Abstract
The BMP and Wnt signalling pathways determine axis specification during embryonic development. Our previous work has shown that PAWS1 (also known as FAM83G) interacts with SMAD1 and modulates BMP signalling. Here, surprisingly, we show that overexpression of PAWS1 in Xenopus embryos activates Wnt signalling and causes complete axis duplication. Consistent with these observations in Xenopus, Wnt signalling is diminished in U2OS osteosarcoma cells lacking PAWS1, while BMP signalling is unaffected. We show that PAWS1 interacts and co‐localises with the α isoform of casein kinase 1 (CK1), and that PAWS1 mutations incapable of binding CK1 fail both to activate Wnt signalling and to elicit axis duplication in Xenopus embryos.
Keywords: BMP, CK1, FAM83G, PAWS1, Wnt
Subject Categories: Signal Transduction
Introduction
PAWS1 (protein associated with Smad1) interacts with Smad1, an intracellular mediator of bone morphogenetic protein (BMP) signalling, and in particular, it modulates SMAD4‐independent BMP activity 1. The protein is conserved in vertebrates, and it belongs to the poorly characterised FAM83 family (FAMily with sequence similarity 83; FAM83A‐H), which is defined by an N‐terminal DUF1669 domain of unknown function and contains a pseudo‐phospholipase D catalytic motif 2. Beyond the DUF1669 domain, there is no sequence similarity between FAM83 members 1, 3. There are two known conditions mapped to mutations in FAM83G. In mice, the wooly mutation (wly), which results in a matted and rough coat in 3‐ to 4‐week‐old pups, is due to aberrant splicing of PAWS1 resulting in a severely truncated protein 4. The second is a recessive missense mutation (p.R52P) within the DUF1669 domain of FAM83G in dogs suffering from hereditary footpad hyperkeratosis (HFH) 5. The affected dogs develop hard and cracked footpads that over time become severely debilitating 5. However, our knowledge of the molecular function of FAM83G/PAWS1 is limited.
BMP signalling plays important roles in development and in adult tissue homeostasis. In the developing Xenopus embryo, a gradient of BMP activity helps pattern the dorso‐ventral axis, with the highest levels of BMP signalling promoting formation of the most ventral tissues 6, 7. In an effort to explore the function of PAWS1 in more detail, we overexpressed the protein in early Xenopus embryos. To our surprise, PAWS1 did not cause embryos to be ventralised but instead induced complete secondary axes, including well‐formed heads. Such a response is typically obtained after ectopic activation of the Wnt signalling pathway 8, and we confirmed both in Xenopus and in U2OS osteosarcoma cells that PAWS1 does regulate Wnt signalling.
Mass spectrometric analysis revealed that PAWS1 interacts with casein kinase 1, and we show that this association is critical for PAWS1 to impact Wnt signalling in cells and embryos.
Results
PAWS1 induces the formation of a secondary axis in Xenopus embryos
In an effort to explore the biological activity of PAWS1, we injected 500 pg of mRNA encoding Xenopus PAWS1 into the animal hemispheres of Xenopus embryos at the one‐cell stage. Such embryos went on to display axial defects, including dorsalisation and the formation of partial secondary axes (Fig EV1A–C). To explore this phenomenon in more detail, we injected a single ventral blastomere at the four‐cell stage with xPAWS1 mRNA. Such embryos went on to form complete secondary axes, resembling those formed in response to ectopic xWnt8 (Fig 1A and B). Similar results were obtained with human PAWS1 (hPAWS1; Fig 1C).
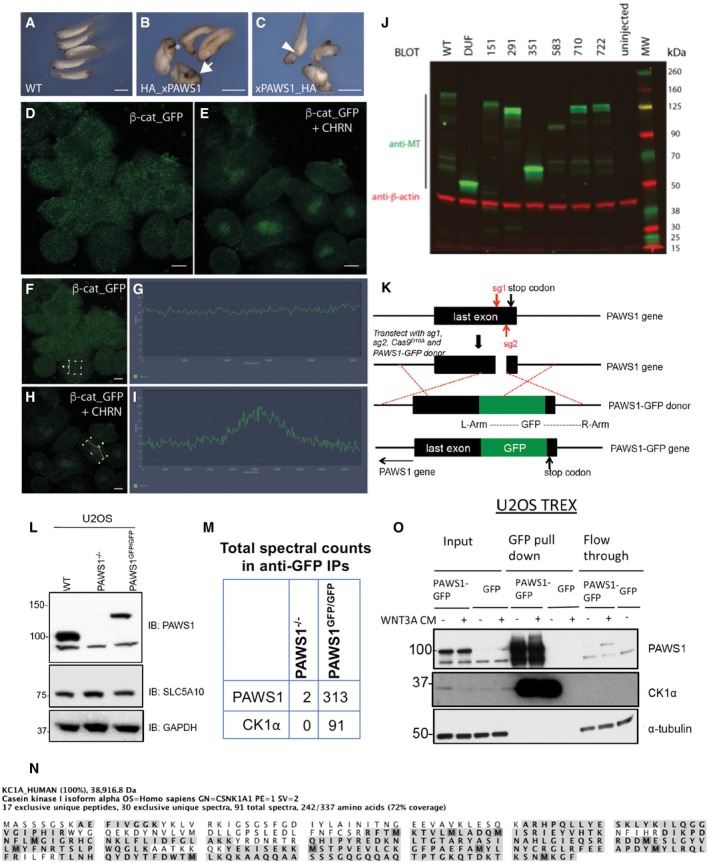
Figure EV1. Manipulation of PAWS1 in Xenopus embryos and human U2OS cells.
-
A–CEctopic axis induction in Xenopus embryos following xPAWS1 mRNA injection. Xenopus embryos were injected at the one‐cell stage with 500 pg of either HA_xPAWS1 (B) or xPAWS_HA mRNA(C). A variety of dorsalised phenotypes were observed including enlarged cement glands (asterisk), partial (arrowhead) and complete secondary axis (arrow). Scale bars are 2 mm.
-
D–IDissociated animal caps injected with 50 pg of β‐catenin_GFP mRNA were imaged over 3 h following treatment with the GSK3β inhibitor CHIR99021. Maximum intensity projection of β‐catenin_GFP‐injected cells before (D) and 3 h (E) after CHIR99021 treatment, demonstrating stabilisation and nuclear localisation of β‐catenin_GFP in the absence of xPAWS1. Single z‐section of a β‐catenin_GFP expressing cell and corresponding fluorescence intensity profile across the nucleus before (F and G) and following 3 h of CHIR99021 treatment (H and I). Cells were imaged using a Zeiss LSM710 microscope, and intensity measurements from a single z‐section were taken using Zen Black software. Scale bars are 20 μm.
-
JExpression level of Myc‐tagged(MT)xPAWS1 and MTxPAWS1 mutants at stage 10. Extracts from embryos injected with 250 pg of MTxPAWS1 and MTxPAWS1 mutants were immunoblotted with antibodies against Myc‐tag (green) and α‐tubulin (red). The image was captured with a Li‐Cor Odyssey scanner using Image Studio software (Li‐Cor).
-
KSchematic illustration of the strategy employed to generate PAWS1‐GFP knock‐ins in U2OS cells. A pair of guide RNAs which recognise a genomic sequence upstream of the stop codon of PAWS1 gene was used in combination with a donor vector which inserts GFP in frame with the c‐terminus of PAWS1.
-
LCell extracts from PAWS1GFP/GFP cells compared with the PAWS1−/−, confirmed that the gene in the reverse DNA strand of PAWS1, SLC5A10 is not disturbed.
-
MMass fingerprinting analysis of PAWS1‐GFP interactors from PAWS1GFP/GFP‐knock‐in U2OS cells compared with PAWS1−/− U2OS cells (from Fig 5A) identified CK1α as a major interactor. The table shows total spectral counts for PAWS1 and CK1α tryptic peptides identified in anti‐GFP IPs.
-
NThe highlighted tryptic peptides identified by mass spectrometry on CK1α indicate the overall protein coverage. The included image was obtained using Scaffold V4.3 analysis of the LC‐MS/MS data.
-
OStable U2OS Flp‐In Trex cells were subjected to 20 ng/ml doxycycline for inducing PAWS1‐GFP expression or GFP expression alone for 24 h. Wnt3A or control medium was added to the cells for 6 h before lysis. 20 mg of cell extract was subjected to GFP‐trap IP. Input (20 μg protein), 5% of the pull down and flow‐through extract (20 μg protein) were subjected to SDS–PAGE followed by Western blot analysis with the indicated antibodies.
Source data are available online for this figure.
Figure 1. PAWS1 causes axis duplication in Xenopus embryos.
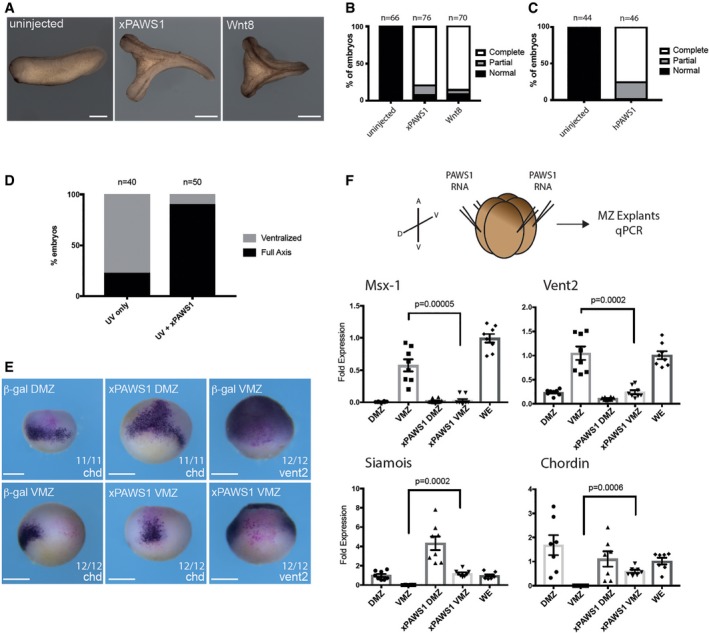
- Representative images of an uninjected embryo, and embryos injected with 250 pg xPAWS1 mRNA or 5 pg xWnt8 mRNA. Scale bars are 1 mm.
- Quantification of (A). Complete axis denotes embryos with a secondary axis with a cement gland, while a partial axis does not.
- Quantification of axis‐inducing activity of 250 pg of hPAWS1.
- 250 pg of xPAWS1 mRNA rescues UV‐ventralised embryos.
- xPAWS1 induces dorsal and represses ventral gene expression in the whole embryo. A single blastomere at the four‐cell stage was injected with either 200 pg nuclear β‐gal mRNA alone or with 250 pg of xPAWS1 and 200 pg of nuclear β‐gal mRNA. At stage 10, embryos were fixed, stained for β‐gal and then developed for in situ hybridisation using probes specific for Chordin or Vent2. In dorsal blastomeres, xPAWS1 induces expression of Chordin, while in ventral blastomeres it represses Vent2 expression. The number of embryos tested is indicated. Scale bars are 500 μm.
