Abstract
Objective
Antibodies against citrullinated fibrinogen (anti-cit-fibrinogen) have been implicated in both rheumatoid arthritis (RA) and cardiovascular (CV) risk in RA. The objective of this study was to examine the association between anti-cit-fibrinogens and coronary artery disease (CAD) outcomes.
Methods
We performed the study in an RA cohort based in a large academic institution linked with electronic medical record data (EMR) containing data on CAD outcomes from medical record review. Using a published bead assay method, we measured 10 types of anti-cit-fibrinogens. We applied a score test to determine the association between the anti-cit-fibrinogens as a group with CAD outcomes. Principal components analysis (PCA) was performed to assess whether the anti-cit-fibrinogens clustered into groups. Each group was then also tested for association with CAD. Sensitivity analyses were also performed using a published ICD9 code group for ischemic heart disease (IHD) as the outcome.
Results
We studied 1,006 RA subjects with mean age 61.0 (SD 13.0) years and 72.2% anti-CCP positive. As a group, anti-cit-fibrinogen was associated with CAD (p=1.1×10−4). From the PCA analysis, we observed 3 main groups, of which only one group, containing 7 of the 10 anti-cit-fibrinogens, was significantly associated with CAD outcomes (p=0.015). In the sensitivity analysis, all anti-cit-fibrinogens as a group remained significantly associated with IHD (p=2.9×10−4).
Conclusion
Anti-cit-fibrinogen antibodies as a group were associated with CAD outcomes in our RA cohort, with the strongest signal for association arising from a subset of the autoantibodies.
INTRODUCTION
Cardiovascular disease (CVD) is a leading cause of death for patients with rheumatoid arthritis (RA)(1). Several studies have demonstrated that traditional CV risk calculators significantly underestimate CV risk in RA(2). These findings have motivated studies to examine whether biomarkers can assist rheumatologists in identifying RA patients at elevated CV risk. Anti-citrullinated protein antibodies (ACPAs) are used in the diagnosis of RA, and recent studies have identified epitope targets several epitope targets implicated in the disease(3–5). A specific class of ACPAs, autoantibodies targeting citrullinated fibrinogen (anti-cit-fibrinogen) have been studied as potential markers for cardiovascular disease (CVD) in RA(6).
In a previously published study, Sokolove an colleagues observed that protein lysates of atherosclerotic plaques from aortic specimens were enriched for citrullinated fibrinogen (cit-fibrinogen)(6). Further, they found that RA patients with higher titers of anti-cit-fibrinogens also had higher atherosclerotic burden as measured by coronary artery calcium (CAC) scores. These data suggested a mechanistic pathway whereby inflammation promoted cit-fibrinogen in atherosclerotic plaques, and thus the development of anti-cit-fibrinogen may be a useful marker of inflammation, atherosclerotic burden and CV risk in RA. Interestingly, a subsequent study from an independent cohort using a different platform to measure anti-cit-fibrinogen found no association between carotid intima media thickness or CAC, and titers of anti-cit-fibrinogen(7).
Since previous studies have only examined surrogate CV outcomes, the objective of this study was to examine the association between anti-cit-fibrinogen and the outcome of CAD. Additionally, fibrinogen is a relatively large molecule(8) with autoantibodies targeting different portions of the citrullinated fibrinogen. Thus, a secondary objective of this study was to determine whether there was specificity to the type(s) of anti-cit-fibrinogen associated with CAD.
METHODS
Study population
We studied a validated RA cohort identified in the electronic medical records (EMR) of two large tertiary care hospitals in Boston, MA, Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH). Briefly, the RA cases were identified using a validated RA phenotype algorithm utilizing a combination of structured data, e.g. International Classification of Diseases, 9th Revision (ICD9), and concepts extracted from text notes, e.g. smoking status, using natural language processing (NLP). The positive predictive value (PPV) for RA using the algorithm was 94%(9). Discarded blood samples were collected using a biospecimen repository linked to anonymized EMR data and all samples were measured for antibodies to cyclic citrullinated peptide (anti-CCP) using a commercial kit as described in a previous publication(10). We studied all RA subjects with clinical data and available blood samples.
CAD Outcome definition
Subjects were classified as having CAD if they had diagnosis of CAD in the medical record with supporting evidence of disease through documentation of CABG, percutaneous coronary intervention (PCI) with stent or balloon angioplasty, positive stress test, or EKG changes consistent ischemia(11). Subjects with a diagnosis of CAD without supporting documentation or subjects with no mention of CAD were considered not to have the disease.
