Abstract
Background
Whether nonsustained ventricular tachycardia (NSVT) is a marker of increased risk of sustained ventricular tachyarrhythmias (VTAs) remains to be established in patients receiving cardiac resynchronization therapy with a defibrillator (CRT‐D) for primary prevention.
Methods
Among the follow‐up data of the Japan cardiac device treatment registry (JCDTR) with an implantation date between January 2011 and August 2015, information regarding a history of NSVT before the CRT‐D implantation for primary prevention had been registered in 269 patients. Outcomes were compared between two groups with and without NSVT: NSVT group (n = 179) and No NSVT group (n = 90).
Results
There was no significant difference with regard to age, gender, and NYHA class between the two groups. Left ventricular ejection fraction (LVEF) was 25.6% in the NSVT group and 28.0% in the No NSVT group (P = .046). The rate of appropriate therapy at 24 months was 26.0% and 18.4% in the NSVT and No NSVT groups (P = .22), respectively. Survival free from heart failure death was reduced in the NSVT group, as compared with the No NSVT group, with the rate of 90.2% vs 97.2% at 24 months (P = .030). A multivariate analysis identified a history of NSVT, anemia, and no use of angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin‐receptor blocker (ARB) as predictors of heart failure death.
Conclusions
NSVT appears to be a surrogate marker of severe heart failure rather than a substrate for subsequent sustained VTAs in patients with CRT‐D for primary prevention.
Keywords: appropriate therapy, cardiac resynchronization therapy with a defibrillator, heart failure death, nonsustained ventricular tachycardia, primary prevention
1. INTRODUCTION
The significance of nonsustained ventricular tachycardia (NSVT) in symptomatic heart failure patients had been evaluated in the late 1990s and early 2000s. In the Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina‐Grupo de Estudios Multicéntricos en Argentina (GESICA‐GEMA) study, amiodarone reduced all‐cause mortality and sudden cardiac death in patients with severe heart failure independently of the presence of NSVT, whereas NSVT was an independent marker for increased rate of mortality and sudden cardiac death.1, 2 The increased mortality associated with the presence of NSVT was similar in the ischemic and nonischemic cardiomyopathy.2 However, the Congestive Heart Failure‐Survival Trial of Antiarrhythmic Therapy (CHF‐STAT) study showed that NSVT had a trend as an independent predictor of all‐cause mortality but not for sudden death in patients with congestive heart failure and ventricular arrhythmias.3 The Prospective Randomized Milrinone Survival Evaluation (PROMISE) study demonstrated that the presence of NSVT was not a significant predictor of overall mortality or sudden death in patients with moderate‐to‐severe heart failure (New York Heart Association(NYHA) class III or IV) and a left ventricular ejection fraction (LVEF) of 35% or less.4 These studies were performed before widespread use of beta‐blocker, which can significantly reduce sudden cardiac death. For example, the proportion of patients taking beta‐blockers was <10% in the CHF‐STAT study and 0% in the PROMISE study. Therefore, the prognostic significance of NSVT in patients with heart failure is ambiguous, and little is known about the clinical implication of NSVT especially in patients receiving contemporary care with guideline‐directed medical therapy (GDMT) including angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin‐receptor blocker (ARB) and beta‐blocker.
The aim of the present study is to evaluate the prognostic significance of NSVT and to examine the relationship between a history of NSVT and implantable cardioverter–defibrillator (ICD) therapy in heart failure patients receiving cardiac resynchronization therapy with a defibrillator (CRT‐D) for primary prevention based on data from the Japan Cardiac Device Treatment Registry (JCDTR).
2. METHODS
2.1. Study population
The JCDTR was established in 2006 by the Japanese Heart Rhythm Society (JHRS) for a survey of actual conditions in patients undergoing implantation of cardiac implantable electronic devices (ICD/CRT‐D/CRT‐P) as described previously.5, 6, 7 Members of the JHRS are encouraged to register their data under a unified protocol, which was normally approved by each facility. The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. In Hokkaido University Hospital, the protocol was approved on September 20, 2012, by the Ethics Committee (approval number: 012‐0156). Among 2714 CRT‐D recipients for primary prevention with an implantation date between January 2011 and August 2015,8 the follow‐up data were available in 620 patients as of September 16, 2015.9 Information regarding presence or absence of NSVT had been registered at the CRT‐D implantation in 269 patients (43%). These 269 patients were analyzed in the present study. NSVT was defined as 3 or more consecutive beats arising below the atrioventricular node with a rate of >100 beats/min and lasting <30 seconds.
2.2. Outcomes
The analyzed events were (i) death from any cause, (ii) heart failure death, (iii) sudden cardiac death, (iv) noncardiac death, and (v) appropriate and inappropriate ICD therapies including shock and/or antitachycardiac pacing. The diagnosis of the cause of death was made by attending physicians.
2.3. Statistical analysis
All data are expressed as mean ± SD. Simple between‐group analysis was conducted using Student's t‐test. Categorical variables were compared using Fisher's exact test. Kaplan–Meier curves were constructed to estimate event‐free outcomes in the two study groups with comparison using the log‐rank test. A multivariate Cox proportional‐hazards regression model was used to estimate hazard ratios for clinical events. Among the variables that reached a significance level of P < .1 in univariate models, a stepwise selection was used to determine the most agreeable model. Differences with P < .05 were considered significant. Statview version 5.0 for Windows (SAS Institute Inc, Cary, NC, USA) or R software ver.3.2.3 (https://www.r-project.org/) was used for all statistical analyses.
