. Decreased expression of DUSP4 is associated with liver and lung metastases in colorectal cancer. Med Oncol. 2013;30:620. doi: 10.1007/s12032-013-0620-x. [DOI] [PubMed] [Google Scholar]
- Scaglioni F, Ciccia S, Marino M, Bedogni G, Bellantani S. ASH and NASH. Digestive Diseases. 2011;29:202–10. doi: 10.1159/000323886. [DOI] [PubMed] [Google Scholar]
- Shen H, French BA, Tillman BC, Jun L, et al. Increased DNA methylation in the livers of patients with alcoholic hepatitis. Exp Mol Pathol. 2015;99:326–29. doi: 10.1016/j.yexmp.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden MS, Brody JR, Gallmeier E, Cunningham SC, Dezentje DA, et al. Functional defects in the Fanconi anemia pathway in pancreatic cancer cells. Am J Pathol. 2004;165:651–657. doi: 10.1016/S0002-9440(10)63329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanamee ES, Faustman DL. TNFR2: A novel target for cancer immunotherapy. Trends Mol Med. 2017;23:1037–46. doi: 10.1016/j.molmed.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang Z, Fan Y, Si Y, Wang J. DNA methyltransferase 3b silencing affects locus-specific DNA methylation and inhibits proliferation, migration and invasion in human hepatocellular carcinoma SMMC-7721 and BEL-7402 cells. Oncol Lett. 2015;9:2499–4506. doi: 10.3892/ol.2015.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wei X, Shi L, Chen B, Zhao G, et al. Integrative genomic analyses of the histamine H1 receptor and its role in cancer prediction. Int J Mol Med. 2014;33:1019–26. doi: 10.3892/ijmm.2014.1649. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Kushihata F, Honda K, Mominoki K, Matsuda S, et al. Bcl-xL overexpression in hepatocellular carcinoma. Int J Oncol. 2002;21:515–9. [PubMed] [Google Scholar]
- Watanabe J, Kushihata F, Honda K, Sugita A, Tateishi N, et al. Prognostic significance of Bcl-xL in human hepatocellular carcinoma. Surgery. 2004;135:604–612. doi: 10.1016/j.surg.2003.11.015. [DOI] [PubMed] [Google Scholar]
- White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359. e2. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, Hou PS, Tseng SF, Chien CL, Wu KJ, et al. Kruppel-like transcription factor 4 contributes to maintenance of telomerase activity in stem cells. Stem Cells. 2010;28:1510–7. doi: 10.1002/stem.477. [DOI] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. Folate and Thiamine Transporters mediated by Facilitative Carriers (SLC19A1-3 and SLC46A1) and Folate Receptors. Mol Aspects Med. 2013;34 doi: 10.1016/j.mam.2012.07.006. 10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Li H, Liu Z. Tumor necrosis factor receptor 2 promotes growth of colorectal cancer via the PI3K/AKT signaling pathway. Oncol Letters. 2017;13:342–346. doi: 10.3892/ol.2016.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]