Abstract
Pancreatic adenocarcinoma remains a major therapeutic challenge, as the poor (<8%) 5-year survival rate has not improved over the last three decades. Our previous preclinical data showed cooperative attenuation of pancreatic tumor growth when dasatinib (Src inhibitor) was added to erlotinib (EGFR inhibitor) and gemcitabine. Thus, this study was designed to determine the maximum-tolerated dose of the triplet combination. Standard 3+3 dose escalation was used, starting with daily oral doses of 70 mg dasatinib and 100 mg erlotinib with gemcitabine on days 1, 8, and 15 (800 mg/m2) of a 28-day cycle (L0). Nineteen patients were enrolled, yet 18 evaluable for dose-limiting toxicities (DLTs). One DLT observed at L0, however dasatinib was reduced to 50 mg (L−1) given side effects observed in the first two patients. At L–1, a DLT occurred in 1/6 patients and dose was re-escalated to L0, where zero DLTs reported in next four patients. Dasatinib was escalated to 100 mg (L1) where 1/6 patients experienced a DLT. Although L1 was tolerable, dose escalation was stopped as investigators felt L1 was within the optimal therapeutic window. Most frequent toxicities were anemia (89%), elevated aspartate aminotransferase (79%), fatigue (79%), nausea (79%), elevated alanine aminotransferase (74%), lymphopenia (74%), leukopenia (74%), neutropenia (63%), and thrombocytopenia (63%), most Grade 1/2. Stable disease as best response was observed in 69% (9/13). Median progression-free and overall survival was 3.6 and 8 months, respectively. Dasatinib, erlotinib, and gemcitabine was safe with manageable side effects, and with encouraging preliminary clinical activity in advanced pancreatic cancer.
Keywords: dasatinib, erlotinib, dual Src and EGFR inhibition, pancreatic cancer
INTRODUCTION
Pancreatic adenocarcinoma is a major therapeutic challenge, as the overall five-year survival rate of 8% has remained relatively unchanged for the past three decades [1]. Until recently, gemcitabine-based therapies were the standard treatment option for patients with advanced or metastatic disease and remain standard for patients with a poor performance status. These regimens, however, result in minimal responses [2–4]. The addition of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor erlotinib to gemcitabine showed a modest but significant improvement in overall survival leading to FDA approval for patients with advanced disease [4]. However, a significant difference in tumor responses was not observed between treatment arms emphasizing the need for developing novel treatment strategies to improve clinical outcomes.
Src, a non-receptor tyrosine kinase encoded by the proto-oncogene c-Src, participates in numerous signaling pathways, including EGFR-mediated signaling networks [5]. The expression and activity of Src has been associated with malignant transformation and cancer progression in a variety of human cancers, including pancreatic cancer [6–9]. We have previously shown a stepwise increase in cytoplasmic and membranous staining of Src from normal pancreas to chronic pancreatitis to advancing tumor grade of pancreatic adenocarcinoma, suggesting that Src expression increases with pancreatic neoplasia progression [10]. Studies from multiple laboratories [8,11,12], including ours [10], have previously established a mechanistic rationale for targeting Src in pancreatic carcinoma. We have shown that pharmacologic inhibition of Src with dasatinib resulted in attenuation of cell proliferation and induced cell cycle arrest and apoptosis in pancreatic cancer cell lines [10]. This outcome was recapitulated in pancreatic tumor xenografts as dasatinib significantly inhibited tumor growth. Despite the observed antitumor activity, dasatinib had no effect on the phosphorylation of specific proteins in cell survival and angiogenic pathways (e.g., STAT3 and MAPK), suggesting possible resistance mechanisms to dasatinib [10].
A recent global genomic analysis observed that pancreatic tumors are highly heterogeneous with genetic alterations in multiple, overlapping pathways, which provided rationale that treatment strategies towards the disease should target multiple signaling pathways rather than just the products of one gene [13]. Thus, we sought to characterize the underlying mechanisms of targeting two tyrosine kinases, EGFR and Src, in combination with gemcitabine, with the hypothesis that targeting multiple tumor-associated pathways would enhance effect and potentially minimizing inherent and acquired resistance. Indeed, the combination of dasatinib with erlotinib and gemcitabine resulted in synergistic inhibition of proliferation and viability in pancreatic cancer cell lines, as well as xenograft tumor growth compared to individual or two drug combinations of these agents [14]. Furthermore, only the three drug combination was shown to overcome constitutive activation of STAT3-mediated signaling [14]. This latter observation is especially important given our previous preclinical data that provided compelling evidence to suggest that STAT3 is a key mediator of chemoresistance in pancreatic carcinoma [10,14]. Based on this preclinical data, we designed this phase I study to evaluate safety and tolerability of dasatinib, erlotinib, and gemcitabine in patients with advanced pancreatic cancer. Secondary objectives included assessing antitumor activity and correlating changes in CA19-9 with tumor response. We also explored the feasibility (and potential utility) of performing serial diffusion-weighted imaging (DWI).