3. RESULTS
3.1. Patient characteristics
The characteristics of patients receiving CRT‐D for primary prevention with (n = 179; NSVT group) or without (n = 90; No NSVT group) NSVT are shown in Table 1. These data were derived from the status of each patient just before the implantation of CRT‐D. Proportion of nonischemic etiology was higher, and LVEF was lower in patients with NSVT than in those without NSVT. Patients with NSVT were less likely to have a history of hypertension. The level of BNP was higher in the NSVT group, as compared with that in the No NSVT group, although it was missing in about 10% of the patients. The distribution of NYHA functional class was not significantly different between the two groups.
Table 1.
Characteristics of the patients
| NSVT (n = 179) | No NSVT (n = 90) | P value | |
|---|---|---|---|
| Age (y) | 64.9 ± 12.1 | 66.1 ± 11.0 | .436 |
| Male | 141 (78.8) | 64 (71.1) | .164 |
| Underlying heart disease | |||
| Ischemic | 37 (20.7) | 33 (36.7) | .0048 |
| Nonischemic | 142 (79.3) | 57 (63.3) | |
| LVEF (%) | 25.6 ± 9.0 | 28.0 ± 9.9 | .046 |
| LVEF ≦30% | 134 (74.9) | 62 (68.9) | .299 |
| NYHA class | |||
| I | 1 (0.6) | 3 (3.3) | .267 |
| II | 44 (24.6) | 26 (28.9) | |
| III | 117 (65.4) | 53 (58.9) | |
| IV | 17 (9.5) | 8 (8.9) | |
| Heart rate (/min) | 72.1 ± 16.6 | 69.2 ± 14.7 | .162 |
| QRS duration (ms) | 149.0 ± 32.7 | 152.9 ± 27.9 | .338 |
| QT interval (ms) | 445.8 ± 53.4 | 454.2 ± 52.7 | .226 |
| Atrial lead | |||
| Absent | 29 (16.2) | 9 (10.0) | .168 |
| Present | 150 (83.8) | 81 (90.0) | |
| AF | 26 (14.5) | 12 (13.3) | .791 |
| Diabetes mellitus | 45 (25.1) | 31 (34.4) | .110 |
| Hypertension | 64 (35.8) | 44 (48.9) | .038 |
| Dyslipidemia | 57 (31.8) | 34 (37.8) | .332 |
| Hyperuricemia | 34 (19.0) | 18 (20.0) | .844 |
| Cerebral infarction | 14 (7.8) | 8 (8.9) | .763 |
| Peripheral artery disease | 3 (1.7) | 5 (5.6) | .077 |
| BNP (pg/mL)a | 690.1 ± 706.4 | 474.5 ± 567.6 | .017 |
| Hemoglobin (g/dL) | 13.2 ± 1.9 | 12.8 ± 1.9 | .196 |
| Creatinine (mg/dL) | 1.28 ± 0.99 | 1.41 ± 1.69 | .415 |
| Goldenberg scoreb | 2.2 ± 1.1 | 2.3 ± 1.1 | .501 |
LVEF, left ventricular ejection fraction; AF, atrial fibrillation.
Values are means ± SD, or number (%).
The value of BNP was missing in 15 patients with NSVT and 8 patients without NSVT.
The original risk score model comprised 5 clinical factors including (i) NYHA class > II, (ii) AF, (iii) QRS duration >120 ms, (iv) age >70 years, and (v) blood urea nitrogen (BUN) >26 mg/dL. Because BUN was not collected in the JCDTR database, blood creatinine >1.5 mg/dL was used as a risk factor instead of BUN.
Pharmacological therapy in the NSVT and No NSVT groups is shown in Table 2. Use of aldosterone antagonists and oral anticoagulant agents was higher in the NSVT group vs No NSVT group. The rate of having antiplatelet agents was lower in the NSVT group than in the No NSVT group.
Table 2.
Pharmacological therapy
| NSVT (n = 179) | No NSVT (n = 90) | P value | |
|---|---|---|---|
| Ia | 1 (0.6) | 1 (1.1) | .619 |
| Ib | 2 (1.1) | 1 (1.1) | .996 |
| Ic | 0 (0.0) | 1 (1.1) | .158 |
| β‐blockers | 141 (78.8) | 63 (70.0) | .113 |
| III | 67 (37.4) | 26 (28.9) | .165 |
| Ca2+ antagonists | 12 (6.7) | 6 (6.7) | .991 |
| Digitalis | 24 (13.4) | 10 (11.1) | .593 |
| Diuretics | 147 (82.1) | 66 (73.3) | .094 |
| ACEI/ARB | 125 (69.8) | 67 (74.4) | .430 |
| Aldosterone antagonists | 93 (52.0) | 34 (37.8) | .028 |
| Nitrates | 24 (13.4) | 10 (11.1) | .593 |
| Statins | 53 (29.6) | 37 (41.1) | .059 |
| Oral anticoagulant agents | 99 (55.3) | 36 (40.0) | .018 |
| Antiplatelet agents | 58 (32.4) | 43 (47.8) | .014 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II‐receptor blocker.
Data are given as number (%).
3.2. Outcomes
During a mean follow‐up of 21 ± 12 months, death from any cause occurred in 40 of 179 patients (22.3%) in the NSVT group and 9 of 90 patients (10.0%) in the No NSVT group. These events included 21 heart failure deaths (11.7%) and 6 sudden cardiac death (3.3%) in the NSVT group and 2 heart failure death (2.2%) and 2 sudden cardiac death (2.2%) in the No NSVT group.