- 250 pg of xPAWS1 and 200 pg of CFP_gpi mRNAs was injected into the marginal zone of each blastomere at the four‐cell stage. The dorsal/ventral (D/V) and animal/vegetal (A/V) axes are indicated. At stage 10, the dorsal marginal zone (DMZ) and ventral marginal zone (VMZ) were isolated and dorsal and ventral marker expression was assessed by qPCR. xPAWS1 inhibits ventral marker expression (Msx‐1 and Vent2), while concomitantly upregulating the dorsal markers Chordin and Siamois in the VMZ. Expressions of Msx‐1, Vent2, Siamois, and Chordin were normalised to the expression levels of Histone H4, and presented as a fold change with respect to the average levels in whole embryos (WE), (n = 8, error bars represent ± SEM; t‐test, unpaired, two‐tailed with unequal variance Mann–Whitney test, P‐values are as indicated).
Early UV exposure of Xenopus embryos inhibits cortical rotation and prevents formation of a dorsal inducing centre 9. UV‐ventralised embryos of this sort can be rescued by ectopic activation of the Wnt signalling pathway 10, 11, 12, 13, and consistent with its strong dorsalising activity, we found that xPAWS1 is indeed able to rescue UV‐treated embryos (Fig 1D).
We also examined gene expression in the early embryo following xPAWS1 overexpression. When injected into the marginal zone, xPAWS1 suppressed expression of the ventral genes Msx1 (Fig 1F), and Vent2 in the ventral marginal zone (VMZ; Fig 1E and F), and increased expression of dorsal markers Siamois (Fig 1F), and Chordin (Fig 1E and F). Together, these results suggest that PAWS1 promotes Wnt signalling.
PAWS1 does not inhibit canonical BMP signalling
Components of the Wnt signal transduction pathway can induce the formation of a complete secondary axis in Xenopus 14, 15, 16, 17. However, inhibition of BMP signalling can induce partial secondary axes 18, and it is possible that as a SMAD1‐interacting protein, PAWS1 might also exert some effects by inhibiting BMP signalling. However, xPAWS1 did not inhibit BMP‐induced nuclear translocation of SMAD1 in dissociated animal cap cells, nor did it affect expression of the BMP responsive ventral genes Msx1 and Vent1 (Fig 2A and B). Together, these data suggest that the ability of PAWS1 to induce secondary axes occurs solely through the Wnt pathway.
Figure 2. BMP signalling pathway is not affected by PAWS1.
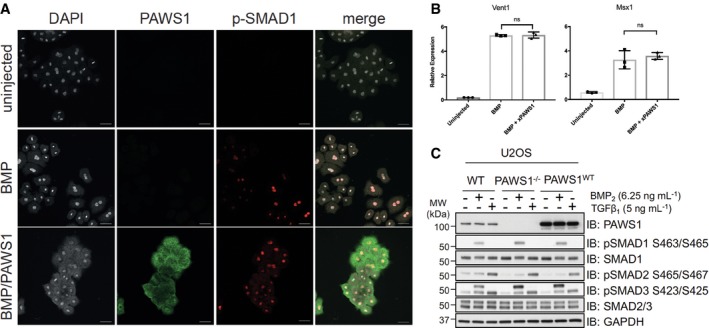
- Expression of pSMAD1 in dissociated animals cap cells. Embryos were injected into the animal pole of both blastomeres at the two‐cell stage with a total of either 1 ng of BMP4 or 1 ng BMP4 and 500 pg of Myc‐tagged(MT)xPAWS1 mRNAs. Dissociated cells were stained with antibodies against MYC‐tag (xPAWS1, green) and for phospho‐SMAD1 (p‐SMAD1, red). Scale bars are 50 μm.
- Expression of ventral markers in animal caps cells injected at the two‐cell stage with a total of either 1 ng of BMP4 or 1 ng BMP4 and 500 pg of MTxPAWS1 mRNA (n = 3, error bars represent ± SD, ns—not significant, t‐test, unpaired, two‐tailed with unequal variance).
- U2OS wild‐type (WT), PAWS1−/− and PAWS1WT rescue cells were serum‐deprived for 16 h. Cells were subsequently stimulated with either 6.25 ng/ml BMP2 or 50 pM TGFβ1 for 1 h prior to lysis. Cell extracts (15 μg) were resolved by SDS–PAGE and immunoblotted with the indicated antibodies. Note that the upper band in the P‐SMAD3 blot is a result of the antibody cross‐reacting with P‐SMAD1.
Source data are available online for this figure.
Consistent with this conclusion, we showed previously that the introduction of PAWS1 in PC3 prostate cancer cells (that normally do not express PAWS1) does not affect BMP‐induced phosphorylation of SMAD1, nor the transcription of SMAD4‐dependent BMP target genes 1. Similarly, BMP‐induced phosphorylation of SMAD1 in PAWS1−/− U2OS cells created by CRISPR/Cas9 genome editing 19 resembles that in wild‐type U2OS cells as well as that in PAWS1−/− cells in which PAWS1 has been restored (Fig 2C). The same is true for TGFβ‐induced phosphorylation of SMAD2/3 in the three cell lines (Fig 2C). Thus, PAWS1 has no significant effect on canonical BMP signalling in Xenopus embryos and nor in human cells.
PAWS1 enhances canonical Wnt signalling in Xenopus embryos and human cells
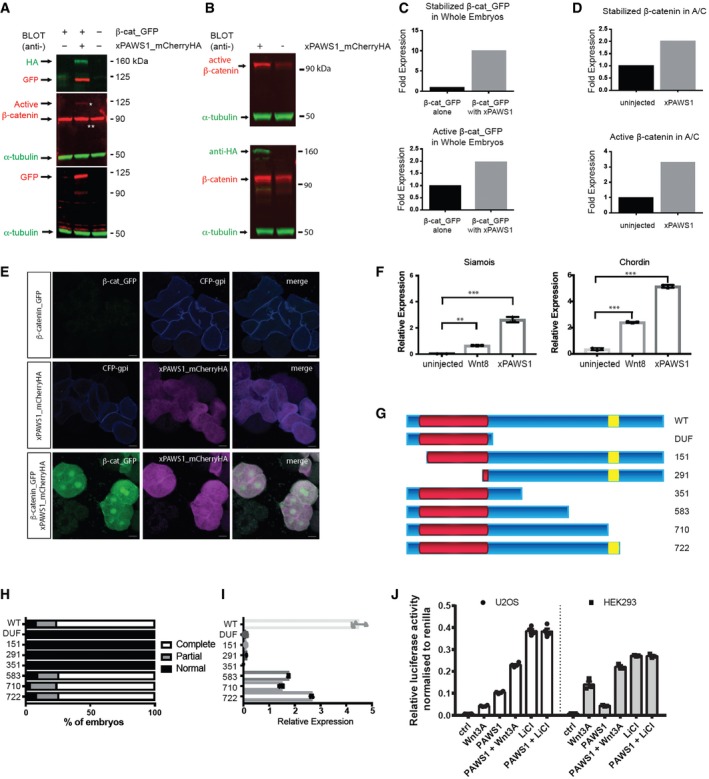
Our data suggest that PAWS1 activates Wnt signalling. To confirm this, we tested its ability to stabilise GFP‐tagged β‐catenin in Xenopus animal pole regions. GFP fluorescence was undetectable in animal pole cells derived from embryos injected with 50 pg mRNA encoding β‐catenin‐GFP, either by Western blot (Fig 3A and C) or by confocal imaging (Figs 3E and EV1D, F and G). Some stabilised β‐catenin‐GFP was detectable after treatment of caps with the Wnt agonist CHIR99021 (Fig EV1E, H and I). Approximately ten times more β‐catenin‐GFP was present in animal caps co‐expressing xPAWS1 (Fig 3A and C), some of which was in an active unphosphorylated state (Fig 3A and C). β‐Catenin‐GFP could also be detected by confocal microscopy in the nuclei of dissociated animal cap cells (Fig 3E). Levels of endogenous and active β‐catenin also increased following injection of xPAWS1 mRNA into naïve animal caps (Fig 3B and D). Finally, animal caps expressing xPAWS1 activated the direct Wnt target Siamois 20, 21, 22 and the dorsalising factor Chordin 18, confirming that PAWS1 can activate the canonical Wnt pathway (Fig 3F). We mapped the domain of PAWS1 that is required for Wnt signalling by testing the ability of different PAWS1 fragments to induce secondary axes in Xenopus and to activate Siamois expression in animal caps (Fig 3G–I). The DUF1669 domain proved to be necessary but not sufficient to activate canonical Wnt signalling, while the 583 fragment that displays activity suggests that the conserved BMPR1 phosphorylation sites S610, S613 and S614 do not impact activity (Fig 3G–I). The expression of these fragments in embryos was monitored by Western blotting (Fig EV1J).
Figure 3. PAWS1 activates Wnt signalling.
-
AxPAWS1 stabilises exogenous β‐catenin in whole embryos. Xenopus embryos were injected into the animal pole at the one‐cell stage with mRNAs encoding either 50 pg of β‐cat_GFP or with 50 pg of β‐cat_GFP and 250 pg xPAWS1_mCherryHA mRNAs. At stage 10, embryo extracts were immunoblotted with the indicated antibodies. Top panel: anti‐HA and anti‐GFP antibodies; middle panel: anti‐active β‐catenin (single white asterisk is active β‐catenin_GFP; double white asterisk is endogenous active β‐catenin) and anti‐α‐tubulin antibodies; bottom panel: anti‐GFP and anti‐α‐tubulin antibodies.
-
BxPAWS1 stabilises endogenous β‐catenin in naïve animal caps. Embryos were injected at the one‐cell stage with 250 pg of xPAWS1_mCherryHA mRNA. At stage 8.5, animal caps were collected from injected and uninjected embryos and cultured until control embryos reached stage 10. Extracts were immunoblotted with the indicated antibodies. Top panel: anti‐active β‐catenin and anti‐α‐tubulin antibodies; bottom panel: anti‐HA, anti‐β‐catenin and anti‐α‐tubulin antibodies.
-
C, DQuantification of (A and B), respectively. In (C), β‐catenin_GPF or active β‐catenin_GFP bands were normalised to α‐tubulin and then expressed as a fold change relative to the expression of β‐catenin_GFP and active β‐catenin_GFP respectively from embryos injected with β‐catenin_GFP alone. In (D), endogenous β‐catenin and active β‐catenin were normalised to α‐tubulin and then expressed as a fold change relative to the expression of β‐catenin and active β‐catenin (respectively) from uninjected cells.
