Abstract
Background
Effects of nonparoxysmal atrial fibrillation (non‐PAF) ablation targeting complex fractionated atrial electrogram (CFAE) areas and/or low voltage areas (LVAs) are still controversial.
Methods and Results
A recently developed online real‐time phase mapping system (ExTRa Mapping) was used to conduct LVA mapping and simultaneous ExTRa and CFAE mapping in 28 non‐PAF patients after pulmonary vein isolation (PVI). Nonpassively activated areas, in the form of meandering rotors and/or multiple wavelets assumed to contain non‐PAF drivers, partly overlapped with CFAE/LVAs but not always coincided with them.
Conclusion
Real‐time rotor imaging, rather than conventional indirect indicators only, might be very useful for detecting non‐PAF drivers.
Keywords: atrial fibrillation, catheter ablation, driver, mapping, rotor
1. INTRODUCTION
As atrial fibrillation (AF) is a major cause of cerebral infarction and heart failure, and the prevalence of AF increases with age, its treatment is an urgent task in this aging society. Pulmonary vein isolation (PVI) is widely accepted as the standard ablation strategy for paroxysmal AF (PAF).1 In contrast, a PVI alone appears to be an insufficient ablation strategy for patients with nonparoxysmal AF (non‐PAF), including both persistent AF (lasting more than 7 days but less than 1 year) and long‐standing persistent AF (lasting more than 1 year).
Thus, modulation of AF drivers (or perpetuators) by catheter ablation targeting indirect indicators of AF drivers, such as complex fractionated atrial electrograms (CFAEs),2 has been proposed as one of the effective ablation strategies for non‐PAF. However, a recent clinical study, the STAR AF II,3 showed that a CFAE‐targeted ablation after a PVI has no apparent additional benefit in patients with non‐PAF.
As an alternative ablation strategy for non‐PAF, ablation targeting low voltage areas (LVAs) detected by voltage mapping during sinus rhythm or coronary sinus pacing has been proposed.4 However, because such an indirect indicator of AF drivers is not based on the excitation wave dynamics during AF, the relationship of the LVA to the location of the real AF drivers is still unclear.
For these reasons, real‐time imaging of AF wave dynamics in clinical practice is required for detecting real AF drivers and improving the outcome of the non‐PAF ablation. Thus, the goals of this study were to visualize the excitation wave dynamics during AF in patients with non‐PAF by employing our novel online real‐time phase mapping system and to investigate whether the above‐mentioned indirect indicators really reflected the real AF drivers.
2. METHODS
2.1. Novel real‐time phase mapping system
We have developed an online real‐time phase mapping system (ExTRa Mapping™, Nihon Kohden Co., Tokyo, Japan), as shown in Figure 1. Because this phase mapping system was based on 41 bipolar intra‐atrial electrograms (including 9 virtual electrograms) recorded by a 20‐pole spiral‐shaped catheter with a diameter of 2.5 cm (Reflexion HD™, St. Jude Medical Inc., MN, USA), the mapping system achieved a high signal density (~8 signals/cm2). Based on the 5‐second wave dynamics during AF, each phase map movie was fully automatically created with the ExTRa Mapping within a few seconds and was immediately played at a 1/10 speed for 50 seconds.
Figure 1.
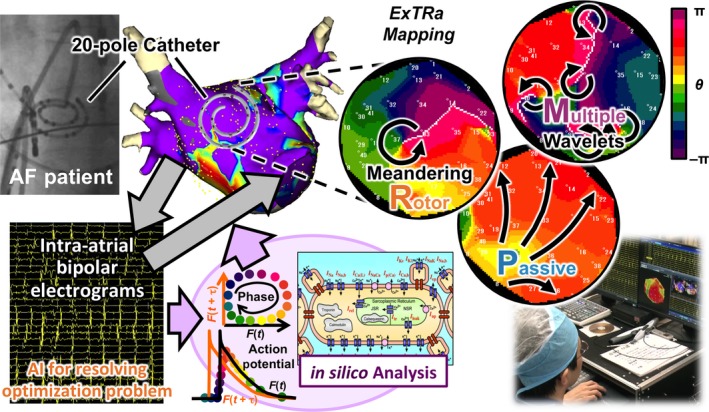
Novel online real‐time phase mapping system (ExTRa Mapping™). Intra‐atrial bipolar electrograms recorded by a 20‐pole spiral‐shaped catheter during atrial fibrillation (AF) in patients with nonparoxysmal AF (non‐PAF) are transferred into an in silico analysis system to calculate virtual action potentials and phase values. Then, the mapping system combines the intra‐atrial bipolar electrograms with the virtual action potentials to create spatial phase map movies within a few seconds. Snapshots of the typical non‐PAF wave dynamics visualized by the ExTRa Mapping are shown in the top right 3 panels
To improve the detection and spatiotemporal interpolation of the intra‐atrial signals and to achieve a rapid prediction of the atrial excitations during AF, both computer simulation (in silico) part and specialized artificial intelligence part were incorporated into the system. Combined with the timing of the action potential onset determined by the intra‐atrial signals, the in silico part calculated the virtual atrial action potentials based on the in silico model of human non‐PAF.5 Therefore, the virtual action potentials during non‐PAF could faithfully reproduce the restitution properties of human atria under structural remodeling.