Kaplan–Meier estimates of event‐free survival in the two groups are shown in Figure 1. There was an increased trend in the risk of death (P = .074) and a significant increase in the risk of heart failure death (P = .030) in the NSVT group as compared with the No NSVT group (Figure 1A,B). The rate of sudden cardiac death and noncardiac death did not differ between the two groups (Figure 1C,D). With regard to the rate of appropriate and inappropriate ICD therapy (shock and/or antitachycardiac pacing), there was no significant difference between the two groups (Figure 2A,B).
Figure 1.
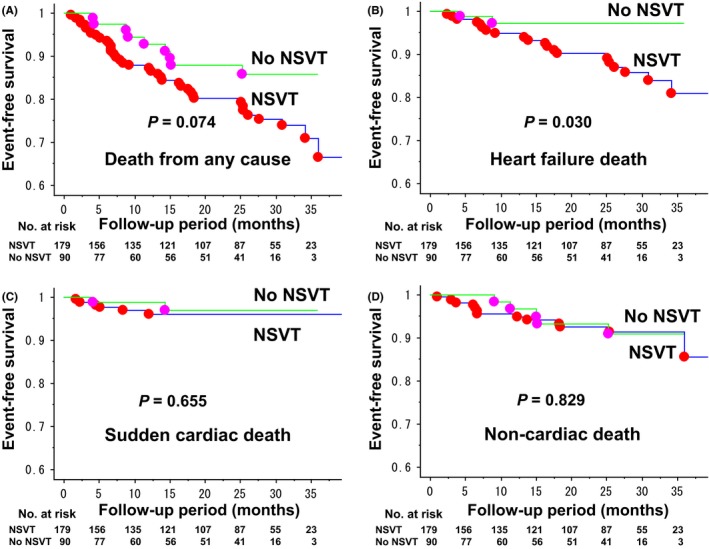
Kaplan–Meier estimates for event‐free survival in CRT‐D recipients for primary prevention of sudden cardiac death with and without prior history of NSVT. Outcome events were death from any cause (A), heart failure death (B), sudden cardiac death (C), and noncardiac death (D). The mean follow‐up period was 22 ± 12 months in the NSVT group and 19 ± 11 months in the No NSVT group (P = .078). A, The rate of death from any cause at 12 and 24 months was 12.8% and 19.8% in the NSVT group, and 7.1% and 12.1% in the No NSVT group (NSVT vs No NSVT, P = .074 by log‐rank test). B, The rate of heart failure death at 12 and 24 months was 5.2% and 9.8% in the NSVT group, and 2.8% and 2.8% in the No NSVT group (NSVT vs No NSVT, P = .030 by log‐rank test). C, The rate of sudden cardiac death at 12 and 24 months was 3.1% and 3.9% in the NSVT group, and 1.2% and 3.0% in the No NSVT group (NSVT vs No NSVT, P = .655 by log‐rank test). D, The rate of noncardiac death at 12 and 24 months was 4.4% and 7.5% in the NSVT group, and 3.3% and 6.8% in the No NSVT group (NSVT vs No NSVT, P = .829 by log‐rank test)
Figure 2.
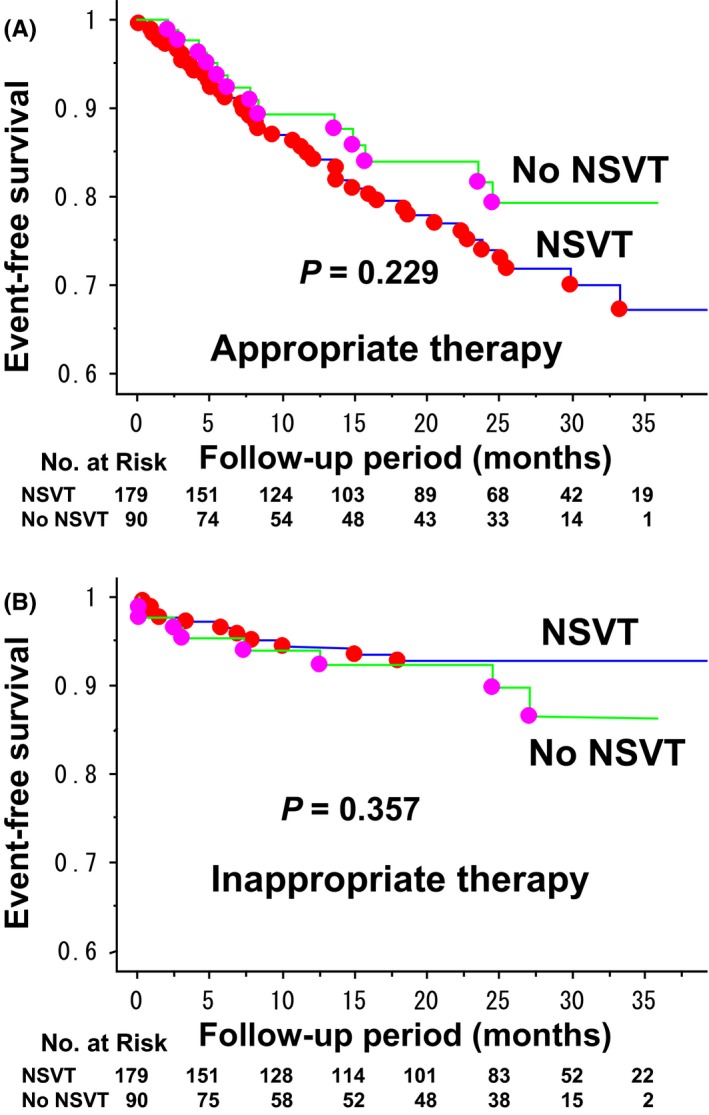
Kaplan–Meier curves comparing first occurrence of ICD therapy between CRT‐D recipients for primary prevention of sudden cardiac death with vs without prior history of NSVT. A, The rate of appropriate ICD therapy at 12 and 24 months was 15.1% and 26.0% in the NSVT group, and 10.6% and 18.4% in the No NSVT group (NSVT vs No NSVT, P = .229 by log‐rank test). B, The rate of inappropriate ICD therapy at 12 and 24 months was 5.7% and 7.3% in the NSVT group, and 6.0% and 7.8% in the No NSVT group (NSVT vs No NSVT, P = .357 by log‐rank test)
The variables associated with the risk of heart failure death obtained by univariate models (P < .1) were presence of NSVT (P = .047), hemoglobin (P = .090), use of angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin II‐receptor blocker (ARB) (P = .019), and use of aldosterone antagonists (P = .078). In this selected population, LVEF was not significantly associated with a risk of heart failure death by a univariate analysis (hazard ratio 0.96; 95% confidence interval [CI], 0.91 to 1.01; P = .124) and it was not included in the multivariate analysis. A stepwise regression modeling was used to identify factors associated with heart failure death. They were presence of NSVT, hemoglobin, and use of angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin II‐receptor blocker (ARB), and hazard ratios determined by a multivariate Cox proportional‐hazards regression model are shown in Figure 3.