Covariates
Information on covariates and potential confounders were extracted from the structured EMR data including age, gender, self-reported race classified as white or non-white, hypertension (ICD9 401.x, 402.x. 403.x. 404.x, 405.x), diabetes (249.xx or 250.xx), hyperlipidemia (272.xx), and RA treatments (ever/never use): disease modifying anti-rheumatic drugs (DMARDs), categorized into non-biologic and biologic DMARDS, and prednisone. Smoking status was extracted using NLP(9).
Laboratory testing
All plasma samples were measured for anti-cit-fibrinogen using a bead-based antigen microarray as described previously(6). In total, we measured autoantibodies directed against 10 epitopes of cit-fibrinogen (Table 2). Anti-CCP positivity was determined using a commercially available assay (CCP3 IgG ELISA, INOVA Diagnostics, San Diego, CA).
Table 2.
The association between anti-cit-fibrinogens and CAD outcomes*.
| Group | Anti-cit-fibrinogen | Association with CAD p-value |
Association with IHD p-value |
|---|---|---|---|
| Group 1 (red) | 1: FA (616635) cit3 sm-cyclic 2: FA (556–575) cit sm cyclic |
0.33 | 0.65 |
| Group 2 (orange) | 3: FA (211–230) cit sm cyclic 4: FB (54–72) cit1 5: FA (41–60) cit3 cyclic 6: FB (36–52) cit 7: FA (27–43) cit 8: FB (246–267) cit 9: FA (582–599) cit |
0.015 | 0.0040 |
| Group 3 (blue) | 10: Fibrinogen cit | 0.34 | 0.29 |
| All anti-cit-fibrinogen | All 10 autoantibodies above | 0.00011 | 0.00029 |
Adjusted by age, gender, self-reported race, hypertension, diabetes mellitus, hyperlipidemia, nbDMARD ever use, bDMARD ever use, smoking status and prednisone ever use.
Note: FA= Fibrinogen alpha, FB=Fibrinogen beta, sm=small
Statistical analysis
To test the association between autoantibodies as a group with the primary outcome CAD, we used the score test (13,14). The use of the score test allowed us to integrate information from multiple correlated ACPAs at once (which individually may not be statistically significant) to determine the overall association of the group with the outcome. The p-value from the score test provides a summary statistic for whether the biomarkers as a group are associated with the outcome. Prior studies on ACPAs tested the association between individual ACPAs with an outcome. Due to the high degree of correlation between the ACPAs, this approach cannot account for collinearity between ACPAs, and can further result in a loss of power to detect associations. As well, each individual ACPA may have a modest effect size, however, as a group, the ACPAs may have a significant association with an outcome that may be missed with individual testing. The score test provides information about whether there is a significant association between a group and an outcome. Notably, since it is testing a group, a single effect size cannot be provided for the group.
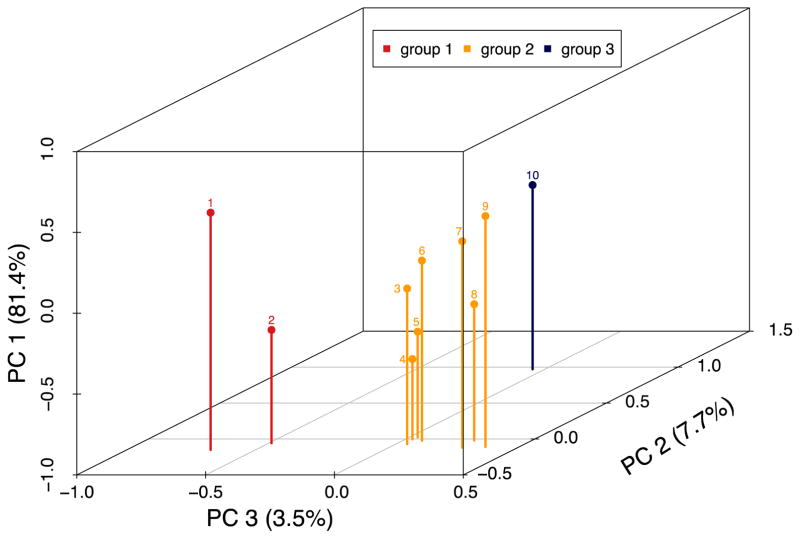
Since the anti-cit-fibrinogens are known to be highly correlated, we performed a Principal Component Analysis (PCA) to determine whether anti-cit-fibrinogen data cluster into groups based on the data. Briefly, PCA is a factor analysis method that highlights linear relationship between correlated variables while extracting a low number of key Principal Components (PC). The first PC explained more than 80% of the anti-cit-fibrinogen antibody variability. PC2 explained an additional 7.7% of variability and PC3 an additional 3.5% of variability. Together the 3 PCs explained over 90% of the variability in anti-cit-fibrinogen levels. From the PCA, anti-cit-fibrinogen autoantibodies clustered into three groups (Figure 1). In addition, the same clustering was found when performing a hierarchical clustering of the data (data not shown). We then tested the association between each group of anti-cit fibrinogen with CAD outcomes using a score test in a logistic regression model, adjusting for traditional CV risk factors.