2.2. Patient characteristics and study protocol
Forty‐nine consecutive patients with non‐PAF scheduled to undergo catheter ablation in the Shiga University of Medical Science hospital were enrolled in this study. Then, ExTRa Mapping was applied to the patients with non‐PAF if AF inducibility was confirmed after the PVI. Antiarrhythmic drugs, except for amiodarone, were discontinued at least 3 weeks before the ablation, and no antiarrhythmic drugs were added after the ablation. Informed consent was obtained from each patient and was approved by the ethics committee in Shiga University of Medical Science.
Then, in the whole left atrium (LA) during AF, phase mapping using the ExTRa Mapping and CFAE mapping using the electro‐anatomical mapping 3D navigation system (EnSite NavX™, St. Jude Medical Inc., MN, USA) were performed simultaneously. To avoid the excess overlap of the ExTRa Mapping regions, the CFAE mapping was conducted simultaneously. As for the CFAE mapping, a definition of the excitation cycle length of below 120 ms was employed.
To rapidly find the location of non‐PAF drivers, nonpassively activated areas (NPAs), in which rotational activations were frequently observed, were automatically detected by the ExTRa Mapping. In this study, the NPAs in each patient were determined according to the value of the “nonpassively activated ratio (%NP)” (the ratio of the nonpassively activated period to the recording time) automatically calculated based on the 5‐second real‐time phase map movies created by the ExTRa Mapping.
Also voltage mapping after the electrical cardioversion was performed in the same patients with non‐PAF. In this study, the LVA was defined as an area, of which the intra‐atrial bipolar signal amplitude was below 0.5 mV.
The overlap of NPA with LVAs was determined if the LVA occupied ≥20% of each NPA. Also, that with CFAE areas was determined if the CFAE areas occupied ≥50% of each NPA.
2.3. NPA‐targeted ablation procedure
Based on a dozen or more phase map movies recorded in the entire LA in each patient with non‐PAF, several NPAs that had the top 4 or top 5 highest %NP values were chosen as the ablation targets. This approach was based on our idea that real AF drivers were contained in the NPAs where rotational activations in the form of rotors and/or multiple wavelets were most frequently observed during the 5‐second recording time. The recording time was decided according to the %NP reproducibility in our preliminary study (unpublished).
Both the NPA detection and NPA‐targeted ablation were performed only if AF was still sustained (or sustained AF was easily initiated) even after an extensive encircling PVI and cavotricuspid isthmus (CTI) ablation. The end point of the NPA‐targeted ablation was to modify the electrical and/or anatomical properties of the NPAs. The ablation was achieved by dragging technique filling the NPA (25‐30 W for 10‐20 seconds per ablation point, thus 4‐5 minutes per NPA).
To confirm the AF inducibility after the ablation, an isoproterenol infusion (0.3‐0.4 mcg/h/kg) and an atrial burst pacing protocol (10‐15 mA, ~30 beats, minimum 180 ms or 2:1 response) were employed. For the pacing sites, coronary sinus distal and proximal parts, superior vena cava, and lower lateral wall of right atrium were employed.
2.4. Evaluation of the outcome of non‐PAF ablation
After the ablation, the patients were monitored every 1‐3 months using the 12‐lead ECG and the event ECG monitors for 2‐4 weeks. AF recurrence was defined as AF of duration ≥30 seconds was documented more than several times after the 3‐month blanking period.
To evaluate the outcome of non‐PAF ablation with a Kaplan‐Meier estimate, EZR software6 was employed.
3. RESULTS
3.1. Clinical characteristics
The clinical characteristics of all 49 consecutive non‐PAF patients and of the 28 non‐PAF patients with AF inducibility after PVI are shown in Table 1. It is noteworthy that the non‐PAF duration in the patients who underwent an ExTRa Mapping‐guided ablation was 38 ± 63 months, and 15 patients (54%) had long‐standing persistent AF. Both the mean LA diameter, measured by transthoracic echocardiography (45 ± 6 mm), and the mean blood concentration of the brain natriuretic peptide (BNP) (159 ± 105 pg/mL) in the ExTRa Mapping‐guided ablation patients clearly exceeded each normal range (40 mm and 18.4 pg/mL, respectively). Such LA dilation and BNP increase did not conflict with the typical features of patients with non‐PAF.
Table 1.