Figure 3.
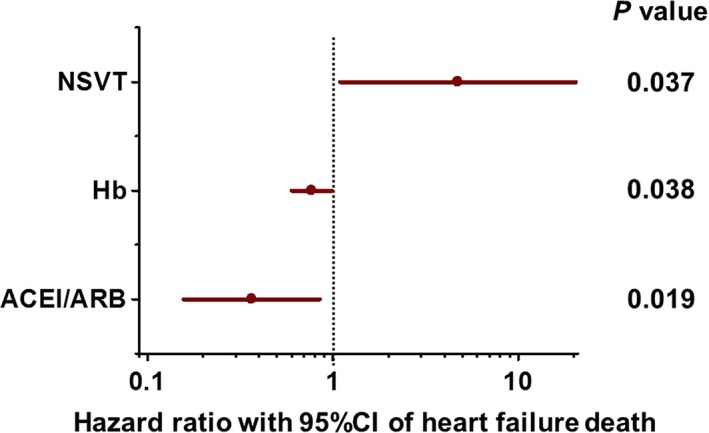
Hazard ratio for heart failure death determined by a stepwise Cox regression for possible factors in CRT‐D recipients for primary prevention of sudden cardiac death. The presence of NSVT (hazard ratio with NSVT vs without NSVT, 4.73; 95% confidence interval [CI], 1.09 to 20.43; P = .037), lower hemoglobin level (hazard ratio per unit (g/dl) hemoglobin, 0.77; 95% CI, 0.60 to 0.99; P = .038), and no use of angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin II‐receptor blocker (ARB) (hazard ratio with ACEI/ARB vs without ACEI/ARB, 0.37; 95% CI, 0.16 to 0.85; P = .019) were significantly associated with heart failure death in CRT‐D recipients for primary prevention
4. DISCUSSION
The present study demonstrated, with analyses of the JCDTR database, a history of NSVT was associated with increased risk of heart failure death in patients undergoing a CRT‐D implantation, whereas it was not a significant predictor of subsequent appropriate ICD therapies. In addition to NSVT, no use of ACEI/ARB and the lower level of hemoglobin were identified as predictors of heart failure death. It is important to adhere to the current GDMT, especially ACEI/ARB for reducing the mortality in heart failure patients receiving CRT‐D.
CRT reduces mortality similarly in both ischemic and nonischemic cardiomyopathy, whereas a defibrillator function could prolong the long‐term survival, especially of CRT recipients with an ischemic etiology for primary prevention of sudden cardiac death.10 In a previous analysis of the JCDTR database, we identified four factors associated with CRT‐D vs CRT‐P implantation in heart failure patients without sustained ventricular tachyarrhythmias.8 They were male gender, younger age, reduced LVEF, and a history of NSVT. In contrast to the COMPANION11 and REVERSE12 studies, no significant superiority of CRT‐D to CRT‐P, in terms of reducing the risk of death, was observed in a recent study of the JCDTR after adjusting the clinical variables.9 This may be in part due to an incomplete adjustment of the study populations. For example, information regarding presence or absence of NSVT was missing in about 60% of the patients, thereby excluding it as a variable for the multivariate analysis.9
The observation that the rate of appropriate ICD therapies was not significantly higher in CRT‐D recipients with NSVT than in those without NSVT is not surprising, as most of the studies in heart failure patients reported that NSVT did not specifically predict sudden cardiac death or major arrhythmic events of sustained VT/VF and that low LVEF was the predictor of these events (Table 3).3, 4, 13 One of the important findings is that NSVT in patients with reduced LVEF represented the higher risk of heart failure death despite the presence of CRT and beta‐blocker therapy with a rate of more than 70%. This is in agreement that nonischemic CRT‐D recipients with NSVT in the MADIT‐CRT study had an increased risk of the combined end point of heart failure and death, as compared with those without NSVT.14 Moreover, in the study population of Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT), rapid‐rate NSVT identified in the ICD log was associated with a >4‐fold higher risk of a subsequent first appropriate shock and a >2‐fold higher risk of death compared with patients without NSVT.15 MADIT‐CRT patients with NSVT had a higher burden of premature ventricular contractions (PVCs), lower percentage of biventricular pacing, and less reverse remodeling.14 These findings may explain the worse outcomes of CRT‐D patients with NSVT, implicating the potential therapeutic interventions for the management of NSVT and PVCs in heart failure patients.