-
ENuclear translocation of β‐catenin_GFP. Dissociated animal cap cells injected with either 50 pg of β‐catenin_GFP or with 50 pg β‐catenin_GFP and 250 pg xPAWS1_mCherryHA mRNAs were plated on coverslips and imaged by confocal microscopy. Only β‐catenin_GFP cells co‐injected with xPAWS1_mCherryHA mRNA accumulated robust levels of β‐catenin in the nucleus. Scale bars are 20 μm.
-
FxPAWS1 induces expression of Siamois and Chordin transcripts in animal caps. Embryos were injected at the one‐cell stage with 250 pg of MT_xPAWS1 mRNA, and then at stage 8.5, animal caps were collected from injected and uninjected embryos and assessed for Chordin and Siamois expression by qPCR (n = 3; error bars represent ± SD, **P = 0.001, ***P = 0.0001; ordinary one‐way ANOVA with multiple comparisons, uninjected as control column).
-
G–IThe DUF1669 domain (G, in red) is necessary but not sufficient to induce a secondary axis (H) and activate Siamois expression (I) while the BMPR1 phosphorylation sites S610, S613 and S614 (yellow) are dispensable (n = 3; error bars represent ± SD). 250 pg of MT_xPAWS1 mRNAs encoding N‐ and C‐terminal truncation fragments were injected into one ventral blastomere at the four‐cell stage. Axis induction was assessed at stage 28. In (I), embryos were injected at the one‐cell stage with 250 pg of MT_xPAWS1 mRNAs encoding N‐ and C‐terminal mutants, and then at stage 8.5, animal caps were collected and assessed for Siamois expression by qPCR.
-
JHEK293 and U2OS cells were transfected with PAWS1 cDNA, or empty vector as a control and TOPFlash luciferase activity was measured after treatment with either conditioned medium (L‐CM), Wnt3A‐conditioned medium (L3‐CM) or 20 mM LiCl for 12 h. Data are normalised to Renilla internal control (n = 4; error bars represent ± SEM).
Source data are available online for this figure.
We also asked whether PAWS1 activates Wnt signalling in mammalian cells. To this end, we overexpressed PAWS1 in HEK293 human kidney cells and in U2OS osteosarcoma cells before stimulating with control or Wnt3A‐conditioned medium and measuring Wnt‐dependent TOPFlash luciferase reporter activity 23. In both cell lines, Wnt3A conditioned medium induced TOPFlash luciferase activity when compared with the control (Fig 3J). Overexpression of PAWS1 substantially enhanced activity under both conditions, as did inhibition of GSK‐3 activity by treatment with LiCl (Fig 3J). Consistent with these observations, Wnt‐induced TOPFlash luciferase activity in PAWS1−/− U2OS cells was significantly less than in wild‐type controls (Fig 4A), as was Wnt‐induced activation of its endogenous target genes AXIN2 and CYCLIN D1 (Fig 4B).
Figure 4. Loss of PAWS1 inhibits Wnt signalling at the level of the destruction complex.
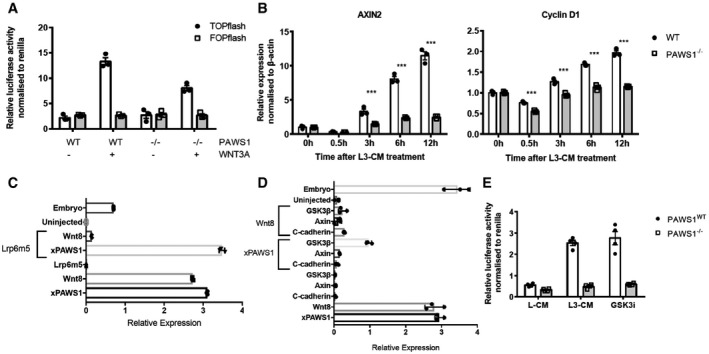
-
ARelative TOPFlash luciferase activity of PAWS1WT and PAWS1−/− U2OS cells after treatment with conditioned media (L‐CM) or Wnt3A‐conditioned media (L3‐CM) (n = 3; error bars represent ± SEM).
-
BWnt3A‐induced activation of the target genes AXIN2 and CYCLIN D1 was examined by qPCR at the indicated time points in PAWS1WT and PAWS1−/− U2OS cells. Transcript expression data are represented as fold induction over unstimulated control and are internally normalised to β‐actin control. Error bars represent ± SEM (***P < 0.0001; two‐way ANOVA with multiple comparisons; n = 3).
-
C, DEpistasis analysis of xPAWS1 in the canonical Wnt pathway. Xenopus embryos were injected at the one‐cell stage with the indicated mRNAs, and then at stage 10, animal caps were assessed by qPCR for the expression of Siamois. xPAWS1 acts downstream of the Wnt receptor LRP6 (C) at the level of the destruction complex (D) (n = 3 error bars represent ± SD).
-
ERelative TOPFlash luciferase activity of PAWS1WT and PAWS1−/− U2OS cells after treatment with either conditioned medium (L‐CM), Wnt3A‐conditioned medium (L3‐CM) or 5 μM of the GSK3 inhibitor CHIR99021 for 6 h. Data are normalised to Renilla‐luciferase internal control (n = 4; error bars represent ± SEM).
To ask where within the Wnt signalling pathway PAWS1 is likely to act, we employed epistasis experiments in animal caps. The induction of Siamois, a direct Wnt target, in naïve animal caps injected with xWnt8 mRNA is inhibited following injection of a dominant negative Wnt receptor Lrp6 m5 (Fig 4C). In contrast, Lrp6 m5 was unable to inhibit Siamois expression in caps injected with xPAWS1 mRNA, suggesting that PAWS1 acts downstream of the Wnt receptor (Fig 4D). Overexpression of C‐cadherin in animal caps can inhibit Wnt signalling by acting as a molecular sink and sequestering stabilised β‐catenin to the cell surface, thereby inhibiting nuclear accumulation of β‐catenin 24. Therefore, agonists that promote Wnt signalling downstream of β‐catenin are unaffected by C‐cadherin overexpression. PAWS1‐dependent induction of Siamois expression was blocked by C‐cadherin (Fig 4D), suggesting that PAWS1‐mediated activation of Wnt signalling requires β‐catenin. Ubiquitin‐mediated degradation of β‐catenin is regulated through the destruction complex, in which CK1, GSK‐3 and Axin1 play key roles 25, 26, 27. Overexpression of Axin1 in animal caps completely blocked xPAWS1‐induced Siamois expression, while overexpression of GSK‐3 did so partially (Fig 4D). Consistent with this notion, in PAWS1−/− U2OS cells, inhibition of GSK‐3 with the selective inhibitor CHIR99021 had no effect on Wnt‐reporter activity (Fig 4E), while in WT cells inhibition of GSK‐3, which stabilises β‐catenin 28, yielded maximal induction of Wnt‐reporter activity, even in the absence of Wnt stimulus. Together, these results suggest that PAWS1 functions in Wnt signalling at the level of the destruction complex or subsequent to it.
PAWS1 interacts with CK1α
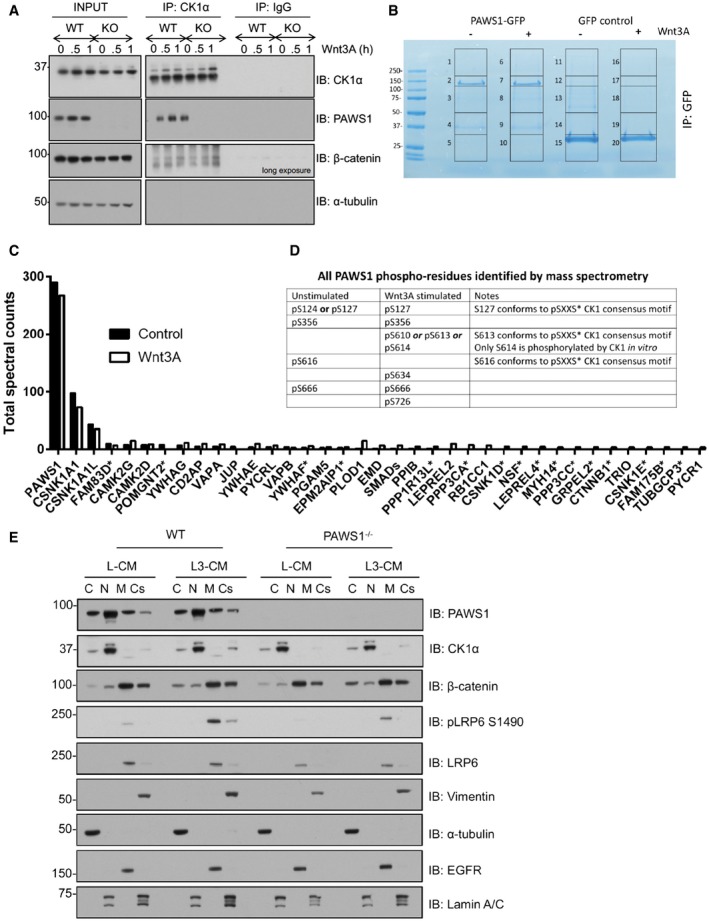
To understand the mechanisms by which PAWS1 stimulates canonical Wnt signalling at the level of the destruction complex, we employed a proteomic approach to identify interactors of endogenous PAWS1. For this, we first generated U2OS cells in which we introduced a GFP tag at the C‐terminus of the endogenous PAWS1 gene on both alleles (Fig EV1K and L). Using these PAWS1GFP/GFP‐knock‐in cells, we performed anti‐GFP IPs to pull down endogenous PAWS1‐GFP and its interacting partners (Fig 5A). Casein kinase 1α (CK1α) isoform was identified as the major interactor of PAWS1 by mass spectrometry (Figs 5A and EV1M and N). CK1α plays a central role in Wnt signalling and phosphorylates many components of the pathway, including β‐catenin at the destruction complex 29, 30. By Western blotting, endogenous CK1α was detected in anti‐GFP IPs from PAWS1GFP/GFP knock‐in cells but not from PAWS1−/− U2OS cells (Fig 5B). Similarly, endogenous PAWS1 was detected in endogenous CK1α IPs from wild‐type but not PAWS1−/− U2OS cells (Fig 5C). Stimulating cells with Wnt3A did not affect the ability of PAWS1 to interact with endogenous CK1α (Fig EV1O), suggesting that the interaction between PAWS1 and CK1α does not depend on Wnt stimulation.
Figure 5. PAWS1 interacts with CK1 at the endogenous level.