MATERIALS AND METHODS
Patient Selection
This investigator-initiated, single institution phase I study (NCT01660971) of dasatinib, erlotinib, and gemcitabine was conducted in patients with locally advanced or metastatic pancreatic carcinoma. The study was approved by the institutional review board. Informed consent was obtained from all individual participants included in the study. Eligible patients had pathologically confirmed pancreatic adenocarcinoma (excluding islet cell or ampullary tumors) that was metastatic or locally advanced and unresectable, had measurable disease as defined by the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 [15], no prior gemcitabine treatment for advanced disease, adequate organ function (hematologic, renal, and hepatic), and were ≥18 years of age with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1. Patients who received prior adjuvant therapy including gemcitabine were eligible if their last dose of gemcitabine was greater than six months prior to enrollment and they had fully recovered from side effects. Additional inclusion criteria and patient exclusion criteria are listed in the clinical trial protocol included in the Supplement.
Study Treatment
This is a phase I study that investigated the standard dose of erlotinib with escalating doses of dasatinib and gemcitabine (Table 1). Dose escalation began with 70 mg of dasatinib once daily, 100 mg of erlotinib once daily, and 800 mg/m2 gemcitabine infusion over 30 minutes administered weekly for three weeks of each four-week cycle. Starting doses for dasatinib and gemcitabine were chosen based on results from previous studies. A phase I/II study by Haura, et al. investigated dasatinib in combination with erlotinib in advanced non-small cell lung cancer, and found 150 mg once daily of erlotinib and 70 mg twice daily of dasatinib was tolerated [16]. A phase I study by Uronis and colleagues found 1000 mg/m2 gemcitabine administered weekly for three weeks of each four-week cycle plus 50 mg twice daily dosing of dasatinib to be tolerable [17]. Since this study evaluated the triplet combination, the investigators felt a safe starting dose for gemcitabine and dasatinib would be one step lower than that of the Uronis trial in combination with the standard dose of erlotinib in pancreatic cancer.
Table 1.
Dose Escalation Schedule (standard 3+3 design) with Dose Limiting Toxicities
| Dose Level | Gemcitabine (mg/m2) |
Erlotinib (mg QD) |
Dasatinib (mg QD) |
Cycle 1 (28-days) Toxicity |
|---|---|---|---|---|
| Level -1 | 800 | 100 | 50 | 6 patients; 1/6 with DLTa |
| Level 0 (starting) | 800 | 100 | 70 | 6 patients; 1/6 with DLTb |
| Level 1 | 800 | 100 | 100 | 7 patients (6 evaluable); 1/6 with DLTc |
| Level 2 | 1000 | 100 | 100 | Not Evaluated |
| Level 3 | 1000 | 100 | 140 | Not Evaluated |
Grade 3 dyspnea;
Grade 3 nausea and vomiting;
Grade 3 elevated AST
QD: once daily; AST: aspartate aminotransferase
Dose escalation was planned for groups of three patients until the maximum tolerated dose (MTD) was established in a standard 3+3 design. No intra-patient dose escalation was allowed. Dose limiting toxicity (DLT) was defined as any treatment-related, first cycle (28 days): Grade ≥3 nonhematologic toxicity, except nausea, vomiting, and hypertension. Nausea and vomiting were considered dose-limiting if hospitalization or repeated hydration was required despite the use of antiemetic agents; Grade 4 neutropenia lasting > seven days or Grade 4 neutropenia of any duration associated with fever >38.5°C; Grade 4 thrombocytopenia for ≥ seven consecutive days, bleeding in the face of Grade 3–4 thrombocytopenia, or Grade 3 fatigue of ≥ three days. Additionally, any treatment-related toxicity that required holding treatment for >14 days or the inability to administer 75% of the planned total dose of any one of the three agents (due to toxicity) during the first treatment cycle was also dose-limiting. Patients continued on study until unacceptable toxicity, disease progression, patient withdrawal, or specific changes in patient’s condition that in the judgment of the investigator rendered the patient unacceptable for further treatment. Patients were followed for adverse events for 30 days or until-treatment-related adverse events resolved, whichever was greater. All patients were followed for survival until death.
Study Assessments
Toxicity assessments were performed at each cycle and graded according to the NCI Common Toxicity Criteria, Version 4.0. All patients who received at least one treatment were evaluable for safety. Patients that were removed from the study during the first four weeks of treatment for reasons other than progressive disease or drug-related adverse events were not considered evaluable for DLT and were replaced. Disease assessments were performed at baseline and every eight weeks using RECIST v1.1. Only those patients who received at least one cycle of therapy and had a post baseline disease assessment were considered evaluable for response. Patients were followed for survival endpoints until death. Tumor marker CA19-9 was measured at baseline and every four weeks (± seven days) during therapy.