Table 3.
Prognostic significance of NSVT in patients with heart failure
| Incidence of NSVT | Isch | NYHA class | LVEF | NSVT in heart failure | |
|---|---|---|---|---|---|
| GESICA‐GEMA (1996) (n = 516)2 | 34% | 38% | II 20% | ≤35% |
RR for death 1.69; 95%CI 1.27‐2.24, P < .0002 RR for SD 2.77; 95% CI 1.78‐4.44, P < .001 |
| III 48% | |||||
| IV 32% | |||||
| CHF STAT (1998) (n = 674)3 | 78% | 70% | II 55% | ≤40% | NSVT showed a trend (P = .07) as a predictor for mortality but not for SD |
| III, IV 45% | |||||
| PROMISE (2000) (n = 1080)4 | 61% | 54% | III 58% | ≤35% | NSVT did not specifically predict SD |
| IV 42% | EF was the most powerful predictor of SD | ||||
| MACAS (2003) (n = 343)13 | 32% | 0% | I 12% | ≤45% | RR of NSVT for MAEs 1.71; 95%CI 0.88‐3.31, P = .106 |
| II 63% | RR of EFa for MAEs 2.28; 95%CI 1.55‐3.33, P = .0001 | ||||
| III 25% | RR of betab for MAEs 0.59; 95%CI 0.29‐1.19, P = .134 |
N, number of patients; Isch, ischemic cardiomyopathy; RR, relative risk; 95%CI, 95% confidence interval; SD, sudden death; MAEs, major arrhythmic events defined as sustained VT, VF or sudden death; GESICA‐GEMA, Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA) ‐Grupo de Estudios Multicéntricos en Argentina (GEMA); CHF‐STAT, Congestive Heart Failure‐Survival Trial of Antiarrhythmic Therapy; PROMISE, Prospective Randomized Milrinone Survival Evaluation; MACAS, Marburg Cardiomyopathy Study.
10% decrease of LVEF.
beta: beta‐blockers.
NSVT beyond 24‐48 hours after acute myocardial infarction and/or acute coronary syndrome is associated with the risk of cardiovascular death (Table 4),16, 17, 18 especially during the first 30 days after presentation.16 The MERLIN‐TIMI 36 study reported an interesting relationship between the rate of sudden cardiac death and the number of beats during NSVT for the first 7 days of non‐ST elevation acute coronary syndrome (Table 4).18 In the DINAMIT19 and IRIS20 studies, prophylactic ICD therapy reduced arrhythmic and sudden cardiac death, but it did not reduce overall mortality in patients with a reduced LVEF (≦40% or ≦35%) and <40 days after a myocardial infarction. One of the enrollment criteria for the IRIS study was to detect NSVT on days 5‐31 after infarction. Those studies concluded that the factors associated with arrhythmia requiring ICD therapy are also associated with an increased risk of nonsudden death.19, 20, 21 Therefore, it is reasonable to consider that NSVT in patients with a reduced LVEF represents a sign of severe failing hearts.
Table 4.
Prognostic significance of NSVT in patients after myocardial infarction and acute coronary syndrome
| Study (year) | F/U (mo) | Incidence of NSVT | Post‐MI | Age (y) | LVEF (%) | Mortality or others |
|---|---|---|---|---|---|---|
| Bigger et al (1984) (n = 766)32 | 22 | 11% | LVEF <30% vs LVEF ≧30%, HR 3.5, P < .001 | |||
| NSVT vs No NSVT, HR 1.9, P < .05 | ||||||
| Cheema et al (1998) (n = 224)17 | 34 | 72 h | 65 | 49% | NSVT ≦24 h had no risk | |
| 64 | 50% | NSVT >24 h had poor survival, P < .0001 | ||||
| Hohnloser et al (1999) (n = 325)33 | 30 | 9% | 10 d | 58 | 49 | No predictive value of NSVT for SCD or AEs |
| Buxton et al (2000) (n = 1750)34 | 39 | >4 d | 67 | ≦40% | NSVT with vs without inducible VT during EPS | |
| HR 1.3, P = .005 | ||||||
| HR for SCD or CA 1.5, P < .001 | ||||||
| La Rovere et al (2001) (n = 1071)35 | 21 | 13% | 30 d | 59 | 49 | NSVT, RR for CD 3.1, P < .001 |
| LVEF <35% & NSVT, RR for AEs 9.0, P < .001 | ||||||
| Makikallio et al (2005) (n = 2130)24 | 34 | AMI | 59 | NSVT in pts with an EF >35%, HR for SCD 3.5, P < .001 | ||
| NSVT in pts with an EF ≦35%, HR for SCD NS | ||||||
| Huikuri et al (2009) (n = 312)36 | 24 | 13% | 5‐21 d & 6 wk | 65 | ≦40% (31%) | No predictive value of NSVT for fatal or near‐fatal AEs |
| Scirica et al (2010) (n = 6345)18 , a | 12 | 57%b | 3‐7 d | 63 | NSVT 3 beats, HR for SCD 1.1, P = .74, 1.4%/y | |
| NSVT 4‐7 beats, HR for SCD 2.3, P < .001, 2.9%/y | ||||||
| NSVT ≧8 beats, HR for SCD 2.8, P = .001, 4.3%/y | ||||||
| Bui et al (2016) (n = 2866)16 , c | 9 | 36% & 22%d | 0‐7 d & 30 d | 63 | NSVT ≦48 h had no risk of CD | |
| NSVT >48 h, HR for CD 1.87, P = .02 | ||||||
| NSVT at 30 d showed an increased risk of CD for an additional several months |
N, number of patients; F/U, follow‐up period; mo, months; d, day; wk, week; y, year; MI, myocardial infarction; AMI, acute MI; LVEF, left ventricular ejection fraction; HR, hazard ratio; RR, relative risk; SCD, sudden cardiac death; AEs, arrhythmic events; pts, patients; NS, not significant; CD, cardiac death; EPS, electrophysiological study; CA, cardiac arrest.