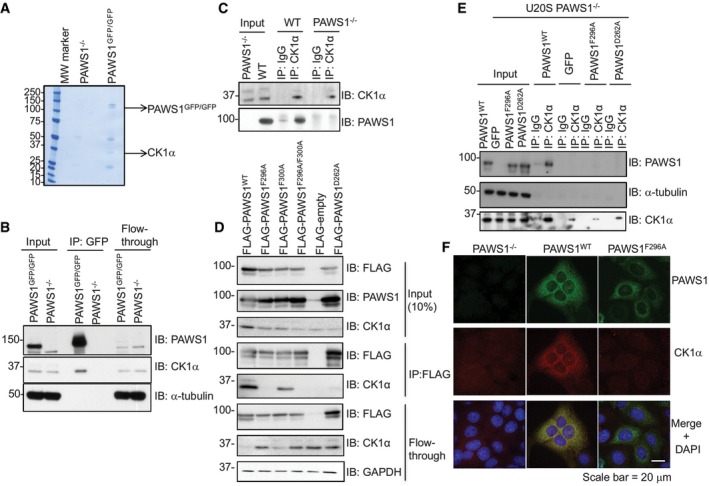
- GFP pull downs from U2OS PAWS1−/− and PAWS1GFP/GFP cells were resolved by SDS–PAGE, and the gel was stained with Coomassie. Each lane was cut into six pieces, which were subsequently processed for protein identification by mass spectrometry.
- GFP pull downs from U2OS PAWS1−/− and PAWS1GFP/GFP cells were resolved by SDS–PAGE and analysed by Western blotting using the indicated antibodies.
- Anti‐CK1α IPs from U2OS PAWS1WT and PAWS1−/− cells were resolved by SDS–PAGE and analysed by Western blotting using the indicated antibodies.
- U2OS cells were transiently transfected with cDNA encoding FLAG‐tagged PAWS1WT, PAWS1F296A, PAWS1F300A, PAWS1F296A/F300A, PAWS1D262A and FLAG‐empty vector. Anti‐FLAG IPs were immunoblotted with the indicated antibodies.
- Anti‐CK1α IPs and IgG‐IPs from U2OS PAWS1WT, GFP, PAWS1F296A and PAWS1D262A rescue cells were resolved by SDS–PAGE and analysed by Western blotting using the indicated antibodies.
- PAWS1 co‐localises with CK1α in U2OS cells. PAWS1−/− cells in which PAWS1WT or PAWS1F296A expression was restored were fixed for immunofluorescence using antibodies against PAWS1 and CK1α. Scale bar is 20 μm. Images from one field of view representative of three biological replicates are included.
Source data are available online for this figure.
To map the interaction sites between PAWS1 and CK1α, we co‐expressed truncated fragments of PAWS1 with CK1α and performed co‐IP interaction assays. From these assays, we determined that the region spanning residues 165‐307 within the DUF1669 domain was sufficient for mediating CK1α interaction 31. Within this region, PAWS1 contains an F296‐X‐X‐X‐F300 motif that has been reported to mediate interaction between CK1α and NFAT1, PER1 and PER2 proteins 32. In order to determine whether the F296‐X‐X‐X‐F300 motif within PAWS1 indeed mediates its association with CK1α, we generated FLAG‐PAWS1 F296A, F300A or F296A/F300A mutants and overexpressed them in U2OS cells. Endogenous CK1α was detected in anti‐FLAG IPs from wild‐type FLAG‐PAWS1 and FLAG‐PAWS1F300A but not from FLAG‐PAWS1F296A and FLAG‐PAWS1F296A/F300A (Fig 5D), suggesting F296 in PAWS1 contributed to the association with CK1α. For PER1/2, it has been shown that both Phe residues within the F‐X‐X‐X‐F motif are essential for interaction with CK1α, as mutating either contributed to the loss of interaction with CK1 32. However, for PAWS1, the fact that PAWS1F300A was still able to interact with CK1α suggested that the mode of interaction with CK1α is mediated through additional mechanisms. We tested further point mutations on conserved residues along the aa165‐aa307 stretch of PAWS1 for their ability to abolish the interaction with CK1α and found that mutating the conserved D262 to A (PAWS1D262A) also abolished the association with CK1α (Fig 5D). We tested the ability of GFP control, PAWS1WT, PAWS1F296A or PAWS1D262A to interact with endogenous CK1α when these were stably restored in PAWS1−/− cells. IPs of endogenous CK1α pulled down PAWS1 only from PAWS1WT rescue cells but not others (Fig 5E).
To test whether, and if so where, PAWS1 and CK1α co‐localise in cells, we restored the expression of PAWS1WT or PAWS1F296A in PAWS1−/− U2OS cells. By immunofluorescence, we observed that PAWS1WT and CK1α displayed overlapping cytoplasmic staining, while PAWS1F296A and CK1α displayed non‐overlapping cytoplasmic staining and, as expected, no PAWS1 was detected in PAWS1−/− cells (Fig 5F). Interestingly, the CK1α staining in PAWS1−/− and PAWS1F296A rescue cells was much weaker than in PAWS1WT rescue cells (Fig 5F), suggesting that PAWS1 might play a role in cellular CK1α protein levels.
PAWS1 regulates cellular CK1α protein levels
The robust association and co‐localisation of PAWS1 with CK1α has several implications for the role of PAWS1 in the Wnt signalling pathway. These include (i) PAWS1 is a substrate of CK1α; (ii) PAWS1 controls CK1α activity; and (iii) PAWS1 controls CK1α stability and/or levels in cells. We sought to test these possibilities.
In vitro, CK1α phosphorylated PAWS1 and we mapped the CK1α phosphorylation site on PAWS1 to Ser614 using Edman sequencing and mass spectrometry (Fig EV2A–C). Consistent with this, CK1α was no longer able to phosphorylate PAWS1S614A mutant (Fig EV2D). However, bearing in mind that the PAWS1(1‐583) fragment, which lacks Ser614, was still able to cause axis duplication and activate Wnt signalling in Xenopus embryos (Fig 3G–I), the phosphorylation of PAWS1 at Ser614 by CK1α is unlikely to explain its role in Wnt signalling. Furthermore, the PAWS1S610A/S614A mutant was still able to induce axis duplication (Fig EV2E). As yet, we have not determined whether endogenous PAWS1 is phosphorylated at Ser614 by CK1α in vivo.
Figure EV2. PAWS1 is phosphorylated at Ser614 by CK1α in vitro .
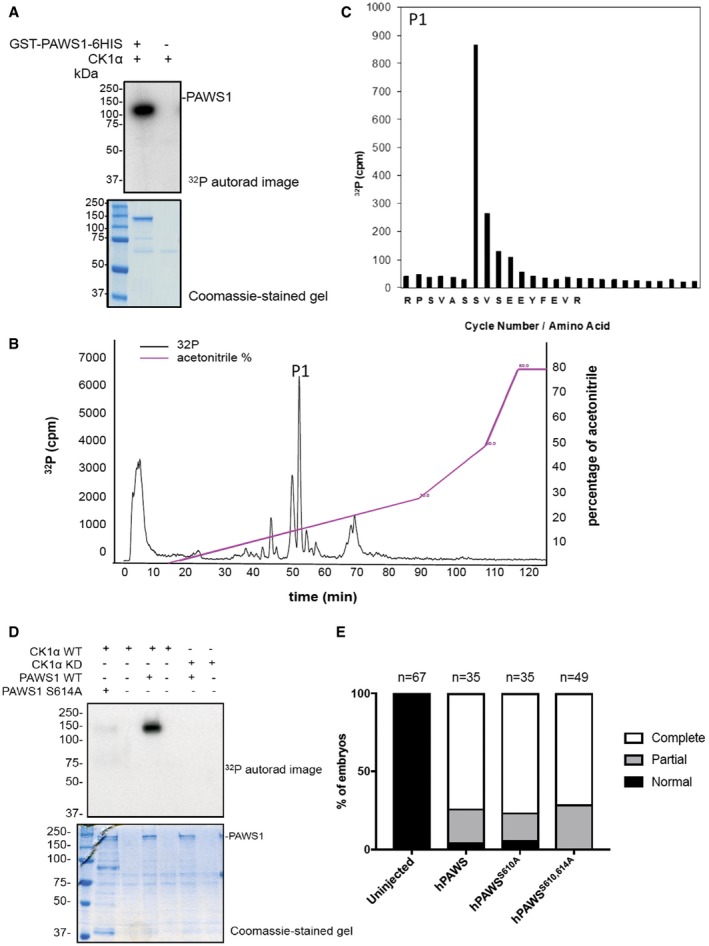
- 32P autoradiography and Coomassie stain of SDS–PAGE gel after an in vitro kinase assay with GST‐CK1α and GST‐PAWS1‐6xHis as a substrate.
- GST‐PAWS1‐6xHis phosphorylated by CK1α in A was excised from the gel, digested with trypsin and resolved by HPLC on a C18 column using increasing acetonitrile gradient. Analysis of the [γ32P] radioactivity peak at 54.1 min (P1) by LC‐MS/MS revealed the phospho‐peptide RPSVASSVSEEYFEVR.
- Analysis of the [γ32P] radioactivity peak P1 by LC‐MS/MS revealed various phospho‐peptides, of which RPSVASS(P)VSEEYFEVR was the only one to match the solid‐phase Edman sequencing data. Together, they reveal that CK1α phosphorylates PAWS1 at Ser614.
- 32P autoradiography and Coomassie stain of SDS–PAGE after an in vitro kinase assay with CK1αWT or CK1αKD (kinase dead) and PAWS1WT or PAWS1S614A as substrates.
- Human PAWS1WT, hPAWS1S610A and hPAWS1S610A/S614A induce axis duplication in Xenopus embryos.
We also tested the ability of PAWS1 to influence CK1α activity against a peptide substrate (CK1tide) 33. First, we detected robust CK1α kinase activity in endogenous PAWS1 IPs from wild‐type but not PAWS1−/− U2OS cell extracts (Fig 6A). The detection by Western blotting of endogenous CK1α in PAWS1 IPs from wild‐type but not PAWS1−/− cell extracts correlated with the observed in vitro kinase activities against CK1tide (Fig 6A). These observations suggest that the association of PAWS1 with CK1α does not inhibit intrinsic CK1α kinase activity. Next, we tested whether PAWS1 potentiated the kinase activity of recombinant CK1α through association. Human recombinant CK1α expressed in E. coli displayed in vitro kinase activity against CK1tide, while the catalytically inactive (KD; D136N) mutant of CK1α did not (Fig 6B). The addition of recombinant human PAWS1WT or the CK1‐interaction‐deficient PAWS1F296A mutant to the kinase reaction did not significantly affect the ability of CK1α to phosphorylate CK1tide (Fig 6B). Collectively, these data suggest that PAWS1 is unlikely to directly regulate the intrinsic CK1α kinase activity.
Figure 6. PAWS1 does not affect CK1α activity but affects the protein levels of CK1α in cells.