Diffusion-Weighted MRI
DWI is an emerging MRI technique that is sensitive to the microscopic, thermally-induced self-diffusion of water molecules within a system [18]. DWI returns estimates of the apparent diffusion coefficient (ADC), and in well-controlled situations, the ADC has been shown to correlate inversely with tissue cellularity.[19] Multiple preclinical and clinical studies have shown that chemotherapy and radiation therapy lead to measurable changes in ADC that are associated with tumor response [20–23]. Therefore, it is reasonable to hypothesize that a relationship would exist between changes in ADC and tumor response to dasatinib, erlotinib, and gemcitabine.
As a pilot study, we collected pre- and post-treatment (i.e., week 4 of Cycle 1) DWI data on three patients. DWI examinations were performed on a Philips 3T Achieva scanner with a 16-channel torso coil (Philips Healthcare, Best, The Netherlands), and data were collected using a respiratory-gated, single-shot echo planar sequence in three orthogonal diffusion encoding directions with two b-values (200 and 750 s/mm2) and the following parameters: repetition time = 1650 ms; echo time = 75 ms; diffusion time Δ = 36.5 ms; diffusion gradient duration Δ = 19.2 ms; number of signals averaged = 4 for b = 200 s/mm2 and 8 for b = 750 s/mm2; acquisition matrix = 120×103×13 sections and a reconstructed voxel size = 1.25×1.25×5 mm3. ADC values were computed for each voxel via: ADC = ln[S1/S2)]/(b2-b1), where S1 is the signal acquired at b1 and S2 is the signal acquired at b2. T2-weighted anatomical images were also collected for tumor localization and region of interest (ROI) delineation. The mean ADC value was collected from each tumor ROI.
Statistical Analysis
Due to the nature of this study, descriptive statistics were used to summarize demographics, adverse events, and tumor response. Confidence intervals were estimated using the Wilson method, and survival functions were estimated using the Kaplan-Meier method. Summary statistics were used to describe the changes in CA19-9 and ADC. Linear and Cox (proportional hazards) regression models were used to evaluate the relationship between tumor response and CA19-9; p<0.05 was considered statistically significant.
RESULTS
Patient Characteristics
Between July 2012 and October 2015, 19 patients were enrolled and three dose levels were investigated. Baseline patient characteristics are listed in Table 2. One patient exhibited disease progression prior to completing the DLT observation window, and thus was replaced in the dose escalation cohort. Median age was 61 years (range, 48–77), 53% were male, and 74% of patients had metastatic disease. A majority (95%) of patients were either treatment naïve or had one prior therapy for advanced disease, and six (32%) patients received prior adjuvant therapy containing gemcitabine. Nine patients came off study due to unacceptable toxicity, three of which came off study during the first treatment cycle; two patients withdrew consent after starting treatment, and one patient each withdrew due to a protocol non-compliance or exhibited a poor performance status. The other six patients came off study due to disease progression.
Table 2.
Patient Characteristics (N = 19)
| Data | Results |
|---|---|
| Gender -No. (%) | |
| Male | 10 (53) |
| Female | 9 (47) |
| Median age -No. (range) | 61 (48–77) |
| Race -No. (%) | |
| White | 19 (100) |
| Ethnicity -No. (%) | |
| Non-hispanic | 19 (100) |
| Disease Status -No. (%) | |
| Locally Advanced | 5 (26) |
| Metastatic | 14 (74) |
| Baseline Median CA 19-9 Level -No. (quartile) | 318 (62-9470) |
| PS (ECOG) -No. (%) | |
| 0 | 3 (16) |
| 1 | 16 (84) |
| Number of Patients Receiving Adjuvant Gemcitabine -No. (%) | 6 (32) |
| Number of Previous Lines of Therapy for Advanced Disease -No. (%) | |
| 0 | 15 (79) |
| 1 | 3 (16) |
| 2 | 1 (5) |
PS: performance status; ECOG: Eastern Cooperative Oncology Group
Treatment and Toxicities
Dose escalation proceeded according to Table 1. At a dose of 70 mg dasatinib, 100 mg erlotinib, and 800 mg/m2 gemcitabine (Level 0), one of two patients experienced two DLTs (Grade 3 nausea and vomiting with hospitalization). The second patient did well but experienced fatigue she deemed unacceptable, yet was not dose limiting. Additionally, both patients experienced Grade 3 neutropenia once during the first two cycles. Thus, based on the side effects observed in the first two patients at Level 0, dasatinib was de-escalated to 50 mg. At this dose, one of six patients experienced a DLT (Grade 3 dyspnea). Dasatinib was re-escalated to the starting dose, and four additional patients were evaluated. Among those patients, no DLTs were observed and thus dasatinib was escalated to 100 mg (Level 1). At this dose, one DLT (Grade 3 elevated aspartate aminotransferase) was observed in the next six patients enrolled. Although Level 1 was tolerable, dose escalation was stopped as the investigators felt the next dose level, which only escalated gemcitabine to 1000 mg/m2, would increase toxicity, especially hematological toxicities, without improving benefit.