Figure 1.
Principal component analysis of anti-cit-fibrinogen antibodies demonstrating 3 main groupings of anti-cit-fibrinogen antibodies. (FA=fibrinogen alpha, FB= fibrinogen beta)
We performed 3 sensitivity analyses. First, we performed an association study using a published group of ICD9 codes for ischemic heart disease (IHD, ICD9 411.x or 414.x)(12), where subjects with ≥1 ICD9 code for any of those codes was defined as having IHD. Second, we tested for association between the anti-cit-fibrinogen group with CAD outcome adjusting for the same covariates in the primary analysis with the addition of high sensitivity CRP (hsCRP) levels. Third, we further added anti-CCP to the adjusted model.
Statistical analyses were performed in R 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria). All aspects of this study were approved by the Partners Institutional Review Board (IRB).
RESULTS
We studied a total of 1006 RA patients with mean age 61.0 (SD) years, 79.0% females, and 72.2% anti-CCP positive (Table 1).
Table 1.
Clinical characteristics of cohort (n=1006).
| Clinical characteristics | CAD yes, n=93 (9.2%) | CAD no, n=913 (90.8%) |
|---|---|---|
| Mean age, years (SD) | 71.0 (10.0) | 60.0 (12.8) |
| Female, n (%) | 56 (60.2) | 739 (80.9) |
| Anti-CCP positive, n (%) | 69 (74.2) | 657 (72.0) |
| HTN | 74 (79.6) | 321 (35.2) |
| Hyperlipidemia | 64 (68.8) | 246 (26.9) |
| Diabetes mellitus | 29 (31.2) | 84 (9.2) |
| Mean hsCRP*, mg/L (SD) | 13.1 (17.3) | 11.4 (18.0) |
| DMARD, ever use, n (%) | 87 (93.5) | 865 (94.7) |
| Biologic DMARD, ever use, n (%) | 43 (46.2) | 437 (47.9) |
| Non-biologic DMARD, ever use, n (%) | 62 (66.7) | 564 (61.8) |
| Methotrexate | 50 (53.8) | 589 (64.5) |
| TNFi | 41 (44.1) | 417 (45.7) |
| Smoking, ever, n (%) | 73 (78.5) | 496 (54.3) |
| Prednisone, ever use, n (%) | 82 (88.2) | 682 (74.7) |
hsCRP value available for n=897 subjects
From the PCA analysis PC1 accounts for the majority of variability, 81.4%, among all anti-cit fibrinogen antibodies(Figure 1). PC2 explains 7.7% and PC3 3.5% of the variability across all anti-cit fibrinogens respectively. From this PCA analysis, we observed that the anti-cit-fibrinogen antibodies clustered into three main groups (Table 2). The anti-cit-fibrinogens (10 autoantibodies total) as a group were associated with a higher risk of CAD, adjusted for age, gender, race, diabetes, hypertension, hyperlipidemia, smoking, non-biologic DMARD, biologic DMARD and prednisone use, p=1.1×10−4 (Table 2). When the associations were examined by subgroups, the strongest association was observed in Group 2 (p=0.015).
The sensitivity analysis using ICD9 codes showed a similar association between anti-cit-fibrinogen as a group with IHD, as well as between Group 2 and IHD (Table 2). We observed no association between anti-CCP positivity (commercial assay) with CAD, OR 1.3 (95% CI 0.7, 2.3), using the same covariates for adjustment. Further we studied the subjects with hsCRP data (n=897). The association between anti-cit fibrinogens and CAD remained significant adjusting for CV risk factors and hsCRP (p=2.2×10−4, for Group 2, p=7.1×10−3). As well, the association remained significant after adjusting the model including hsCRP and anti-CCP status (p=5.7×10−8; for group 2 alone, p=2.8×10−4).