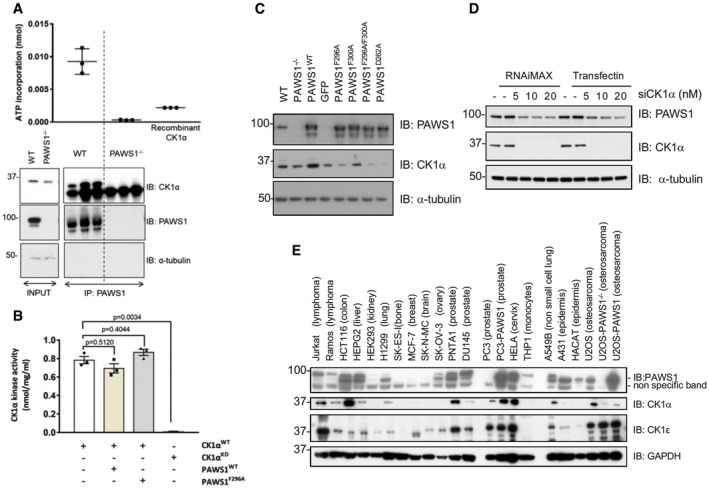
- Endogenous PAWS1 was immunoprecipitated from wild‐type and PAWS1−/− U2OS cells (n = 3), and the associated CK1α kinase activity was measured following a kinase assay using γ32P‐ATP and the CK1tide peptide substrate. A third of the PAWS1 IP samples were resolved by SDS–PAGE and immunoblotted with the indicated antibodies (bottom panel) (n = 3, error bars represent ± SEM).
- In vitro wild‐type (WT) or catalytically inactive (KD) CK1α kinase assay using CK1tide as a substrate in the presence or absence of recombinant PAWS1WT or PAWS1F296A (n = 3; error bars represent ± SEM; one way ANOVA with multiple comparisons, n.s.: no statistical significance).
- Cell extracts from U2OS wild‐type, PAWS1−/− and PAWS1−/− cells in which PAWS1, GFP control, PAWS1F296A, PAWS1F300A, PAWS1F296A/F300A or PAWS1D262A was restored, were resolved by SDS–PAGE and immunoblotted with the indicated antibodies.
- CK1α expression was silenced in U2OS cells with indicated amounts of siRNA using two different transfection reagents (RNAi‐Max or TransFectin) and PAWS1 protein levels monitored by Western blotting.
- Expression of PAWS1 and CK1α protein in the indicated cell lines was monitored by Western blotting. The protein levels of CK1α mirror the levels of PAWS1 in the majority of the cancer cell lines tested.
Source data are available online for this figure.
Strikingly, when we analysed the levels of CK1α protein in extracts from wild‐type and PAWS1−/− U2OS cells, we observed decreased CK1α protein levels in PAWS1−/− cells compared to the WT controls (Fig 6C). When we rescued PAWS1WT in PAWS1−/− cells, we noticed a remarkable increase in the endogenous CK1α protein levels compared to the re‐introduction of GFP control (Fig 6C). Interestingly, the re‐introduction of the CK1α‐binding‐deficient mutants PAWS1F296A and PAWS1F296A/F300A did not result in any increased CK1α protein levels, while the PAWS1F300A mutant, which interacts with CK1α, resulted in increased CK1α protein levels similar to PAWS1WT rescue (Fig 6C), suggesting that the binding of PAWS1 to CK1α regulates CK1α protein levels in cells. The reduction in levels of CK1α protein in PAWS1−/− U2OS cells compared to the WT controls could not be rescued by either the proteasomal inhibitor Bortezomib or the lysosomal inhibitor Bafilomycin A1 (Fig EV3A and B). Similarly, the mRNA levels of CK1α were comparable in PAWS1WT, PAWS1−/− and PAWS1F296A rescue cells (Fig EV3E), suggesting that the PAWS1 regulation of CK1α protein levels is not through its transcriptional regulation. In U2OS cells in which we transiently depleted endogenous CK1α protein with siRNAs, we observed a concomitant reduction in endogenous PAWS1 protein levels (Fig 6D), suggesting a reciprocal effect of PAWS1 and CK1α on controlling cellular protein levels.
Figure EV3. PAWS1 regulates the CK1α protein but not mRNA levels in cells.
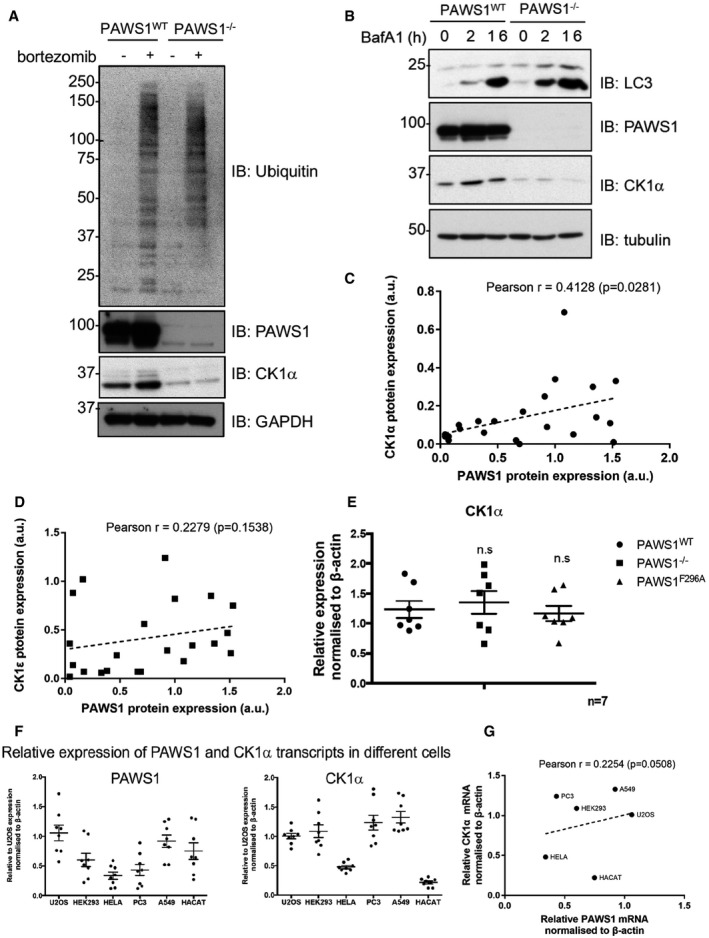
- PAWS1WT and PAWS1−/− U2OS cells were treated with 10 μM of the proteasome inhibitor Bortezomib for 6 h and extracts were analysed by Western blotting with the indicated antibodies.
- PAWS1WT and PAWS1−/− U2OS cells were treated with 50 μM Bafilomycin A1 (BafA1) for 2 or 16 h, and extracts were analysed by Western blotting with the indicated antibodies.
- Correlation plots of PAWS1 and CK1α protein expression from Fig 6E based on densitometry (using ImageJ) quantification of immunoblots normalised to GAPDH levels (a.u.: arbitrary units). Each dot represents a cell line. Pearson r coefficient and one‐tailed P‐values were calculated with Prism6 software.
- As in (C), except that PAWS1 and CK1ε protein levels were quantified and plotted.
- CK1α mRNA in PAWS1−/− cells rescued with PAWS1WT or GFP control analysed by quantitative RT–PCR. Data are represented as fold induction over controls and normalised internally to β‐actin control. Error bars represent ± SEM (n.s.: no statistical significance; n = 3).
- Relative expression of PAWS1 and CK1α transcripts in the indicated cancer cell lines was measured by RT–PCR (n = 7; error bars represent ± SEM).
- Correlation plot of relative PAWS1 and CK1α mRNA expression (normalised to β‐actin) in a subset of the indicated cancer cell lines. Pearson r coefficient and one‐tailed P‐values were calculated with Prism6 software.
Source data are available online for this figure.
Prompted by these observations, we examined the potential correlation in expression of PAWS1 and CK1α proteins in a number of mammalian cell lines. We observed that PAWS1 protein expression was absent in many human cancer cells, including SK‐ES (bone), MCF‐7 (breast), SK‐N‐MC (brain), and PC3 (prostate) cells while robust expression was observed in HCT116 (colorectal), HEPG2 (liver), PNTA1 (prostate) and HeLa (cervical) cells. Strikingly, the expression levels of PAWS1 in these cells correlated with the levels of CK1α protein (Figs 6E and EV3C). Cells that lacked PAWS1 protein expression generally correlated with no detectable or low levels of CK1α protein, whereas cells that had relatively higher levels of PAWS1 also expressed relatively higher levels of CK1α (Figs 6E and EV3C). No correlation in expression was evident between PAWS1 and CK1ε, which does not interact with PAWS1 (Figs 6E and EV3D). In both PC3 and PAWS1−/− U2OS cells, which lack endogenous PAWS1, the restoration of WT PAWS1 increased the protein levels of endogenous CK1α but not CK1ε (Fig 6E) 31. In order to test whether the correlation in PAWS1 and CK1α protein levels was due to a similar correlation in mRNA expression, we assessed the PAWS1 and CK1α mRNA levels in six of these mammalian cell lines. No discernible correlation was observed with the expression of PAWS1 and CK1α mRNAs (Fig EV3F–H), indicating that the correlation between PAWS1 and CK1α protein levels occurs post‐transcriptionally.
Interaction between PAWS1 and CK1α is essential for PAWS1‐dependent axis duplication and the activation of Wnt signalling
Armed with the knowledge that PAWS1 associates and co‐localises with CK1α, we asked whether the association between PAWS1 and CK1α was essential for PAWS1‐dependent activation of Wnt signalling in Xenopus embryos and in U2OS cells. PAWS1‐induced axis duplication in Xenopus embryos was scored following microinjection with wild‐type PAWS1, three separate CK1α‐interaction‐deficient PAWS1 mutants, PAWS1D262A, PAWS1F296A and PAWS1F296A/F300A as well as PAWS1F300A mutant that interacts with CK1α (Fig 7A). Both PAWS1WT and the PAWS1F300A mutant induced partial or complete axis duplication in ~80% of embryos. In contrast, PAWS1D262A, PAWS1F296A and PAWS1F296A/F300A failed to induce secondary axes in the embryos (Fig 7A). These results demonstrate that the interaction between PAWS1 and CK1α is essential for PAWS1‐mediated axis duplication in Xenopus embryos. To explore whether the induction of the Wnt‐reporter activity in U2OS cells also required interaction between PAWS1 and CK1α, we assessed the Wnt‐reporter activity in wild‐type, PAWS1−/− and PAWS1−/− cells rescued with either PAWS1WT or the PAWS1F296A mutant. The treatment of wild‐type cells with Wnt3A substantially enhanced the luciferase reporter activity over unstimulated controls, while in PAWS1−/− cells it did not (Fig 7B). In PAWS1−/− cells rescued with wild‐type PAWS1, the Wnt3A‐induced luciferase reporter activity was restored. In contrast, it was not restored in PAWS1−/− cells rescued with the PAWS1F296A mutant (Fig 7B), suggesting that PAWS1 activation of Wnt signalling necessitates PAWS1: CK1α interaction.
Figure 7. PAWS1 association with CK1α is essential for induction of Wnt signalling.