The most common treatment-related toxicities were anemia (89%), elevated aspartate aminotransferase (79%), fatigue (79%), nausea (79%), elevated alanine aminotransferase (74%), lymphocytopenia (74%), leukopenia (74%), neutropenia (63%), diarrhea (58%), and hypophosphatemia (58%); a majority of these were Grade 1–2. Other frequently observed (≥20%) adverse events are listed in Table 3. Fifteen patients experienced at least one Grade 3 adverse event, with the most common being neutropenia (32%), lymphocytopenia (26%), and leukopenia (21%). Grade 4 neutropenia and lymphocytopenia occurred in three patients and one patient, respectively. All Grade 3–4 adverse events are listed in Table 3.
Table 3.
Most common (frequency ≥ 20%) treatment-related adverse events categorized by highest grade experienced per patient (N = 19)
| All Grades | Grade 1 | Grade 2 | Grade 3a | Grade 4 | |
|---|---|---|---|---|---|
| Non-Hematological | |||||
| Elevated AST | 15 (79%) | 10 | 2 | 3 | 0 |
| Fatigue | 15 (79%) | 2 | 11 | 2 | 0 |
| Nausea | 14 (74%) | 8 | 5 | 2 | 0 |
| Elevated ALT | 14 (74%) | 10 | 2 | 2 | 0 |
| Diarrhea | 11 (58%) | 9 | 2 | 0 | 0 |
| Dysgeusia | 9 (47%) | 3 | 6 | 0 | 0 |
| Rash | 9 (47%) | 9 | 0 | 0 | 0 |
| Anorexia | 8 (42%) | 2 | 6 | 0 | 0 |
| Oral Mucositis | 7 (37%) | 5 | 1 | 1 | 0 |
| Vomiting | 6 (32%) | 3 | 1 | 2 | 0 |
| Peripheral Edema | 6 (32%) | 3 | 3 | 0 | 0 |
| Elevated Alkaline Phosphatase | 5 (26%) | 3 | 2 | 0 | 0 |
| Fever | 4 (21%) | 4 | 0 | 0 | 0 |
| Hematological | |||||
| Anemia | 17 (89%) | 3 | 11 | 3 | 0 |
| Lymphocytopenia | 14 (74%) | 2 | 6 | 5 | 1 |
| Leukopenia | 14 (74%) | 2 | 8 | 4 | 0 |
| Neutropenia | 12 (63%) | 0 | 3 | 6 | 3 |
| Thrombocytopenia | 12 (63%) | 7 | 3 | 2 | 0 |
| Hypophosphatemia | 11 (58%) | 1 | 10 | 0 | 0 |
AST: aspartate aminotransferase; ALT: alanine aminotransferase
Additional Grade 3 dyspnea observed by one patient
Antitumor Activity
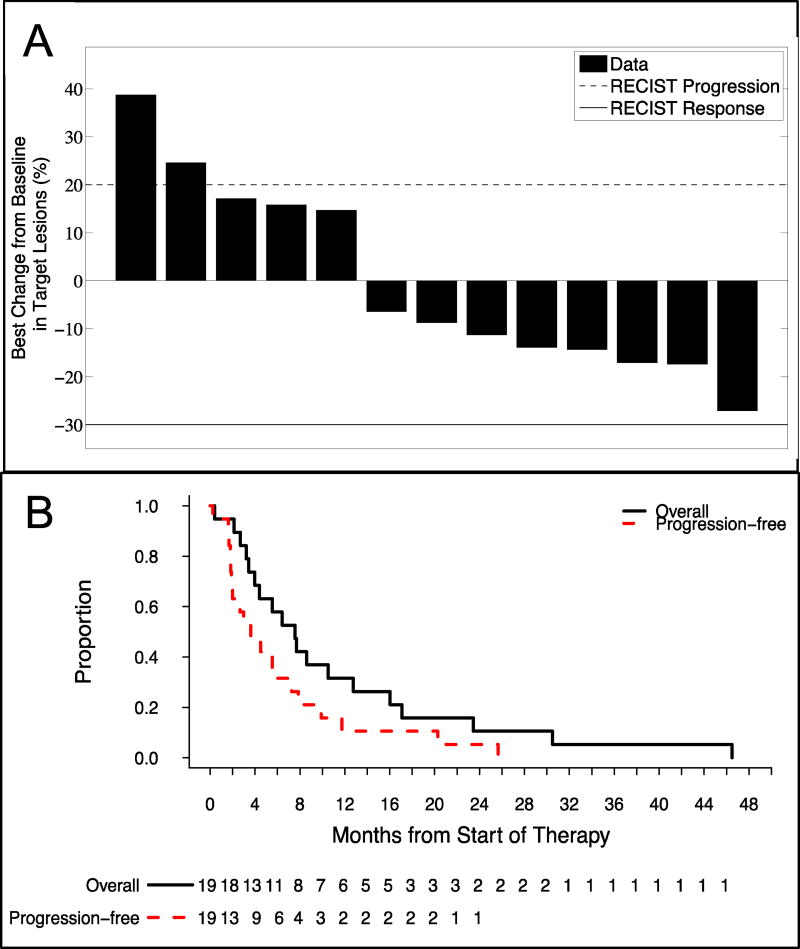