Patient characteristics
| All non‐PAF patients (N = 49) | Non‐PAF patients with AF inducibility after PVI (N = 28) | |
|---|---|---|
| Age, mean±SD, y | 63 ± 9 | 64 ± 10 |
| Male, n (%) | 37 (76) | 21 (75) |
| Duration of all types AF, mean±SD, mo | 78 ± 107 | 83 ± 121 |
| Duration of persistent AF, mean±SD, mo | 29 ± 52 | 38 ± 63 |
| Long‐standing persistent AF, n (%) | 21 (43) | 15 (54) |
| CHA2DS2‐VASc score, mean±SD | 2.3 ± 1.7 | 2.4 ± 1.8 |
| Echocardiographic parameters | ||
| Left ventricle ejection fraction, mean±SD, % | 55 ± 9 | 56 ± 8 |
| Interventricular septum, mean±SD, mm | 10 ± 1 | 10 ± 1 |
| LA diameter, mean±SD, mm | 43 ± 6 | 45 ± 6 |
| LA appendage flow, mean±SD, m/s | 0.31 ± 0.15 | 0.33 ± 0.17 |
| BNP, mean±SD, pg/mL | 173 ± 132 | 159 ± 105 |
| Previous session, n (%) | 8 (16) | 6 (25) |
CHA2DS2‐VASc score was defined in the 2010 European Society of Cardiology (ESC) guidelines.
AF, atrial fibrillation; BNP, brain natriuretic peptide; LA, left atrium; Non‐PAF, nonparoxysmal AF.
Among the 28 patients with non‐PAF who underwent the ExTRa Mapping, 5 typical cases that provided us some hints on clarifying the mechanisms of AF persistency and on proposing a novel ablation strategy were chosen.
3.2. Five typical cases who underwent ExTRa Mapping for non‐PAF ablation
3.2.1. Case 1: Long‐standing persistent AF
Case 1 was a 66‐year‐old female with non‐PAF duration of 2.2 years. As the AF did not spontaneously terminated after PVI, voltage mapping was conducted following electrical cardioversion. Then, the AF inducibility was confirmed by burst pacing at LA and simultaneous ExTRa and CFAE mapping during the AF was performed.
LVAs were widely distributed within the LA (Figure 2A), but no obvious CFAEs were observed in the entire LA (Figure 2B). The 4 black open circles in Figures 2A, B represent NPAs (areas with the top 4 highest %NP values) detected by the ExTRa Mapping. Figure 2C shows a snapshot of a phase map movie (Video S1) in one of the NPAs. In the NPA, counterclockwise rotational activations in the form of meandering rotors frequently appeared, and multiple wavelets were shortly observed. Surprisingly, this NPA was neither an LVA nor a CFAE area.
Figure 2.
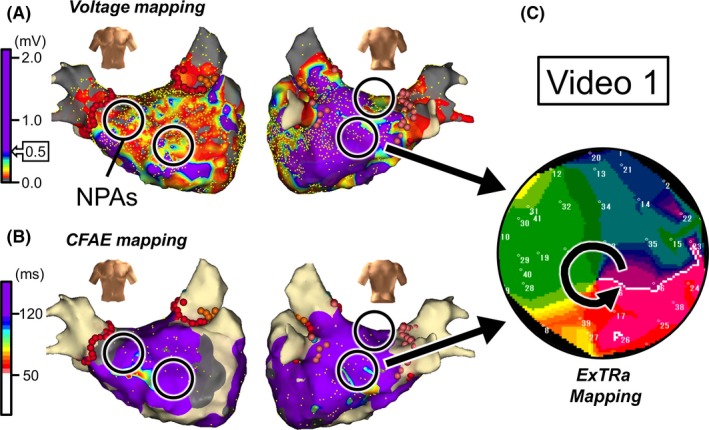
Case 1: 66‐year‐old female, long‐standing persistent AF (2.2 years). A, Voltage map after cardioversion. B, CFAE map during AF. Black open circles in panels A and B represent nonpassively activated areas (NPAs) detected by the ExTRa Mapping. The %NP values in the 4 NPAs were 82%, 53%, 51%, and 51%. C, An example of a phase map in one of the NPAs. Video S1 demonstrates the relevant phase map movie
Thus, 2 of the 4 NPAs did not overlap with LVAs (Figure 2A), whereas all 4 NPAs did not overlapped with CFAE areas (Figure 2B). However, it was experienced that ablation targeting such 4 NPAs, where conventional indirect indicators did not predict the existence of AF drivers, was effective in those patients to maintain sinus rhythm during a 9‐month follow‐up. Please note that in this case, the CFAE areas were not ablated and only a part of the LVAs was ablated.
3.2.2. Case 2: Long‐standing persistent AF
Case 2 was a 49‐year‐old male with non‐PAF duration of 7 years. In this case as well, AF did not spontaneously terminate after PVI, and voltage mapping was conducted after cardioversion. Then, simultaneous ExTRa and CFAE mapping was performed after confirming the AF inducibility.
LVAs were distributed only in the region from mitral isthmus to bottom left of the LA posterior wall (Figure 3A), and CFAE areas were distributed in a patchy fashion over the entire LA (Figure 3B). Again, the 5 black open circles in Figures 3A, B denote NPAs (areas with the top 5 highest %NP values) detected by the ExTRa Mapping. Figure 3C shows a snapshot of the phase map movie (Video S2) in one of the NPAs. In the NPA, clockwise or counterclockwise rotational activations in the form of meandering rotors or multiple wavelets were observed. This NPA was not an LVA but a CFAE area.