Discussion
In this study, we observed a significant association between anti-cit-fibrinogens with CAD outcomes in a cohort of RA patients. Using a data-driven approach, we observed that the association was driven by a subset of autoantibodies in Group 2 (Table 2). These findings demonstrated an association between anti-cit-fibrinogen with the clinical outcome of CAD, adding to previous studies examining surrogate measures of CV risk. Moreover, this study may explain why differences have been observed with regards to association between anti-cit-fibrinogen and surrogate measures of CV risk(6, 7). While we detected a significant association between all anti-cit-fibrinogens with CAD, the signal appeared to be driven by a group of targets within the large fibrinogen molecule. Thus, if a particular ACPA assay does not measure antibodies targeting specific regions of fibrinogen, an association with CAD, may not be detected. Additionally, an association may not be detected using individual ACPA tests.
In a study by Montes et al(7) no relationship was observed between anti-cit-fibrinogen and cIMT and CAC scores. Their examined antibodies directed against citrullinated fibrinogen beta and not alpha. In the present study, we found the strongest signal to be comprised of a group of autoantibodies directed at citrullinated chains for both fibrinogen alpha and beta. Using the same biomarker measurement platform, the results of this study corroborates the previous associations observed between anti-cit fibrinogen and CAC scores(6). This association remained significant after adjusting for potential confounders including age, gender, race, and traditional CV risk factors. The association remained significant after further adjustment for CRP and anti-CCP status. Thus, the concentrations of anti-cit fibrinogen provided additional information in identifying which RA patients had CAD in addition to anti-CCP. These data also support the notion that specificity for epitope target may be important for identifying CAD in RA; the commercial anti-CCP assay is a mixture of cyclic antibodies against a proprietary set of epitope targets, whereas the anti-cit fibrinogen assays were developed using tissue from RA patients with characterized epitope targets. As found in a previous study, cit-fibrinogen is present in atherosclerotic plaques and are recognized by ACPAs from RA patients(6).
We designed this study as a “Phase 3” biomarker validation study. Phase 3 biomarker studies focus on the association between previously identified candidate biomarkers, anti-cit fibrinogens with CAD outcome, rather than novel biomarker identification(13). Therefore, we did not perform screening for other ACPAs with the CAD outcome. However, our recently published results from a Phenome-Wide Association Study (PheWAS) corroborate findings from previous studies as well as our own. This hypothesis generating screen performed for all ACPAs and all potential phenotypes (defined using billing code data), showed a signal for association between anti-cit fibrinogens with billing codes for ischemic heart disease (14). We did not observe association of other ACPAs with CV-related phenotypes.
The potential mechanism or biology behind the association between cit-fibrinogen, anti-cit-fibrinogen and CV risk is beyond the scope of this study. However, fibrinogen is a large multi-domain glycoprotein and is one of the best characterized citrullinated protein targets of ACPAs in RA(8, 15). Fibrinogen is a known constituent of the atherosclerotic plaque for which at least a portion has been found to be citrullinated(16). These data suggest a potential pathway connecting citrullination of fibrinogen with CAD. Whether pathology occurs as a result of citrullination of fibrinogen or antibody binding of citrullinated fibrinogen is not known.
A limitation to our study is that the samples were collected in patients who have prevalent CAD. Thus, this study cannot answer the question of whether anti-cit-fibrinogen is predictive of future risk. Notably, the association between the anti-cit fibrinogen as a group remained significant after adjusting for anti-CCP status. Misclassification of RA and CAD is another potential limitation. However, we believe that misclassification, particularly with CAD is more likely to attenuate the association. Additionally, while we adjusted for CRP, we did not have detailed information on disease activity.
In summary, previous studies observed an association between anti-cit-fibrinogen antibodies with pre-clinical CAD, and in this study with actual CAD outcomes supporting its utility as a potential marker for assessing CV risk in RA. While our study adjusted for traditional risk CV risk factors, future Phase 4 biomarker development studies investigating the predictive value of these biomarkers in an independent cohort are needed. Further studies can assess whether these markers as a group improve CV risk stratification for RA patients in the context of validated general population based CV risk estimators.
Significance and Innovations.