Six patients did not undergo a post baseline disease assessment and therefore were not evaluable for response. Of the 13 evaluable patients, no responses were observed, however, nine patients exhibited a best response of stable disease for a disease control rate (DCR) of 69%. Eight patients exhibited some form of tumor regression while on treatment (Figure 2A), and the median duration of stable disease was 5.5 months (95% Confidence Interval [CI], 3.8 to NA). The overall median progression-free survival (PFS) was 3.6 months (Figure 2B; 95% CI, 2.0 to 9.9), while the median overall survival (OS) was 8.0 months (Figure 2B; 95% CI, 4.4 to 17). Additionally, the 12-month OS rate was 32%.
Figure 2. Antitumor Activity.
(A) Best percentage change from baseline in the sum of all target lesions is presented for each efficacy evaluable patient on study. Solid and dashed lines represent the RECIST v1.1 definition for partial response and progressive disease, respectively. Although no responses were observed, eight (62%) of patients had reductions in tumor size suggesting the triplet combination has some antitumor activity in patients with advanced pancreatic carcinoma. (B) Kaplan-Meier analysis of progression-free survival (dashed red line) and overall survival (block solid line). The overall median progression-free survival was 3.6 months whereas the median overall survival was 8.0 months.
CA19-9 Correlative Analysis
Twelve patients had both CA19-9 measurements at baseline and four weeks, as well as a disease response assessment at eight weeks. A moderate but significant positive correlation (Figure 1 in the Supplement; Pearson Correlation; 0.65, p=0.023) was observed between changes in CA19-9 levels at four weeks and tumor size at eight weeks. The reduction in percent change between baseline and 4-week CA19-9 levels was significantly (p<0.001) different between patients that achieved a best response of stable disease versus those who progressed. Additionally, an increase in CA19-9 over time was significantly associated with an increased hazard of death (hazard ratio=1.26; p=0.017).
DWI Correlative Studies
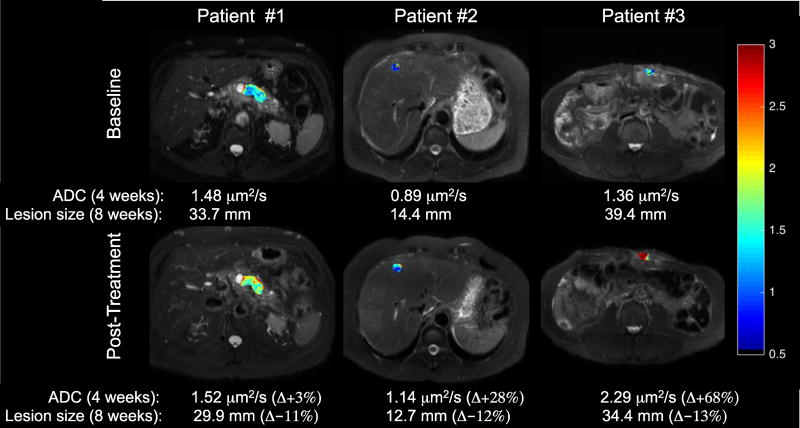
Pre- and post-treatment tumor ADC maps along with mean ADC and percent change from baseline are presented in Figure 3. Corresponding baseline and 8-week tumor RECIST measurements and percent change are also included. The expected inverse relationship between changes in tumor size and ADC (i.e., increase in ADC and decrease in tumor size) was observed for all three patients (Figure 3).
Figure 3. Preliminary DW-MRI results.