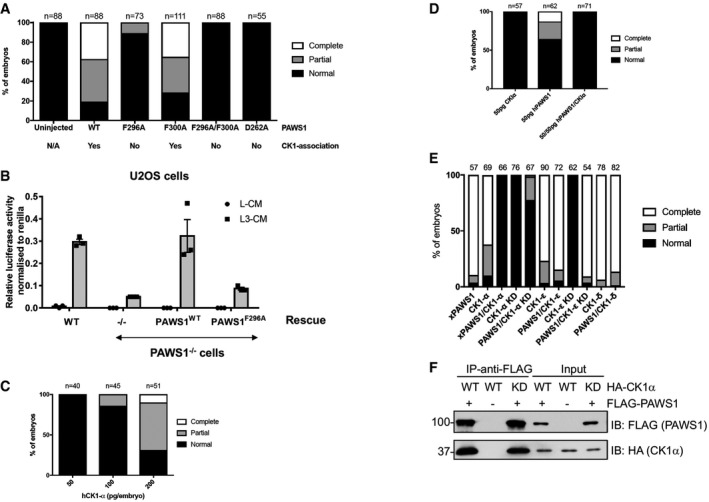
- PAWS1F296A, PAWS1F296A/F300A and PAWS1D262A mutants that do not interact with CK1α fail to induce a secondary axis in Xenopus embryos.
- Relative TOPFlash luciferase activity of U2OS WT, PAWS1−/−, PAWS1WT and PAWS1F296A rescue cells after treatment with control‐conditioned media (L‐CM), Wnt3A‐conditioned media (L3‐CM) (n = 3; error bars represent ± SEM).
- Human CK1α induces a secondary axis in Xenopus embryos in a dose‐dependent manner.
- Co‐expression of PAWS1 and CK1α mRNAs do not induce secondary axis.
- Effect of CK1 isoforms on PAWS1‐dependent axis‐inducing activity. 250 pg of xPAWS1 and 300 pg of the indicated CK1 isoform mRNAs were injected into one ventral blastomere at the four‐cell stage. Axis induction was assessed at stage 28.
- PAWS1 interacts with kinase‐dead CK1α in U2OS cells. U2OS cells were co‐transfected with HA‐CK1α (WT or KD) and FLAG‐PAWS1, and cell extracts were subjected to FLAG IP, followed by SDS–PAGE and Western blot analysis with the indicated antibodies.
Source data are available online for this figure.
The role of PAWS1:CK1α complex in Wnt signalling
In the absence of Wnt ligands, CK1α has been reported to phosphorylate β‐catenin on Ser45 34, 35, which triggers a sequential phosphorylation by GSK‐3 on Thr41, Ser37 and Ser33 28, thereby allowing recruitment of the E3 ubiquitin ligase β‐TrCP to β‐catenin for its ubiquitin‐mediated proteasomal degradation 26. Paradoxically, overexpression of CK1α has been reported to stimulate Wnt signalling 36, and indeed, human CK1α can also induce a secondary axis in a dose‐dependent manner in Xenopus embryos (Fig 7C). However, when co‐expressed with PAWS1, the axis induction was blocked (Fig 7D). A possible explanation is that PAWS1 inhibits CK1α activity; hence, the overexpression of CK1α counters the PAWS1 effects on axis duplication. However, we have shown earlier that PAWS1 IPs from WT but not PAWS1−/− U2OS cells display CK1α catalytic activity and recombinant PAWS1 does not impair CK1α activity in vitro (Fig 6A and B). Therefore, these observations suggest that overexpression of either PAWS1 or CK1α alone in embryos potentially disrupts the endogenous PAWS1:CK1α complex homeostasis, resulting in the interference with Wnt signalling that results in the axis phenotypes observed. In contrast, when these are co‐expressed, this interference on the endogenous PAWS1:CK1α complexes is minimised, so the phenotypes are normal. If this were the case, then one would expect similar outcomes using a catalytically inactive mutant of CK1α (Fig 7E), which we have shown interacts with PAWS1 with similar affinity to the wild‐type CK1α (Fig 7F). Indeed, co‐expression of PAWS1 and the catalytically inactive CK1α mutant in Xenopus embryos also resulted in completely normal embryos (Fig 7E). In similar assays, we also tested the ability of CK1δ and CK1ε, which do not interact with PAWS1 31, to inhibit PAWS1‐dependent axis duplication phenotype in Xenopus embryos. Unlike CK1α, CK1δ and CK1ε or the catalytically inactive CK1ε were unable to block the axis duplication phenotype caused by PAWS1 (Fig 7E). Like CK1α, CK1δ and CK1ε isoforms on their own also induced axis duplication in Xenopus embryos (Fig 7E), suggesting PAWS1‐independent roles in Wnt signalling. Interestingly, catalytically inactive CK1α and CK1ε on their own did not induce axis duplication, suggesting that the catalytic activity is critical to their impact on Wnt signalling. Together, our findings suggest that critical regulation of endogenous PAWS1:CK1α complex is vital in driving Wnt signalling through axis specification during development and subtle imbalances can interfere with normal development. Not surprisingly, this feature is shared with the non‐canonical Wnt/planar cell polarity pathway in which slight perturbations lead to defects in developmental events such as convergent extension and ciliogenesis 37.
Since PAWS1 appears to exist predominantly in a PAWS1:CK1α complex (Fig 5), we asked whether PAWS1:CK1α also regulated other CK1α‐dependent pathways. One potential target of PAWS1:CK1α is the transcription factor NFAT, which has been reported to promote ventral cell fates in the Xenopus embryo 38. Nuclear localisation of NFAT is regulated by CK1α via a SSR‐1 motif in the N‐terminus, in which phosphorylation of the motif by CK1 promotes NFAT1 nuclear export, while its dephosphorylation by calcineurin induces nuclear import. Phospho‐deficient SSR‐1 motif mutants of NFAT1 accumulate in the nucleus 32, as does NFAT in cells treated with a CK1 inhibitor 32, and when overexpressed in Xenopus ventralise embryos 38. In dissociated animal cap cells expressing a NFAT_GFP reporter construct, NFAT_GFP accumulated predominantly in the cytoplasm (Fig EV4A–C). However, unlike cells expressing the catalytically inactive CK1α (KD), in which NFAT was distributed in the both the cytoplasm and the nucleus (Fig EV4C), we found that xPAWS1 expression had no effect on the nuclear localisation of the reporter (Fig EV4A–C), nor did it affect nuclear localisation of NFAT_GFP following Ca2+ ionophore treatment (Fig EV4A–C) where no cytotoxicity was detected. These data suggest that, in contrast to the Wnt pathway, the regulation of nuclear localisation of NFAT is insensitive to perturbations of the PAWS1:CK1α complex.
Figure EV4. xPAWS1 does not induce nuclear localisation of NFAT.
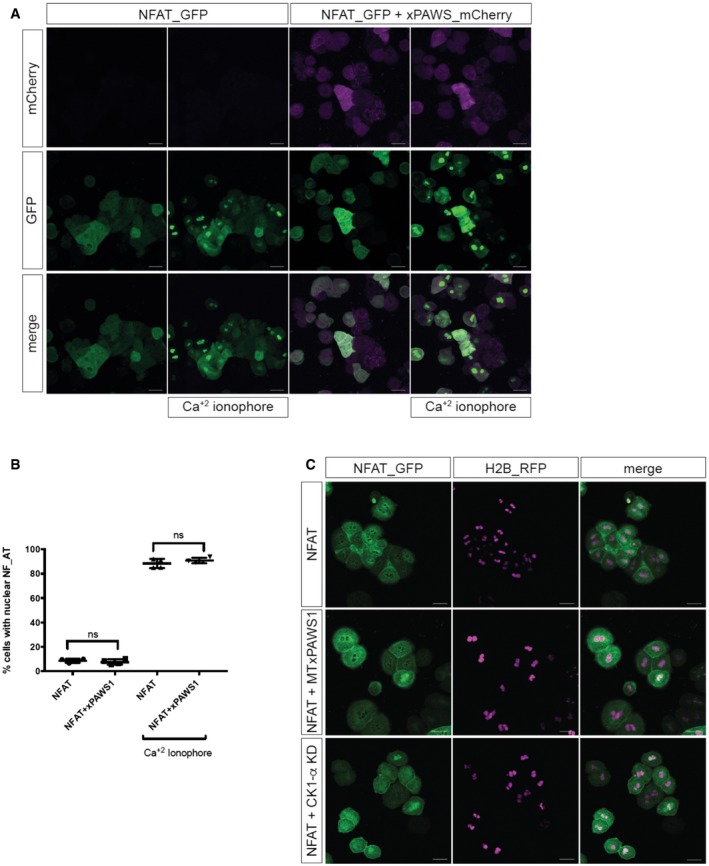
- Dissociated animal cap cells injected with either 500 pg of NFAT_GFP or 500 pg of NFAT_GFP and 250 pg of xPAWS1_mCherrymRNA. Images are maximum intensity projections, scale bars = 50 μm.
- Nuclear localisation of NFAT_GFP was assessed before and after treatment with 0.025 μg/ml calcium ionophore. ns: no statistical significance (n = 4; error bars represent ± SD; Mann–Whitney test, unpaired, two‐tailed with unequal variance, P = 0.6857).
- Expression of a catalytically inactive CK1α leads to Ca2+‐independent nuclear localisation of NFAT_GFP. Dissociated animal cap cells injected with either 500 pg of NFAT_GFP alone and 250 pg of H2B_RFP (top panels), 500 pg of NFAT_GFP, 250 pg H2B_RFP and 250 pg of MTxPAWS1 (middle panels), or 500 pg NFAT_GFP, 250 pg H2B_RFP, and 300 pg CK1α KD (bottom panels). Images represent a single z‐frame at the level of the nucleus. Cells were imaged using a Zeiss LSM710 confocal microscope controlled with Zen Black software. Image analysis was performed using Zen blue software. Scale bars = 50 μm.
Source data are available online for this figure.