Patients with non‐ST‐elevation acute coronary syndrome (NSTEACS) were enrolled.
Continuous ECG monitoring was performed with the median time of 6.0 days.
Patients with acute coronary syndrome (ACS) were enrolled.
Continuous ECG monitoring was performed for ≦7 days.
Risk stratification of sudden cardiac death remains to be established among patients with relatively preserved LVEF (>40%). Although the risk of sudden cardiac arrest was low (0.35% per year) in this population with preserved LVEF of >50%,22 the absolute number of the victims was highest in the preserved LVEF category.22, 23 It is interesting that NSVT was a predictor of sudden cardiac death in patients after acute myocardial infarction only when LVEF was >35%.24 Similarly, in nonischemic dilated cardiomyopathy after optimization of medical treatment, NSVT was associated with a higher risk of major ventricular arrhythmias if LVEF was >35%, but not if LVEF was ≦35%.25 NSVT in combination with other noninvasive evaluations, such as late gadolinium enhancement,26 periodic repolarization dynamics,27 and multiple 12‐lead ECG parameters,28 might identify patients at risk of sudden cardiac death with preserved LVEF.
The second version of JCDTR is now under construction in the ICD Committee of the Japanese Heart Rhythm Society (JHRS), and it will be updated within a year. With voluntary efforts by members of the JHRS, we hope the next generation of JCDTR will be able to provide firm and further evidence of Japanese patients.
4.1. Study limitations
There are several limitations to be considered in this study. First, it is not known how and when the history of NSVT in each patient was determined. Occurrence of NSVT depends on the duration of ECG recording time and the clinical setting. For example, NSVT identified in hospital, compared with that identified out‐of‐hospital, was associated with a higher risk of mortality.29 Second, the number of events may have been small for adjusting the large number of clinical variables for the proper multivariate regression analysis. Third, the ICD programming was left on the discretion of each attending doctor and appropriate ICD therapies included both shock and antitachycardiac pacing. Programming of ICD therapies affect all‐cause mortality during long‐term follow‐up and is also associated with occurrence appropriate antitachycardiac pacing.30 Fourth, information with regard to the percent biventricular pacing is lacking in the JCDTR database. The mortality benefit was associated with an increasing percentage of biventricular pacing in CRT recipients.31
5. CONCLUSIONS
The present study demonstrated that a history of NSVT in CRT‐D recipients for primary prevention enrolled in the JCDTR was associated with a higher risk of heart failure death, but with a similar rate of appropriate ICD therapies, as compared with no prior history of NSVT. NSVT in heart failure patients appears to indicate a sign of a more severe failing heart rather than a substrate for subsequent sustained VTAs even in the presence of CRT.
CONFLICTS OF INTEREST
All authors declare no conflict of interest related to this study.
ACKNOWLEDGMENTS
We thank all the members of the JHRS who registered data in the JCDTR on a voluntary basis. As of September 22, 2017, 384 facilities in Japan had enrolled at least one patient. The list of the facilities that enrolled more than 100 patients (103 facilities in alphabetical order) is below:
Akita Medical Center, Anjo Kosei Hospital, Dokkyo Medical University, Edogawa Hospital, Fujita Health University, Fukushima Medical University, Gifu University, Gunma University, Hirosaki University, Hokkaido University Hospital, Hokko Memorial Hospital, Hyogo College of Medicine, IMS Katsushika Heart Center, Ishinomaki Red Cross Hospital, Itabashi Chuo Medical Center, Japanese Red Cross Society Kyoto Daini Hospital, Japanese Red Cross Wakayama Medical Center, JCHO Hokkaido Hospital, JCHO Kyushu Hospital, Jichi Medical University, Juntendo University, Juntendo University Urayasu Hospital, Kakogawa East City Hospital, Kameda Medical Center, Kanazawa Medical University, Kansai Rosai Hospital, Keio University, Kitasato University, Kochi Health Science Center, Kokura Memorial Hospital, Komaki City Hospital, Kumamoto Red Cross Hospital, Kumamoto University, Kurashiki Chuo Hospital, Kyorin University, Kyoto Prefectural University of Medicine, Kyoto‐Katsura Hospital, Maebashi Red Cross Hospital, Matsudo City Hospital, Matsue Red Cross Hospital, Matsumoto Kyoritsu Hospital, Mie University, Mito Saiseikai General Hospital, Nagasaki University, Nagoya University, Nara Medical University, National Hospital Organization Kagoshima Medical Center, National Hospital Organization Kanazawa Medical Center, National Hospital Organization Shizuoka Medical Center, Nihon University, Niigata University, Nippon Medical University, Nippon Medical School Chiba Hokusou Hospital, Odawara Municipal Hospital, Okayama University, Okinawa Prefectural Chubu Hospital, Osaka City General Hospital, Osaka City University, Osaka Medical College, Osaka Police Hospital, Osaka Red Cross Hospital, Osaka