ADC maps overlaid onto T2-weighted anatomical images. The baseline and post-treatment mean ADC values and percent change from baseline are presented for each patient. The corresponding baseline and 8-week RECIST measurement, as well as percent change in tumor size is also presented. The expected inverse relationship between changes in ADC and tumor size is observed in all three patients. Notably, a measureable change in ADC for all three patients is detected after one treatment cycle, which corresponds to later changes in tumor size.
DISCUSSION
Although a greater understanding of the genomic landscape and molecular pathways of tumorigenesis have led to improvements in survival for some cancers, the use of individual targeted therapies have not yet provided a meaningful impact on clinical outcome in pancreatic cancer. Based on our preclinical data that suggested simultaneous inhibition of Src and EGFR in combination with gemcitabine had a synergistic anti-tumor effect in PDAC [14], we designed this phase I study to evaluate the safety of combining dasatinib, erlotinib, and gemcitabine in patients with locally advanced or metastatic pancreatic cancer.
Overall, dasatinib and erlotinib administered with gemcitabine was safe with manageable side effects. The MTD was not officially reached, and the RP2D was determined to be 100 mg dasatinib, 100 mg erlotinib, and 800 mg gemcitabine (Level 1) as the investigators thought the next dose level (Level 2) would only increase toxicities without improving benefit. Although the AEs were manageable, the addition of dasatinib to gemcitabine and erlotinib increased the frequency of multiple toxicities compared to the previous Phase III Canadian study that investigated gemcitabine and erlotinib. Of note, the frequency of severe (Grade ≥3) neutropenia was higher at 47% versus 24% [4]. Severe rash was not observed at all whereas the Phase III study reported 6% of patients had Grade ≥3 rash, and the frequency of mild (Grade ≤2) rash in the current study was slightly less at 47% versus 66% [4]. We note, however, that the dosage and treatment scheme of gemcitabine and erlotinib in the previous study was different thus limiting a direct comparison with the current study. Additionally, caution should be taken when interpreting the observed clinical activity due to the small number of patients evaluated in this study.
The combination of dasatinib, erlotinib, and gemcitabine exhibited clinical activity in patients with advanced pancreatic cancer as evidenced by a 69% DCR. Additionally, eight (62%) patients experienced tumor regression at the time of best response with a median duration of stable disease of 5.5 months. The Phase III Canadian study observed a significant improvement in median OS of 6.2 months compared to 5.9 months with gemcitabine monotherapy, which led to FDA approval for patients with locally advanced, unresectable or metastatic disease [4]. Our study observed a median OS of 8 months, as well as a higher DCR (69% versus 58%), suggesting that adding dasatinib to erlotinib and gemcitabine could represent a viable treatment option to improve disease stabilization and survival.
Current recommended first-line regimens to treat patients with locally advanced or metastatic pancreatic cancer are FOLFIRINOX or gemcitabine plus nab-paclitaxel based on randomized studies that observed significant clinical benefits compared to gemcitabine monotherapy. FOLFIRINOX improved the median OS compared to gemcitabine (11.1 months versus 6.8 months) [2]. Adding nab-paclitaxel to gemcitabine also improved median OS (8.5 months versus 6.7 months) [3]. However, these improvements in survival came at a cost of increased toxicities and therefore these regimens are only recommended for patients with a good performance status. Single-agent gemcitabine or best supportive care is recommended for patients with a poor performance status. Given the inferior clinical activity of gemcitabine monotherapy, the results from the current study provide motivation for further investigation of dasatinib, erlotinib, and gemcitabine in patients with a poor performance status.
The development of blood-based assays and noninvasive imaging techniques that can provide surrogate biomarkers of response is an active area of research. As a secondary objective, we explored the utility of CA19-9 to assess early tumor response and observed a significant (p=0.022) correlation between levels of CA19-9 at four weeks with tumor size at eight weeks. Furthermore, a time dependent covariate analysis resulted in a significant (p=0.017) association between temporal changes in CA19-9 and death, suggesting that CA19-9 could represent a viable biomarker for survival in this setting.
In a pilot study, we explored the feasibility (and potential utility) of performing serial DWI assessments of disease response. Image data from all three patients were collected and analyzed successfully without complications. Additionally, the expected relationship between change in ADC and tumor size was observed. Of special interest, the RECIST measurement after two cycles of treatment indicated stable disease, whereas a measureable change in ADC was observed after one treatment cycle. Unfortunately, due to the small sample size, no formal statistical associations between ADC and response could be performed. Nonetheless, our preliminary results suggest that early changes in ADC could be representative of later changes in tumor size, and perhaps predictive of tumor response. Further optimization is ongoing to improve the robustness, and consequently the reliability, of this imaging technique in serial assessments of disease response.