PAWS1 does not interact robustly with Axin1 and β‐catenin complexes during Wnt signalling
Having earlier established the role of PAWS1 in Wnt signalling at or downstream of the destruction complex, we sought to explore the composition of the destruction complex in the absence of PAWS1. In order to dissect any changes in the make‐up of the destruction complex, we immunoprecipitated CK1α, Axin1 and β‐catenin from unstimulated and Wnt3A (3 h)‐stimulated WT and PAWS1−/− U2OS cells and analysed some of the known components of the destruction complex. In endogenous CK1α IPs from both WT and PAWS1−/− cells, Axin1, GSK‐3α/β and β‐catenin were not detectable, regardless of Wnt3A stimulation, while as expected PAWS1 was identified in CK1α IPs from WT cells but not PAWS1−/− cells (Fig 8). In Axin1 IPs, GSK‐3α/β were detected robustly, while very low levels of β‐catenin were also detected, although neither Wnt3A treatment nor the PAWS1‐status had any effect on these Axin1 complexes (Fig 8). Similarly, in β‐catenin IPs, we were able to detect low levels of GSK‐3β, as well as CK1δ and CK1ε but not CK1α (Fig 8), suggesting that CK1δ and CK1ε, rather than CK1α, may be essential in mediating β‐catenin phosphorylation at Ser45. CK1α IPs from cells treated with Wnt3A for shorter times (30 min and 1 h) did not pull down any significant levels of β‐catenin and no differences were observed between WT and PAWS1−/− U2OS cells (Fig EV5A). With no significant differences in Axin1, GSK‐3α/β and β‐catenin complexes detected in the absence of PAWS1, we undertook an unbiased proteomic approach to identify interactors of PAWS1‐GFP expressed in U2OS cells under unstimulated and Wnt3A (3 h)‐stimulated conditions (Fig EV5B). However, other than CK1α and CK1α‐like, no major components of either the Wnt pathway or the destruction complex were identified in the PAWS1‐GFP IPs under both control and Wnt3A‐stimulated conditions (Fig EV5C). While some PAWS1 interactors, such as PLOD1 and many 14‐3‐3 proteins, were substantially enriched in Wnt3A‐stimulated conditions, albeit with much lower abundance compared to CK1α and CK1α‐like, these still need to be validated further and their roles in Wnt signalling are not well established (Fig EV5B). As 14‐3‐3 proteins are binders of phospho‐proteins 39, we analysed all phospho‐residues on PAWS1 by mass spectrometry. Interestingly, some phospho‐residues on PAWS1, namely Ser127, Ser634 and Ser726, appeared to be enriched by Wnt3A stimulation (Fig EV5D). Clearly, these need to be validated at the endogenous level and the functional relevance of these is unclear.
Figure 8. PAWS1 does not appear to affect Axin1 and β‐catenin complexes upon Wnt signalling.
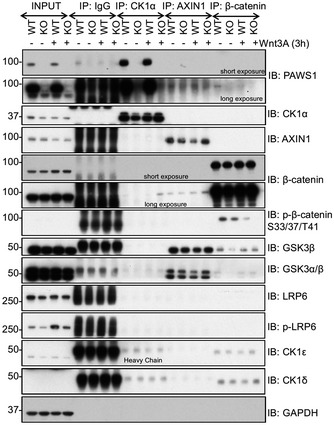
U2OS wild‐type (WT) and PAWS1−/− (KO) cells were treated with control‐conditioned medium or Wnt3A‐conditioned medium, and the extracts (0.5 mg protein) were subjected to immunoprecipitation using antibodies against the endogenous CK1α, AXIN1 and β‐catenin antibodies or anti‐rabbit pre‐immune IgG as a control (10 μg antibodies coupled to 10 μl packed protein‐G sepharose beads). IPs were resolved by SDS–PAGE and immunoblotted with the indicated antibodies.Source data are available online for this figure.
Figure EV5. Effects of PAWS1 on Wnt pathway components.
- U2OS wild‐type (WT) and PAWS1−/− (KO) cells were treated with control‐conditioned medium or Wnt3A‐conditioned medium and lysed at the indicated time points. Extracts (0.5 mg protein) were subjected to IPs using anti‐CK1α or pre‐immune IgG control (10 μg antibodies coupled to 10 μl packed protein‐G sepharose beads). IPs were resolved by SDS–PAGE and immunoblotted with the indicated antibodies.
- Stable U2OS Flp‐In TRex cells were treated with 20 ng/ml doxycycline for 16 h, for induction of PAWS1‐GFP or GFP protein expression, and with Wnt3A conditioned medium or control medium for 3 h prior to lysis. GFP pull downs were resolved by SDS–PAGE and the gel was stained with Coomassie. Each lane was cut into five pieces, which were subsequently processed for protein identification by mass fingerprinting analysis.
- PAWS1‐GFP interacting proteins were plotted using total spectral counts for selected individual protein for both control (filled) and Wnt3A (open) conditions. Total spectral counts are defined as the sum of all the spectra associated with a specific protein within a sample, which includes also those spectra that are shared with other proteins. A spectral count of 3 or more in either control or Wnt3A condition in PAWS1‐GFP IPs and no spectral counts in GFP control IPs were set as threshold for inclusion. All proteins, except those indicated with asterisks, were identified as endogenous PAWS1GFP/GFP interactors as well (Fig 5A).
- PAWS1 phospho‐residues from gel slices 2 and 7 from (B) were identified by mass spectrometry. Residues denoted by “or” indicate that a specific single phospho‐residue on the corresponding tryptic peptide could not be assigned but could be any one of those indicated.
- U2OS wild‐type (WT) and PAWS1−/− cells were treated with control (L‐CM) or Wnt3A conditioned medium (L3‐CM) for 3 h and fractionated into cytoplasmic (C), nuclear (N), membrane (M) and cytoskeletal (Cs) fractions. Extracts (20 μg protein) from each fraction was resolved by SDS–PAGE, transferred onto PVDF membranes, which were probed by Western blotting with the indicated antibodies. α‐Tubulin was used as a cytosolic marker, Lamin A/C as a nuclear marker, LRP6 and EGFR were used as membrane markers, and Vimentin was used as a cytoskeletal marker.
Source data are available online for this figure.
PAWS1 impacts the nuclear translocation of β‐catenin downstream of the destruction complex
Next, we assessed the effect of PAWS1‐loss on the post‐translational regulation of β‐catenin. To this end, we analysed β‐catenin phosphorylation on known CK1 (Ser45) and GSK3 (Thr41, Ser37 and Ser33) phospho‐sites and its subcellular distribution. In WT U2OS cells, Wnt3A stimulation resulted in acute reduction in levels of both phospho‐Ser45 and phospho‐Thr41, ‐Ser37 and ‐Ser33 residues within 30 min, while the levels then recovered in a time‐dependent manner (Fig 9A). This is in agreement with the accumulation of active non‐phosphorylated (Thr41, Ser37 and Ser33) form of either endogenous β‐catenin or ectopic β‐catenin‐GFP observed in Xenopus animal caps injected with xPAWS1 (Fig 3A–D). No significant differences in total endogenous β‐catenin levels were observed over the Wnt3A stimulation time course in either cell type, suggesting that in U2OS cells, only a small pool of total β‐catenin might be regulated by Wnt3A treatment (Fig 9A). Interestingly, under unstimulated basal conditions in which cells were grown in DMEM containing 10% serum, slightly higher levels of both CK1α (Ser45) and GSK3 (Thr41, Ser37 and Ser33) phospho‐sites in β‐catenin were observed in PAWS1−/− extracts compared to the wild type (Fig 9A). No substantial differences between the wild‐type and PAWS1−/− cells were observed when we analysed the levels of Wnt‐induced phosphorylation of LRP6 at Ser1490 (Fig 9A). We also assessed the expression of AXIN2 protein, a target of Wnt, in wild‐type and PAWS1−/− cells over the course of 16 h of Wnt3A stimulation. Like Wnt3A‐induced AXIN2 mRNA expression earlier (Fig 4B), we observed that a time course of Wnt3A stimulation induced an increase in AXIN2 protein levels in wild‐type U2OS cells, while this increase was less pronounced in PAWS1−/− cells (Fig 9A). Since the Wnt3A‐induced phospho‐ and total β‐catenin levels in U2OS cell extracts did not appear to change significantly to account for the inhibition of Wnt signalling caused by loss of PAWS1, we analysed the cytoplasmic and nuclear distribution of β‐catenin in wild‐type, PAWS1−/−, and PAWS1WT and PAWS1F296A rescue U2OS cells upon Wnt stimulation (Fig 9B). We monitored pSer45, pThr41/Ser37/Ser33, non‐phospho Ser45 and Thr41 (so‐called α‐active β‐catenin monoclonal antibody 40) and total β‐catenin levels. Compared to wild‐type and PAWS1WT rescue cells, both basal and Wnt‐induced levels of β‐catenin‐pSer45, β‐catenin‐pThr41/Ser37/Ser33, active β‐catenin and to some extent total β‐catenin in nuclear, but not cytoplasmic, fractions were markedly lower in PAWS1−/− and PAWS1F296A rescue cells (Fig 9B). This was particularly evident when we analysed the levels of active β‐catenin in each fraction relative to that present in the cytoplasmic fraction of WT U2OS cells (Fig 9B, lower panel), suggesting that PAWS1 potentially promotes nuclear accumulation of β‐catenin. In WT and PAWS1WT and PAWS1F296A rescue U2OS cells, PAWS1 was observed in both cytoplasmic and nuclear fractions, with slight enrichment in cytoplasmic fractions. However, more CK1α was detected in the nuclear fractions than cytoplasmic, except that there was less overall CK1α detected in PAWS1−/− and PAWS1F296A rescue cells compared to WT and PAWS1WT rescue cells (Fig 9B). These observations suggest that other FAM83 members that also bind CK1α probably account for the enhanced nuclear pool of CK1α 31. Because most of the β‐catenin protein is reported to be associated with the plasma membrane 41, 42, we assessed the effect of PAWS1‐loss on membrane‐associated pool of β‐catenin. We also found high levels of total β‐catenin in the plasma membrane fractions and quite substantial amounts in the cytoskeletal fractions, when compared to cytoplasmic and nuclear fractions (Fig EV5E). However, neither the loss of PAWS1 nor Wnt3A stimulation changed the levels of β‐catenin protein in both the membrane and cytoskeletal fractions (Fig EV5E). Under these conditions, very low levels of PAWS1 and CK1α were detected in the membrane and cytoskeletal fractions (Fig EV5E).
Figure 9. PAWS1 promotes Wnt signalling through increased accumulation of nuclear active β‐catenin.
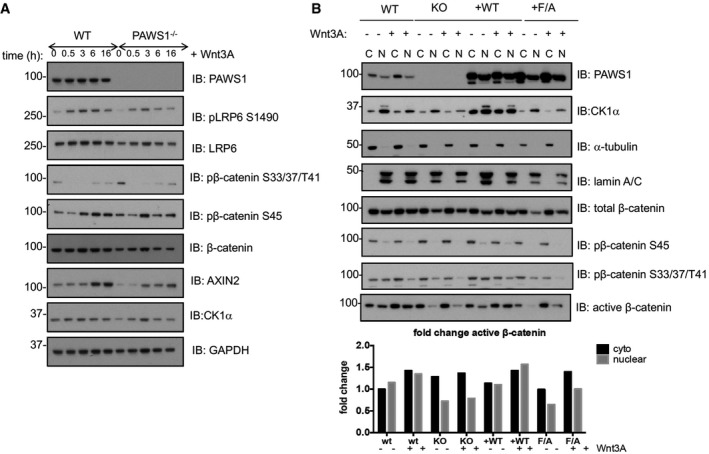
- U2OS wild‐type and PAWS1−/− cells were exposed to either Wnt3A or control medium for the indicated time points. Cell extracts were subjected to SDS–PAGE followed by Western blot analysis with the indicated antibodies.