University, Osaki Hospital Tokyo Heart Center, Saiseikai Fukuoka General Hospital, Saiseikai Kumamoto Hospital, Saiseikai Shimonoseki General Hospital, Saiseikai Yokohamashi Tobu Hospital, Saitama Red Cross Hospital, Sakakibara Memorial Hospital, Sakurabashi Watanabe Hospital, Seirei Hamamatsu General Hospital, Sendai Kosei Hospital, Shiga University of Medical Science, Shinshu University, Shizuoka municipal Hospital, Showa General Hospital, St. Luke's International Hospital, St. Marianna University School of Medicine, Takeda Hospital, Tenri Hospital, The University of Tokyo, Toho University, Tokai University, Tokyo Medical University, Tokyo Metropolitan Bokutoh Hospital, Tokyo Metropolitan Hiroo Hospital, Tokyo Metropolitan Tama Medical Center, Tokyo Women's Medical University, Tottori University, Toyama Prefectural Central Hospital, Toyama University, Toyohashi Heart Center, Tsuchiura Kyodo General Hospital, Tsukuba Medical Center Hospital, University of Fukui, University of Miyazaki, University of Occupational and Environmental Health, University of Tsukuba, Yamagata Prefectural Central Hospital, Yamagata University, Yamaguchi University, Yamanashi Prefectural Central Hospital, Yokohama Rosai Hospital.
Yokoshiki H, Shimizu A, Mitsuhashi T, et al. Prognostic significance of nonsustained ventricular tachycardia in patients receiving cardiac resynchronization therapy for primary prevention: Analysis of the Japan cardiac device treatment registry database. J Arrhythmia. 2018;34:139–147. https://doi.org/10.1002/joa3.12023
REFERENCES
- 1. Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R. Randomised trial of low‐dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet. 1994;344:493–8. [DOI] [PubMed] [Google Scholar]
- 2. Doval HC, Nul DR, Grancelli HO, et al. Nonsustained ventricular tachycardia in severe heart failure. Independent marker of increased mortality due to sudden death. GESICA‐GEMA Investigators. Circulation. 1996;94:3198–203. [DOI] [PubMed] [Google Scholar]
- 3. Singh SN, Fisher SG, Carson PE, Fletcher RD. Prevalence and significance of nonsustained ventricular tachycardia in patients with premature ventricular contractions and heart failure treated with vasodilator therapy. Department of Veterans Affairs CHF STAT Investigators. J Am Coll Cardiol. 1998;32:942–7. [DOI] [PubMed] [Google Scholar]
- 4. Teerlink JR, Jalaluddin M, Anderson S, et al. Ambulatory ventricular arrhythmias in patients with heart failure do not specifically predict an increased risk of sudden death. PROMISE (Prospective Randomized Milrinone Survival Evaluation) Investigators. Circulation. 2000;101:40–6. [DOI] [PubMed] [Google Scholar]
- 5. Shimizu A, Mitsuhashi T, Furushima H, et al. Current status of cardiac resynchronization therapy with defibrillators and factors influencing its prognosis in Japan. J Arrhythmia. 2013;29:168–74. [Google Scholar]
- 6. Shimizu A, Nitta T, Kurita T, et al. Current status of implantable defibrillator devices in patients with left ventricular dysfunction – the first report from the online registry database. J Arrhythmia. 2008;24:133–40. [Google Scholar]
- 7. Shimizu A, Nitta T, Kurita T, et al. Actual conditions of implantable defibrillator therapy over 5 years in Japan. J Arrhythmia. 2012;28:263–72. [Google Scholar]
- 8. Yokoshiki H, Shimizu A, Mitsuhashi T, et al. Trends and determinant factors in the use of cardiac resynchronization therapy devices in Japan: analysis of the Japan cardiac device treatment registry database. J Arrhythmia. 2016;32:486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yokoshiki H, Shimizu A, Mitsuhashi T, et al. Survival and heart failure hospitalization in patients with cardiac resynchronization therapy with or without a defibrillator for primary prevention in Japan‐ analysis of the Japan cardiac device treatment registry database. Circ J. 2017;81:1798–806. [DOI] [PubMed] [Google Scholar]
- 10. Yokoshiki H, Mitsuyama H, Watanabe M, Mitsuhashi T, Shimizu A. Cardiac resynchronization therapy in ischemic and non‐ischemic cardiomyopathy. J Arrhythmia. 2017;33:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. [DOI] [PubMed] [Google Scholar]
- 12. Gold MR, Daubert JC, Abraham WT, et al. Implantable defibrillators improve survival in patients with mildly symptomatic heart failure receiving cardiac resynchronization therapy: analysis of the long‐term follow‐up of remodeling in systolic left ventricular dysfunction (REVERSE). Circ Arrhythm Electrophysiol. 2013;6:1163–8. [DOI] [PubMed] [Google Scholar]
- 13. Grimm W, Christ M, Bach J, Muller HH, Maisch B. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation. 2003;108:2883–91. [DOI] [PubMed] [Google Scholar]
- 14. Mittal S, Aktas MK, Moss AJ, et al. The impact of nonsustained ventricular tachycardia on reverse remodeling, heart failure, and treated ventricular tachyarrhythmias in MADIT‐CRT. J Cardiovasc Electrophysiol. 2014;25:1082–7. [DOI] [PubMed] [Google Scholar]