Figure 3.
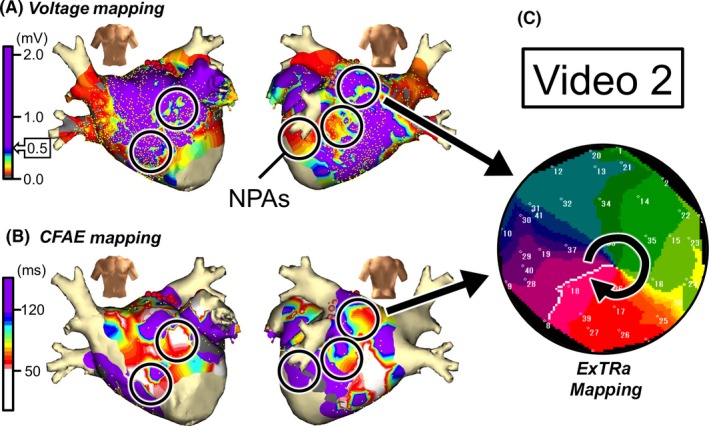
Case 2: 49‐year‐old male, long‐standing persistent AF (7 years). A, Voltage map after cardioversion. B, CFAE map during AF. Black open circles in panels A and B are NPAs detected by the ExTRa Mapping. The %NP values in the 5 NPAs were 100%, 77%, 63%, 61%, and 56%. C, An example of a phase map in one of the NPAs. Video S2 demonstrates the relevant phase map movie
The NPAs (black open circles) did not always overlap with LVAs (Figure 3A) and/or CFAE areas (Figure 3B); 2 of the 5 NPAs were LVAs and 3 of the 5 NPAs were CFAE areas. Again, in this case, the NPAs were chosen as ablation targets regardless of whether the NPAs were LVAs and/or CFAE areas. It was then experienced that the NPA‐targeted ablation was effective in that patient to maintain sinus rhythm during a 10‐month follow‐up. Please note that in this case, only a part of the LVAs and a part of the CFAE areas were ablated.
3.2.3. Case 3: Long‐standing persistent AF
Case 3 was a 69‐year‐old male with non‐PAF duration of 1.2 years. In this case, voltage mapping (Figure 4A) was conducted before PVI because the AF was terminated by electrical cardioversion. After the PVI followed by CTI ablation, the AF inducibility was confirmed by burst pacing, and simultaneous ExTRa and CFAE mapping was conducted (Figure 4B).
Figure 4.
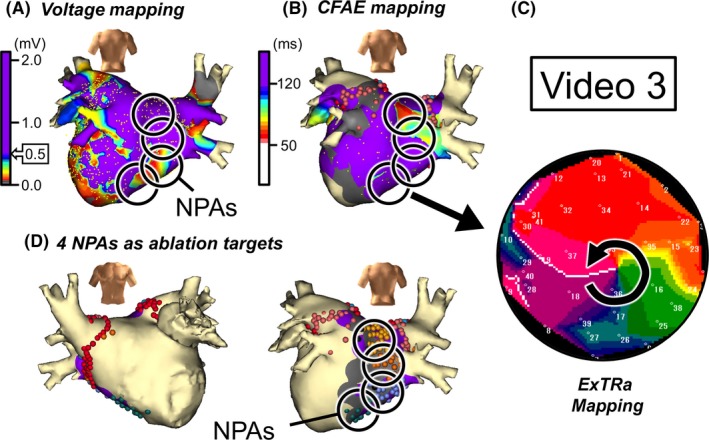
Case 3: 69‐year‐old male, long‐standing persistent AF (1.2 years). A, Voltage map in posterior‐anterior view after cardioversion. B, CFAE map in posterior‐anterior view during AF. Black open circles in panels A and B denote NPAs detected by the ExTRa Mapping. The %NP values in the 4 NPAs were 55%, 49%, 46%, and 43%. C, An example of a phase map in one of the NPAs. Video S3 demonstrates the relevant phase map movie. D, The 4 NPAs were selected as ablation targets
Figure 4C shows a snapshot of the phase map movie (Video S3) in one of the NPAs (areas with the top 4 highest %NP values) detected by the ExTRa Mapping. In the NPA, unstable short‐lived meandering rotors or figure‐of‐eight type reentries were frequently observed. The adjacent NPAs were distributed from posterior wall to bottom of the LA. This suggests that rotors contributing as AF drivers largely meandered only within the adjacent NPAs.
Indeed, the ablation targeting such NPAs as these 4 NPAs (Figure 4D) was effective in maintaining sinus rhythm for the long‐term (free from AF during 18‐month follow‐up). As a matter of fact, nonclinical atrial tachycardia (AT)‐like organized AF in right atrium was induced just after the NPA‐targeted ablation. However, no such an AT‐like arrhythmia as well was not observed during the 18‐month follow‐up.