CONCLUSIONS
Our results suggest that dasatinib in combination with erlotinib and gemcitabine was tolerable, and that this combination exhibited some clinical activity in patients with locally advanced and metastatic pancreatic cancer. Although a direct comparison cannot be made, the improved survival benefit of the current study compared to the erlotinib and gemcitabine combination should not be overlooked, and could represent a viable treatment option for patients with a poor performance status who are not candidates for other, more effective therapies. Additionally, our results provide evidence that further investigation of ADC as a surrogate biomarker of tumor response is warranted.
Supplementary Material
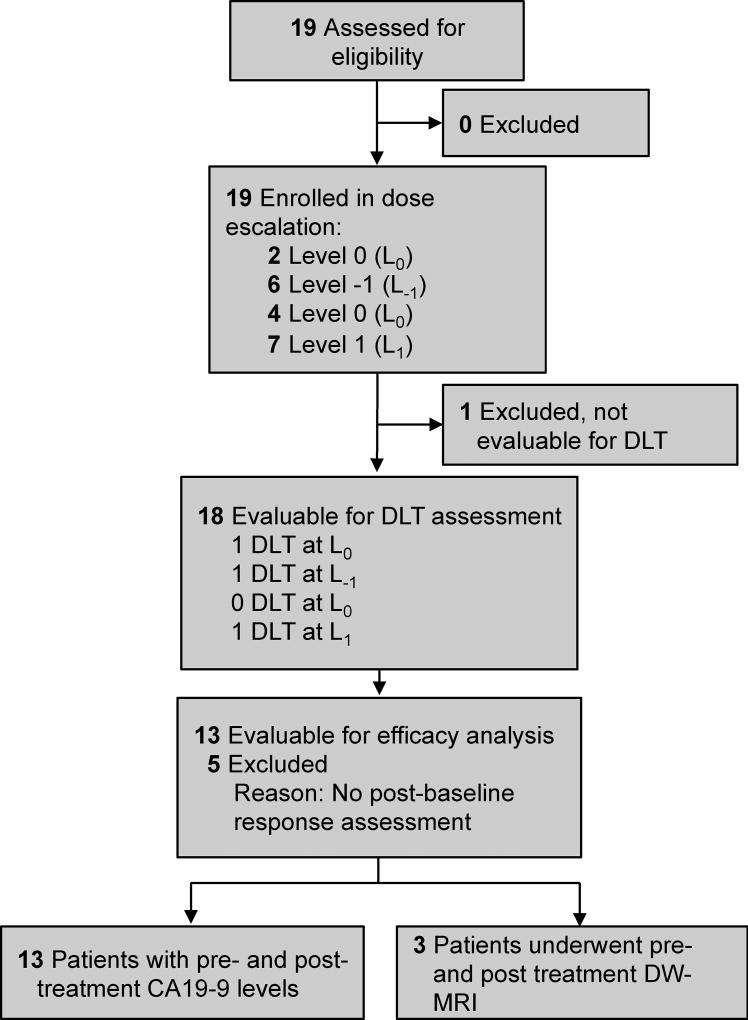
Figure 1. Consolidated Standards of Reporting Trials Diagram.
Study diagram listing number of eligible subjects enrolled onto the study, and numbers of patients in the safety, efficacy, and biomarker correlate analyses.
Acknowledgments
The authors would like to thank the patients who participated in this study and their families, the study investigators, and study staff. We would also like to thank the National Cancer Institute Clinical Trials Evaluation Program (NCI Trial #9043), the Vanderbilt-Ingram Cancer Center (P30 CA068485), and the National Institutes of Health (R01 CA161976 and U01 CA142565) for sponsoring this study.
Footnotes
Conflicts of interest
DBC has served as an advisory board consultant for Merrimack and Raphael Pharmaceuticals and has institutional research funding from Synta, Incyte, Celgene, Hoffman-LaRoxhe, EMD-Serono, and Oncolytics Biotech. LWG has served as a consultant for Celgene and has institutional research funding from Astellas Pharma, Pfizer, Onxy, SunPharma, Lilly, and Bristol-Myers Squibb. EC has served on advisory boards for Castle Biosciences, Taiho, EMD-Serono, Amgen, Lilly, Advaxis, Bayer and Merrimack. EC is currently employed at Amgen and owns Amgen stock; however, this work was done while at Vanderbilt-Ingram Cancer Center prior to her employment with Amgen. JB has served as a consultant for Celgene, Genentech, Aduro, Boston Biomedical, Janssen, Cornerstone, Symphogen, and Bayer and has institutional research funding from Genentech, Abbvie, Taiho, Bayer, 5Prime, Phoenix, Incyte, and Vertex. For the remaining authors, none were declared.