Previous studies found an association between anti-citrullinated protein antibodies (ACPA) directed against citrullinated fibrinogen (cit-fibrinogen) and surrogate markers of cardiovascular (CV) risk; this study examined the association between ACPAs and coronary artery disease (CAD) outcomes
Our study demonstrated a specific association between a subset of anti-cit-fibrinogen antibodies and CAD in RA, present even after adjusting for anti-CCP status
We provide a roadmap for a data driven approach that can be applied to study association between a group of biomarkers with an outcome using state-of-the-art biostatistics methods; the traditional approach of testing individual biomarkers with an outcome and adjusting for multiple comparisons are challenging due to the high degree of correlation between ACPAs
Acknowledgments
Funding sources: Rheumatology Research Foundation Disease Targeted Research Pilot Grant, NIH R01 HL127118, NIH U54 HG007963
References
- 1.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71(9):1524–9. doi: 10.1136/annrheumdis-2011-200726. [DOI] [PubMed] [Google Scholar]
- 2.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110(3):420–4. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7(5):e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brink M, Hansson M, Mathsson-Alm L, Wijayatunga P, Verheul MK, Trouw LA, et al. Rheumatoid factor isotypes in relation to antibodies against citrullinated peptides and carbamylated proteins before the onset of rheumatoid arthritis. Arthritis Res Ther. 2016;18:43. doi: 10.1186/s13075-016-0940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raijmakers R, van Beers JJ, El-Azzouny M, Visser NF, Bozic B, Pruijn GJ, et al. Elevated levels of fibrinogen-derived endogenous citrullinated peptides in synovial fluid of rheumatoid arthritis patients. Arthritis Res Ther. 2012;14(3):R114. doi: 10.1186/ar3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokolove J, Brennan MJ, Sharpe O, Lahey LJ, Kao AH, Krishnan E, et al. Brief report: citrullination within the atherosclerotic plaque: a potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum. 2013;65(7):1719–24. doi: 10.1002/art.37961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montes A, Corrales A, Calaza M, Lopez-Mejias R, Parra JA, Gonzalez-Gay MA, et al. Brief report: lack of replication of an association between anti-citrullinated fibrinogen and subclinical atherosclerosis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2015;67(11):2861–5. doi: 10.1002/art.39302. [DOI] [PubMed] [Google Scholar]
- 8.Kollman JM, Pandi L, Sawaya MR, Riley M, Doolittle RF. Crystal structure of human fibrinogen. Biochemistry. 2009;48(18):3877–86. doi: 10.1021/bi802205g. [DOI] [PubMed] [Google Scholar]
- 9.Liao KP, Cai T, Gainer V, Goryachev S, Zeng-treitler Q, Raychaudhuri S, et al. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62(8):1120–7. doi: 10.1002/acr.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurreeman F, Liao K, Chibnik L, Hickey B, Stahl E, Gainer V, et al. Genetic basis of autoantibody positive and negative rheumatoid arthritis risk in a multi-ethnic cohort derived from electronic health records. Am J Hum Genet. 2010;88(1):57–69. doi: 10.1016/j.ajhg.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao KP, Ananthakrishnan AN, Kumar V, Xia Z, Cagan A, Gainer VS, et al. Methods to Develop an Electronic Medical Record Phenotype Algorithm to Compare the Risk of Coronary Artery Disease across 3 Chronic Disease Cohorts. PLoS One. 2015;10(8):e0136651. doi: 10.1371/journal.pone.0136651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denny JC, Bastarache L, Ritchie MD, Carroll RJ, Zink R, Mosley JD, et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol. 2013;31(12):1102–10. doi: 10.1038/nbt.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–62. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 14.Liao KP, Sparks JA, Hejblum BP, Kuo IH, Cui J, Lahey LJ, et al. Phenome-Wide Association Study of Autoantibodies to Citrullinated and Noncitrullinated Epitopes in Rheumatoid Arthritis. Arthritis Rheumatol. 2017;69(4):742–749. doi: 10.1002/art.39974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes-Cerqueira C, Ossipova E, Gunasekera S, Hansson M, Mathsson L, Catrina AI, et al. Targeting of anti-citrullinated protein/peptide antibodies in rheumatoid arthritis using peptides mimicking endogenously citrullinated fibrinogen antigens. Arthritis Res Ther. 2015;17:155. doi: 10.1186/s13075-015-0666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith EB. Fibrinogen, fibrin and fibrin degradation products in relation to atherosclerosis. Clin Haematol. 1986;15(2):355–70. [PubMed] [Google Scholar]