As for the relationship between the conventional indirect indicators suggesting the existence of AF drivers, it was observed that the spread of LVAs and CFAE areas in the LA was somewhat limited, but 2 of the 4 NPAs overlapped with LVAs (Figure 4A), and 1 of the 4 NPAs overlapped with CFAE areas (Figure 4B). Intriguingly, the NPAs that overlapped with LVAs were not CFAE areas, and the NPAs that overlapped with CFAE areas were not LVAs. As observed in this case, not all AF drivers were detected by the conventional indirect indicators.
3.2.4. Case 4: Long‐standing persistent AF
Case 4 was a 65‐year‐old male with non‐PAF duration of 1.5 years. Voltage mapping was performed after 2 repeated 20‐J cardioversions, and then, PVI was conducted. Simultaneous ExTRa and CFAE mapping was performed after the AF induction because the AF inducibility was confirmed after the PVI.
Four of the 5 NPAs (areas with the top 5 highest %NP values) partially overlapped with LVAs (Figure 5A), whereas only 1 of the 5 NPAs overlapped with obvious CFAE areas (Figure 5B). Figure 5C is a snapshot of the phase map movie (Video S4) of the NPA where that neither overlapped with LVAs nor CFAE areas. In the NPA, following 3 passive excitation waves, counterclockwise rotational activations were observed and followed by multiple wavelets, short‐lived meandering rotors, and figure‐of‐eight type reentries.
Figure 5.
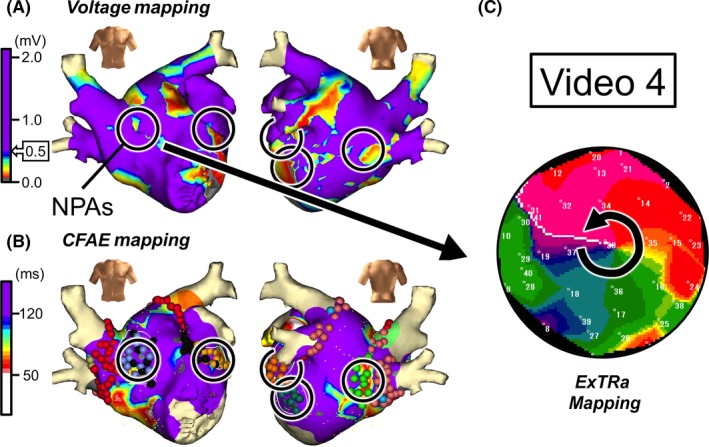
Case 4: 65‐year‐old male, long‐standing persistent AF (1.5 years). A, Voltage map after cardioversion. B, CFAE map during AF. Black open circles in panels A and B denote NPAs detected by the ExTRa Mapping. The %NP values in the 5 NPAs were 70%, 51%, 50%, 46%, and 46%. C, An example of a phase map in one of the NPAs. Video S4 demonstrates the relevant phase map movie
Ablation targeting the 5 NPAs was performed, and the AF inducibility vanished. After a 3‐month blanking period, a recurrence of AF in the form of PAF rather than non‐PAF was observed. Therefore, a second session was performed 11 months later and it was found that there was still no AF inducibility but reconnection of right superior and left superior pulmonary veins (PVs). Indeed, additional ablation to the PV reconnection sites was effective in maintaining sinus rhythm for 5 months after the second session. Such a clinical course might suggest that the NPA‐targeted ablation in the first session was effective in at least modifying the AF maintenance mechanism.
3.2.5. Case 5: Persistent AF
Case 5 was a 77‐year‐old male with non‐PAF duration of 11 months. This patient received both PVI and CTI ablation 9 years prior because of PAF and a second session 8 years prior for the PAF recurrence. During the second session, both additional ablation of the reconnected PVs and that of a box isolation plus mitral isthmus block line were performed. However, 11 months before the third session (this session), AF in the form of non‐PAF rather than PAF began.
As cardioversion did not terminate the AF, no voltage mapping was conducted before the NPA‐targeted ablation. Thus, only simultaneous ExTRa and CFAE mapping was performed during the AF. It was then observed that only 2 of the 5 NPAs (areas with the top 5 highest %NP values) partially overlapped with CFAE areas, but 3 residual NPAs of the 5 NPAs did not overlap with obvious CFAE areas (Figure 6A). Figure 6B shows a snapshot of the phase map (Video S5) of one of the NPAs. In the NPA, a single counterclockwise rotational activation followed by short‐lived meandering rotors and/or multiple wavelets was continuously observed.
Figure 6.