COMPLIANCE WITH ETHICAL STANDARDS
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained for all individual participants included in the study.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Groupe Tumeurs Digestives of U, Intergroup P FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National Cancer Institute of Canada Clinical Trials G Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.Roskoski R., Jr Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res. 2015;94:9–25. doi: 10.1016/j.phrs.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94(2):344–351. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- 7.Chang YM, Bai L, Liu S, Yang JC, Kung HJ, Evans CP. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene. 2008;27(49):6365–6375. doi: 10.1038/onc.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yezhelyev MV, Koehl G, Guba M, Brabletz T, Jauch KW, Ryan A, Barge A, Green T, Fennell M, Bruns CJ. Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res. 2004;10(23):8028–8036. doi: 10.1158/1078-0432.CCR-04-0621. [DOI] [PubMed] [Google Scholar]
- 9.Serrels B, Serrels A, Mason SM, Baldeschi C, Ashton GH, Canel M, Mackintosh LJ, Doyle B, Green TP, Frame MC, Sansom OJ, Brunton VG. A novel Src kinase inhibitor reduces tumour formation in a skin carcinogenesis model. Carcinogenesis. 2009;30(2):249–257. doi: 10.1093/carcin/bgn278. [DOI] [PubMed] [Google Scholar]
- 10.Nagaraj NS, Smith JJ, Revetta F, Washington MK, Merchant NB. Targeted inhibition of SRC kinase signaling attenuates pancreatic tumorigenesis. Mol Cancer Ther. 2010;9(8):2322–2332. doi: 10.1158/1535-7163.MCT-09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, Donato NJ, Abbruzzese JL, Baker CH, Gallick GE. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. The American journal of pathology. 2006;168(3):962–972. doi: 10.2353/ajpath.2006.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajeshkumar NV, Tan AC, De Oliveira E, Womack C, Wombwell H, Morgan S, Warren MV, Walker J, Green TP, Jimeno A, Messersmith WA, Hidalgo M. Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res. 2009;15(12):4138–4146. doi: 10.1158/1078-0432.CCR-08-3021. [DOI] [PubMed] [Google Scholar]
- 13.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaraj NS, Washington MK, Merchant NB. Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res. 2011;17(3):483–493. doi: 10.1158/1078-0432.CCR-10-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. S0959-8049(08)00873-3 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Haura EB, Tanvetyanon T, Chiappori A, Williams C, Simon G, Antonia S, Gray J, Litschauer S, Tetteh L, Neuger A, Song L, Rawal B, Schell MJ, Bepler G. Phase I/II study of the Src inhibitor dasatinib in combination with erlotinib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(8):1387–1394. doi: 10.1200/JCO.2009.25.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uronis HE, Bullock K, Blobe G, Hsu S, Morse M, Nixon A, Haley S, O’Neill M, Hurwitz H, Bendell J. A phase I study of gemcitabine plus dasatinib (GD) or gemcitabine plus dasatinib plus cetuximab (GDC) in refractory solid tumors. Journal of Clinical Oncology. 2009;27(15_suppl):e15506–e15506. doi: 10.1200/jco.2009.27.15_suppl.e15506. [DOI] [Google Scholar]
- 18.Gore JC, Xu J, Colvin DC, Yankeelov TE, Parsons EC, Does MD. Characterization of tissue structure at varying length scales using temporal diffusion spectroscopy. NMR Biomed. 2010;23(7):745–756. doi: 10.1002/nbm.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson AW, Xie J, Pizzonia J, Bronen RA, Spencer DD, Gore JC. Effects of cell volume fraction changes on apparent diffusion in human cells. Magn Reson Imaging. 2000;18(6):689–695. doi: 10.1016/s0730-725x(00)00147-8. doi:S0730-725X(00)00147-8 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Galons JP, Altbach MI, Paine-Murrieta GD, Taylor CW, Gillies RJ. Early increases in breast tumor xenograft water mobility in response to paclitaxel therapy detected by non-invasive diffusion magnetic resonance imaging. Neoplasia. 1999;1(2):113–117. doi: 10.1038/sj.neo.7900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuneo KC, Chenevert TL, Ben-Josef E, Feng MU, Greenson JK, Hussain HK, Simeone DM, Schipper MJ, Anderson MA, Zalupski MM, Al-Hawary M, Galban CJ, Rehemtulla A, Feng FY, Lawrence TS, Ross BD. A pilot study of diffusion-weighted MRI in patients undergoing neoadjuvant chemoradiation for pancreatic cancer. Transl Oncol. 2014;7(5):644–649. doi: 10.1016/j.tranon.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Abramson RG, Arlinghaus LR, Kang H, Chakravarthy AB, Abramson VG, Farley J, Mayer IA, Kelley MC, Meszoely IM, Means-Powell J, Grau AM, Sanders M, Yankeelov TE. Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest Radiol. 2015;50(4):195–204. doi: 10.1097/RLI.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248(3):894–900. doi: 10.1148/radiol.2483071407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.