- 15. Chen J, Johnson G, Hellkamp AS, et al. Rapid‐rate nonsustained ventricular tachycardia found on implantable cardioverter‐defibrillator interrogation: relationship to outcomes in the SCD‐HeFT (Sudden Cardiac Death in Heart Failure Trial). J Am Coll Cardiol. 2013;61:2161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bui AH, Cannon CP, Steg PG, et al. Relationship between early and late nonsustained ventricular tachycardia and cardiovascular death in patients with acute coronary syndrome in the platelet inhibition and patient outcomes (PLATO) trial. Circ Arrhythm Electrophysiol. 2016;9:e002951. [DOI] [PubMed] [Google Scholar]
- 17. Cheema AN, Sheu K, Parker M, Kadish AH, Goldberger JJ. Nonsustained ventricular tachycardia in the setting of acute myocardial infarction: tachycardia characteristics and their prognostic implications. Circulation. 1998;98:2030–6. [DOI] [PubMed] [Google Scholar]
- 18. Scirica BM, Braunwald E, Belardinelli L, et al. Relationship between nonsustained ventricular tachycardia after non‐ST‐elevation acute coronary syndrome and sudden cardiac death: observations from the metabolic efficiency with ranolazine for less ischemia in non‐ST‐elevation acute coronary syndrome‐thrombolysis in myocardial infarction 36 (MERLIN‐TIMI 36) randomized controlled trial. Circulation. 2010;122:455–62. [DOI] [PubMed] [Google Scholar]
- 19. Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter‐defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–8. [DOI] [PubMed] [Google Scholar]
- 20. Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–36. [DOI] [PubMed] [Google Scholar]
- 21. Dorian P, Hohnloser SH, Thorpe KE, et al. Mechanisms underlying the lack of effect of implantable cardioverter‐defibrillator therapy on mortality in high‐risk patients with recent myocardial infarction: insights from the Defibrillation in Acute Myocardial Infarction Trial (DINAMIT). Circulation. 2010;122:2645–52. [DOI] [PubMed] [Google Scholar]
- 22. Gorgels AP, Gijsbers C, de Vreede‐Swagemakers J, Lousberg A, Wellens HJ. Out‐of‐hospital cardiac arrest–the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–9. [DOI] [PubMed] [Google Scholar]
- 23. Stecker EC, Vickers C, Waltz J, et al. Population‐based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two‐year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–6. [DOI] [PubMed] [Google Scholar]
- 24. Makikallio TH, Barthel P, Schneider R, et al. Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Heart J. 2005;26:762–9. [DOI] [PubMed] [Google Scholar]
- 25. Zecchin M, Di Lenarda A, Gregori D, et al. Are nonsustained ventricular tachycardias predictive of major arrhythmias in patients with dilated cardiomyopathy on optimal medical treatment? Pacing Clin Electrophysiol. 2008;31:290–9. [DOI] [PubMed] [Google Scholar]
- 26. Halliday BP, Gulati A, Ali A, et al. Association between midwall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation. 2017;135:2106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rizas KD, McNitt S, Hamm W, et al. Prediction of sudden and non‐sudden cardiac death in post‐infarction patients with reduced left ventricular ejection fraction by periodic repolarization dynamics: MADIT‐II substudy. Eur Heart J. 2017;38:2110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aro AL, Reinier K, Rusinaru C, et al. Electrical risk score beyond the left ventricular ejection fraction: prediction of sudden cardiac death in the Oregon Sudden Unexpected Death Study and the Atherosclerosis Risk in Communities Study. Eur Heart J. 2017;38:3017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pires LA, Lehmann MH, Buxton AE, Hafley GE, Lee KL. Differences in inducibility and prognosis of in‐hospital versus out‐of‐hospital identified nonsustained ventricular tachycardia in patients with coronary artery disease: clinical and trial design implications. J Am Coll Cardiol. 2001;38:1156–62. [DOI] [PubMed] [Google Scholar]
- 30. Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–83. [DOI] [PubMed] [Google Scholar]
- 31. Hayes DL, Boehmer JP, Day JD, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm. 2011;8:1469–75. [DOI] [PubMed] [Google Scholar]
- 32. Bigger JT Jr, Fleiss JL, Kleiger R, Miller JP, Rolnitzky LM. The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation. 1984;69:250–8. [DOI] [PubMed] [Google Scholar]
- 33. Hohnloser SH, Klingenheben T, Zabel M, Schopperl M, Mauss O. Prevalence, characteristics and prognostic value during long‐term follow‐up of nonsustained ventricular tachycardia after myocardial infarction in the thrombolytic era. J Am Coll Cardiol. 1999;33:1895–902. [DOI] [PubMed] [Google Scholar]
- 34. Buxton AE, Lee KL, DiCarlo L, et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 2000;342:1937–45. [DOI] [PubMed] [Google Scholar]
- 35. La Rovere MT, Pinna GD, Hohnloser SH, et al. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life‐threatening arrhythmias: implications for clinical trials. Circulation. 2001;103:2072–7. [DOI] [PubMed] [Google Scholar]
- 36. Huikuri HV, Raatikainen MJ, Moerch‐Joergensen R, et al. Prediction of fatal or near‐fatal cardiac arrhythmia events in patients with depressed left ventricular function after an acute myocardial infarction. Eur Heart J. 2009;30:689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]