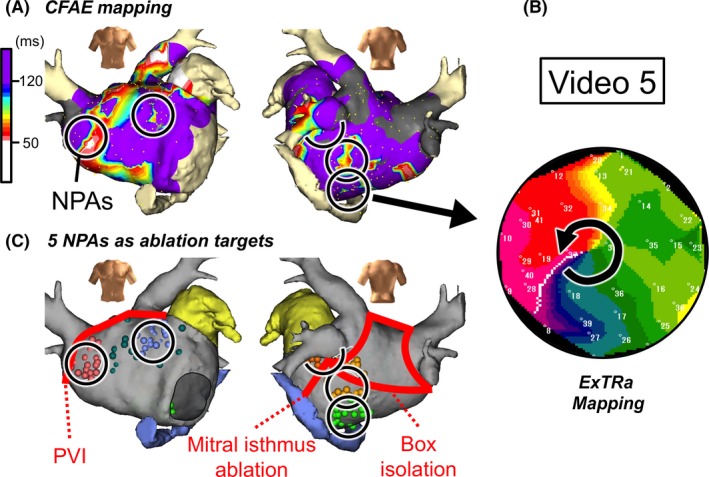
Case 5: 77‐year‐old male, persistent AF (11 months), A, CFAE map during AF. Black open circles are NPAs detected by the ExTRa Mapping. The %NP values in the 5 NPAs were 76%, 64%, 63%, 61%, and 58%. B, An example of a phase map in one of the NPAs. Video S5 demonstrates the relevant phase map movie. C, Locations of the 5 ablated NPAs in the third session are superimposed on the block lines constructed by a box isolation and mitral isthmus ablation in the second session
It is noteworthy that all 5 NPAs detected by the ExTRa Mapping were located outside the region of the box isolation (Figure 6C). In this case, only an NPA‐guided ablation during AF was performed, and after the cardioversion, it was confirmed that there were no PV reconnections. After the last session, sinus rhythm was maintained during a 15‐month follow‐up period.
3.3. NPAs vs conventional indirect indicators of AF drivers
Because we experienced that catheter ablation targeting NPAs instead of LVAs or CFAE areas was effective in the above‐mentioned 5 patients with non‐PAF, we applied the same ablation procedure to the remaining patients with non‐PAF. The phase map movies in all 28 patients with non‐PAF who underwent ExTRa Mapping were analyzed, and the correlation between the NPAs and distribution of the LVAs and CFAE areas was investigated. Consequently, we found that the NPAs did not always coincide with LVAs or CFAE areas and that there was no significant correlation among them (P = .990 and P = .155, respectively).
3.4. Outcome of the ExTRa Mapping‐guided NPA ablation
The freedom from non‐PAF/AT during the 8.1 ± 4.2‐month follow‐up of the ExTRa Mapping‐guided NPA ablation in addition to PVI was 79%, which was markedly higher than that of the CFAE‐targeted ablation in our hospital (47%, unpublished data). Furthermore, the Kaplan‐Meier estimate of the freedom from non‐PAF/AT in all the patients with non‐PAF (N = 49) clearly demonstrated that the long‐term (≥12 months) outcome of the freedom from non‐PAF/AT was above 75% (Figure 7, red line). Even when the patients with non‐PAF who received PVI only because of no AF inducibility after PVI were excluded, the long‐term outcome (N = 28) was also ~75% (Figure 7, black line).
Figure 7.
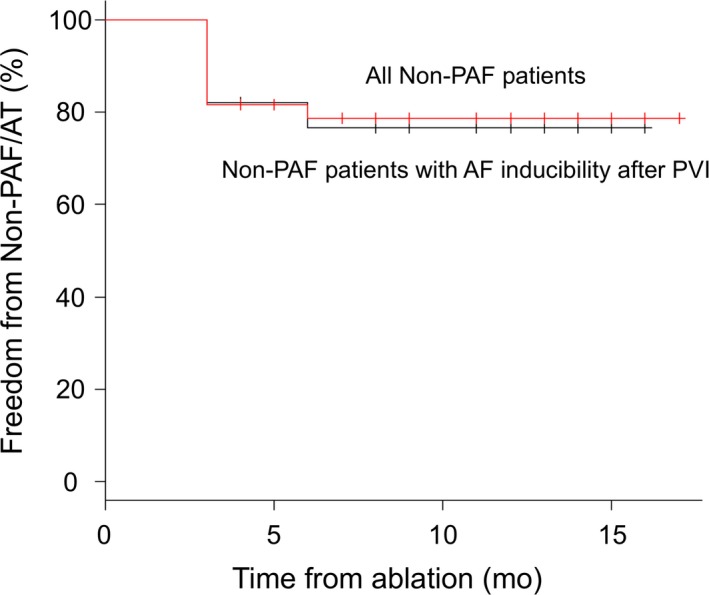
Kaplan‐Meier estimates of freedom from non‐PAF/AT. Red line represents the ratio freedom from non‐PAF/AT in all non‐PAF patients (N = 49); the patients in whom ExTRa Mapping‐guided NPA ablation was performed because of AF inducibility after PVI, and in whom PVI only was performed because of no AF inducibility after the PVI. Black line denotes the ratio freedom from non‐PAF/AT in the non‐PAF patients in whom the ExTRa Mapping‐guided ablation was applied because of AF inducibility even after the PVI (N = 28)
4. DISCUSSION
The major findings of the present study were as follows: (i) our recently developed novel online real‐time phase mapping system, called ExTRa Mapping, was very useful for imaging the AF wave dynamics and detecting NPAs assumed to contain AF drivers in patients with non‐PAF; (ii) the NPAs did not always coincide with the conventional indirect indicators of AF drivers; and (iii) the ablation targeting such NPAs might be effective in patients with non‐PAF for maintaining sinus rhythm.
4.1. Mechanisms on AF persistency in patients with non‐PAF
Although it is accepted that functional reentry with a spiral‐shaped wavefront, called rotor or spiral wave reentry, is involved in the mechanism of AF, the type of excitation wave dynamics leading to AF persistency is still unknown. Even for basic research, in terms of the excitation wave dynamics during AF, several hypotheses, that is, multiple wavelets,7, 8 meandering rotors,9 and stationary stable rotors,10 have been proposed; therefore, it is natural that the mechanisms of non‐PAF persistency in clinical practice are still unclear.
One of the main reasons for the difficulty in elucidating the mechanism of AF persistency in patients with non‐PAF is that the optical mapping techniques11, 12 commonly employed in animal experiments cannot be applied to clinical practice because of both an invasive thoracotomy procedure and the toxicity of the voltage‐sensitive dye. Another reason is that existing high‐density electro‐anatomical mapping systems cannot be applied to unstable random reentries during AF. Therefore, clinically available real‐time AF imaging systems such as this ExTRa Mapping have long been sought.
In the present study, the ExTRa Mapping was applied to patients with non‐PAF and as a result, other than the passively activated planar wave propagation, the typical non‐PAF wave dynamics were rotational activations in the form of meandering rotors and/or multiple wavelets, as shown in Figures 1, 2C, 3C, 4C, 5C, and 6B (Videos[Link], [Link], [Link], [Link], [Link]). Indeed, such rotational activations, which were defined as NPAs, were effective ablation targets at least in most of our patients with non‐PAF. It is generally thought that both the LVAs and CFAE areas contain AF drivers or their substrate. Therefore, such indirect indicators of AF drivers have been used as ablation targets for non‐PAF. However, based on this study, NPAs where meandering rotors and/or multiple wavelets were frequently observed did not necessarily coincide with the indirect indicators (compare panels A and B in Figures 2 to 5). Moreover, no significant correlation between the NPAs and LVAs or CFAE areas was found. Thus, it was conjectured that the mechanism of AF persistency in patients with non‐PAF was multifactorial because all of the following phenomena were observed as follows: (i) NPAs coincided with both LVAs and CFAE areas, (ii) NPAs coincided with either LVAs or CFAE areas, and (iii) NPAs coincided with neither LVAs nor CFAE areas.
The spatial density of the phase map drawn by the ExTRa Mapping was ~8 signals/cm2, which was much higher than that needed to differentiate rotor from focal activation,13 that is, ≥4 signals/cm2. The accuracy of the phase mapping was sufficiently verified with in silico model studies. It was therefore reasonable to think that the non‐PAF encountered in clinical practice was mostly driven by spatially and temporally unstable rotors rather than stationary stable rotors.
4.2. Comparison with other phase mapping systems
In the United States and Europe, several AF visualization systems available in clinical practice have been proposed. One of the systems was proposed by Narayan, et al14 (Topera® Solution, Abbott Lab., CA, USA). The system employs a 64‐pole basket catheter to conduct a panorama mapping of the atria. Another one employs a body surface 252‐electrode vest (CardioInsight™, Medtronic Inc., MN, USA) to solve the inverse problem of AF wave dynamics and was originally developed by Rudy's group15 and applied to AF ablation by Haïssaguerre's group.16
However, as far as we know, the above‐mentioned phase mapping systems contain several limitations as follows: offline use thus requiring a long period for AF visualization, low spatial density of <4 signals/cm2, and insufficient verification of the imaging algorithms, resulting from poor electrode contact17 or constraints due to the inverse problem.18 Moreover, those phase mapping systems are not currently available in our country, Japan.
On the other hand, the ExTRa Mapping online real‐time AF imaging system complements the intra‐atrial signals with a specialized artificial intelligence algorithm and provides a higher density imaging movie within a few seconds. In addition, a notable feature of the present system is that the phase map is based on myocardial action potentials, which are similar to experiments employing optical mapping systems,11 whereas the phase maps produced by the aforementioned systems14, 16 are based only on the timing of the excitation wavefront propagation.
4.3. Comparison with extensive ablation
In this study, limited local ablation suggested the potential benefit beyond PVI. However, it is reported that extensive ablation, such as stepwise ablation, resulted in similar outcome to PVI.19 A possible explanation about this discrepancy is that excessive extensive ablation eliminates most of the rotors, but at the same time, it stabilizes the remaining rotors, resulting in the cancelation of the additional benefits. Whereas, the ExTRa Mapping‐guided NPA ablation might eliminate only a part of rotors but do not stabilize remaining rotors, thus likely to receive additional benefits.
4.4. Study limitations
The novel phase mapping system employed in the present study might include some unknown limitations because the development of the system is still in progress. Although this system is not yet widely available even in Japan, we hope this system will spread to the world in the near future.
Although we directly observed the AF wave dynamics in real‐time by applying our novel phase mapping system in patients with non‐PAF, it was still very difficult to extract the real AF drivers from the various NPAs detected by our system. Although not all NPAs coincided with true AF drivers, we strongly believe that most NPAs with high %NP values contained, more or less, true AF drivers.
As this study was not randomized clinical trial, this study only suggested the potential benefit of NPA‐targeted ablation beyond PVI. Moreover, we did not perform right atrial mapping in the first session. Nevertheless, the ablation outcome was much better than we expected.
Further studies are needed to elucidate these issues.
5. CONCLUSIONS
Not all non‐PAF drivers might be included in CFAE areas or LVAs. To detect AF drivers during a non‐PAF ablation, real‐time imaging of the AF wave dynamics using ExTRa Mapping might be more useful than the conventional indirect indicators of AF drivers only.
CONFLICT OF INTEREST
The authors have received research support from the Nihon Kohden Corporation.
Supporting information
ACKNOWLEDGEMENTS
We would like to thank Mr. John Martin for revising the English text of this manuscript.
Sakata K, Okuyama Y, Ozawa T, et al. Not all rotors, effective ablation targets for nonparoxysmal atrial fibrillation, are included in areas suggested by conventional indirect indicators of atrial fibrillation drivers: ExTRa Mapping project. J Arrhythmia. 2018;34:176–184. https://doi.org/10.1002/joa3.12036
Funding information
This work was supported in part by the Grant‐in‐Aid 16K09431 and 25461106 for Scientific Research (C) (to K.S., T.O., R.H., K.N., and T.A.), 15H01801 for Scientific Research (A) (to K.N. and T.A.), and 15K09077 for Scientific Research (C) (to K.N. and T.A.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1. Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 2. Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–53. [DOI] [PubMed] [Google Scholar]
- 3. Verma A, Jiang CY, Betts TR, et al. STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 4. Rolf S, Kircher S, Arya A, et al. Tailored atrial substrate modification based on low‐voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:825–33. [DOI] [PubMed] [Google Scholar]
- 5. Ashihara T, Haraguchi R, Nakazawa K, et al. The role of fibroblasts in complex fractionated electrograms during persistent/permanent atrial fibrillation: implications for electrogram‐based catheter ablation. Circ Res. 2012;110:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J. 1964;67:200–20. [DOI] [PubMed] [Google Scholar]
- 8. Allessie MA, Lammers WJEP, Bonke FIM, et al. Experimental evaluation of Moe's multiple wavelet hypothesis of atrial fibrillation In: Zipes DP, Jalife J. editors. Cardiac electrophysiology and arrhythmias. Orlando, FL: Grune & Stratton; 1985: p. 265–75. [Google Scholar]
- 9. Ikeda T, Czer L, Trento A, et al. Induction of meandering functional reentrant wave front in isolated human atrial tissues. Circulation. 1997;96:3013–20. [DOI] [PubMed] [Google Scholar]
- 10. Mandapati R, Skanes A, Chen J, et al. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–9. [DOI] [PubMed] [Google Scholar]
- 11. Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–8. [DOI] [PubMed] [Google Scholar]
- 12. Davidenko JM, Kent PF, Chialvo DR, et al. Sustained vortex‐like waves in normal isolated ventricular muscle. Proc Natl Acad Sci U S A. 1990;87:8785–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rappel WJ, Narayan SM. Theoretical considerations for mapping activation in human cardiac fibrillation. Chaos. 2013;23:023113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramanathan C, Ghanem RN, Jia P, et al. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med. 2004;10:422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haïssaguerre M, Hocini M, Shah AJ, et al. Noninvasive panoramic mapping of human atrial fibrillation mechanisms: a feasibility report. J Cardiovasc Electrophysiol. 2013;24:711–7. [DOI] [PubMed] [Google Scholar]
- 17. Benharash P, Buch E, Frank P, et al. Quantitative analysis of localized sources identified by focal impulse and rotor modulation mapping in atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodrigo M, Guillem MS, Climent AM, et al. Body surface localization of left and right atrial high‐frequency rotors in atrial fibrillation patients: a clinical‐computational study. Heart Rhythm. 2014;11:1584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fink T, Schlüter M, Heeger CH, et al. Stand‐alone pulmonary vein isolation versus pulmonary vein isolation with additional substrate modification as index ablation procedures in patients with persistent and long‐standing persistent atrial fibrillation: the randomized Alster‐Lost‐AF trial. Circ Arrhythm Electrophysiol. 2017;10:e005114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