- U2OS wild‐type (WT), PAWS1−/− (KO), PAWS1WT (+WT) and PAWS1F296A (F/A) rescue cells were exposed to either Wnt3A or control medium for 3 h followed by separation and preparation of cytoplasmic and nuclear fractions. The extracts were subjected to SDS–PAGE followed by Western blot analysis with the indicated antibodies. The lower panel represents the fold changes in active β‐catenin intensities in each fraction relative to those seen in the cytoplasmic fraction of control WT U2OS cells. The intensities of the active β‐catenin bands in each fraction were quantified by using the ImageJ software.
Source data are available online for this figure.
Discussion
We have shown previously that PAWS1 modulates a subset of non‐canonical SMAD4‐independent genes in the BMP signalling pathway, yet has no effect on the canonical BMP pathway 1. Consistent with this, we find here that PAWS1 does not affect canonical BMP signalling in either Xenopus embryos or U20S cells. In contrast, we demonstrate here that PAWS1 plays a significant role in regulating Wnt signalling in both developing Xenopus embryos and human cells. Furthermore, we find that the association between PAWS1 with CK1α is necessary to modulate Wnt signalling. The precise mechanisms for the coordinated actions of PAWS1 and CK1α in Wnt signalling, and perhaps beyond, remain to be established but our findings indicate that PAWS1 regulates cellular levels and subcellular localisation of CK1α protein, and potentially the phosphorylation of CK1α substrates. Collectively, our findings establish PAWS1 as a novel player in the Wnt signalling pathway and a critical regulator of CK1α.
It has long been known that CK1 isoforms play crucial roles in the Wnt/β‐catenin signalling 28, 29, 30, 34, 35, 43, 44, 45, 46. In fact, early observations that overexpression of CK1 isoforms in Xenopus embryos caused axis duplication suggested a positive role for CK1 in Wnt signalling 47. However, it has since become evident that CK1 isoforms play both negative and positive roles in the Wnt/β‐catenin signalling 29, and their activities may be regulated in a coordinated fashion 48. The precise mechanisms by which CK1 isoforms are regulated to allow them to execute these opposing outcomes in Wnt/β‐catenin signalling remain elusive. From our data, it is evident that PAWS1 and CK1α coordinate to modulate Wnt signalling but the influence of CK1δ and CK1ε on Wnt signalling is not affected by PAWS1. This suggests that different CK1 isoforms target specific sets of substrates in cells and are uniquely regulated in driving specific cellular processes, including Wnt signalling. In this context, our findings that different FAM83 members, of which PAWS1 is a member, associate and co‐localise with different CK1 isoforms suggest that perhaps coordinated actions of one or more FAM83 members may define CK1 isoform‐specific substrates and actions in the Wnt/β‐catenin signalling and beyond 31.
In the absence of Wnt signalling, it has been reported that autophosphorylation of the C‐terminal domains of CK1δ and ε inhibits their catalytic activity, which is relieved upon Wnt stimulation by yet‐unidentified phosphatases 49, 50. On the other hand, CK1α is constitutively active and therefore must be regulated in a different manner. One attractive model is the “scaffold or proximity effect” (reviewed in 29, 51). In this model, rather than directly regulating catalytic activity, scaffold proteins facilitate kinase activity by regulating substrate proximity to the kinase 52. For example, Axin, which interacts with GSK3β, enhances β‐catenin phosphorylation by CK1α and GSK3β, without altering CK1α kinase activity 53, 54, 55, 56. Similarly, PAWS1 may act as a scaffold by regulating the access of CK1α to its substrates. Consistent with this notion, we find that PAWS1 IPs from cells display CK1α catalytic activity and recombinant PAWS1 has no effect on intrinsic CK1α kinase activity in vitro. Our observations that while PAWS1 and Axin form robust complexes with CK1α and GSK3β, respectively, neither appears to bind β‐catenin or other known Wnt pathway components robustly are consistent with the potential scaffolding roles of PAWS1 and Axin in facilitating the phosphorylation of CK1α and GSK3β substrates respectively, as protein kinases often engage with their substrates only transiently. It is still unclear precisely how PAWS1 acts in concert with CK1α and it remains feasible that PAWS1 regulates CK1α substrate availability in either a positive or negative fashion. From our data, the PAWS1:CK1α complex does not appear to regulate the phosphorylation of β‐catenin at Ser45; however, the nuclear accumulation of β‐catenin appears to require PAWS1:CK1α complex. We have also shown that all members of the FAM83 family can associate and co‐localise with CK1α, while some also interact with CK1δ and CK1ε isoforms, suggesting that other FAM83:CK1 complexes may act redundantly or in a coordinated fashion to control Wnt/β‐catenin signalling 31.
Future work will identify and characterise the PAWS1‐dependent CK1α substrates that may be essential for mediating Wnt signalling, using phospho‐proteomic approaches in wild‐type and PAWS1−/− cells and address the molecular basis for interaction between PAWS1 and CK1α. Similarly, investigations are underway to understand how CK1α protein levels are regulated by PAWS1.
Materials and Methods
Plasmids, antibodies and reagents
All constructs were sequence‐verified by the DNA Sequencing Service, University of Dundee (http://www.dnaseq.co.uk). They included PAWS1‐GFP donor (DU48585), sense (DU48793) and antisense (DU48826) gRNAs, pCMV5‐FLAG‐PAWS1 (DU33274), FLAG‐PAWS1 F296A/F300A (DU28026), FLAG‐PAWS1 F296A (DU28024) and FLAG‐PAWS1 F300A (DU28025), pBabe puro PAWS1 (DU33460), pBabe puro FAM83G F296A (DU28044), pBabe puro FAM83G F300A (DU28045), pBabe puro FAM83G F296A/F300A (DU28046), pcDNA5‐Frt/TO‐PAWS1‐GFP (DU42816) and pcDNA5‐Frt/TO‐GFP (DU41455). For bacterial expression of proteins, PAWS1 wild type and mutants were cloned in pGEXP1 vectors. These include pGEXP‐1 GST‐PAWS1‐6xHis (DU28293), pGEXP‐1 GST‐PAWS1 S614A (DU33321) and pGEXP‐1 GST‐PAWS1 F296A (DU28047). These constructs are available to request from the MRC‐PPU reagents webpage (http://mrcppureagents.dundee.ac.uk) and the unique identifier (DU) numbers indicated above provide direct links to the cloning strategies and sequence details.
Antibodies included PAWS1 (DU33022), SMAD1 (DU19291), CK1α (Bethyl Laboratories #Α301‐991A‐M), CK1ε (CST #12448), HA (Sigma HA‐7 #H3663), FLAG‐HRP (Sigma #A8592), GFP (Abcam, #ab6556), Myc tag (Sigma 9E10 #M5546), α‐tubulin (ThermoScientific #MA1‐80189, Sigma #T5168), AXIN2 (CST #76G6), AXIN1 (CST #2074), β‐catenin (CST #D10A8; Santa Cruz Biotechnology #H‐102), GSK3‐α/β (Santa Cruz #sc‐7291), GSK3β (CST #9315S), SLC5A10 (Abcam, #ab167156), active β‐catenin (Millipore #05‐665, CST D13A1 #8814), LRP6 (CST #C47E12), GAPDH (CST #D16H11), Lamin A/C (CST #2032) and HA‐HRP (Roche 2013819001). All phospho‐antibodies were purchased from Cell Signaling Technologies.
Xenopus Addgene constructs pCS2AxinMT (#16298) pCS2 GSK3β 9SA (#16333) were a kind gift from Peter Klein, Xi He kindly provided pCS2LRP6‐m5 (#27283); Randall Moon pCS2 β‐catenin‐GFP (#16839), Super 8× TOPFlash (#12456), Super 8× FOPFlash (#12457), xCK1ε D128N (#16726); William Hahn and David Root CK1α (#23355); and Barry Gumbiner pCS2C‐cadherin (#17023). The ORFs of Xenopus FAM83G (xPAWS1; Image Clone MGC:98851 Source Bioscience), CK1δ (image clone #5571715 Source Bioscience), CK1ε (Image Clone #6316339, Source Bioscience) were cloned into pENTR‐D‐TOPO (Invitrogen) vector and then transferred into pCS2_N‐MT, pCS2_N‐HA, pCS2_C‐HA, pCS2_c‐mCherryHA destination vectors using LR clonase (Invitrogen). The catalytically inactive forms of CK1α (CK1α KD, D136N) were generated by site‐directed mutagenesis. pCS2nuc β‐gal and pCS2 H2B_RFP were kind gifts from Anna Philpott (University of Cambridge) and Sean Megason (Harvard University), respectively.
Recombinant TGFβ1 and BMP2 were purchased from R&D Systems. Bortezomib was from LC Laboratories. Doxycycline was from Sigma and CHIR99021 from Axon. Recombinant kinases were generated by the MRC‐PPU protein expression team. These include CK1α (DU329) and CK1α(D136N) (DU28371).
Xenopus maintenance and manipulations
Regulations for the use of Xenopus laevis, as outlined in the Animals Scientific Procedures Act (ASPA) and implemented by the Home Office in the UK, were followed. Xenopus laevis embryos were obtained by in vitro fertilisation and staged according to Nieuwkoop and Faber (1975). Embryos were injected with capped RNA synthesised using SP6 mMessage mMachine kit (Invitrogen). Animal caps assays were as described 57. Dissociated animal caps were plated onto μ‐Slide 8‐well glass bottom chamber slides (#80827, Ibidi) or 12‐well μChamber slides (#81201) coated with 3 μg/ml recombinant human E‐cadherin protein (#8505‐EC‐050; R&D Systems) in 0.7× Marc's Modified Ringers (MMR; 0.1 M NaCl, 2 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 5 mM HEPES pH 7.8, 0.1 mM EDTA).
In situ hybridisation
Whole‐mount in situ hybridisation was performed as described 58, except the triethanolamine and acetic anhydride treatments were omitted. β‐Gal staining was developed using Salmon Gal (Apollo Scientific #BIMB1026), as an alternative chromogenic substrate, which produces a light pink colour. Probes were made using DIG labelling mix (Roche, #11277037910), and developed using BCIP (Roche, #11383221001), and NBT (Roche, #11383213001). Images of stained embryos were captured using a Leica DFC310FX camera attached to a Leica M165FC microscope controlled by LAS V4.9 software.
Immunofluorescence
Dissociated animal cap cells injected with β‐catenin_GFP and/or xPAWS1_mCherry mRNAs were imaged live with a Zeiss LSM710 confocal microscope controlled by ZEN Black 2012 software. Post‐acquisition analysis was performed using ZEN Black, Zen Blue 2012 and ImageJ software packages.
For the analysis of SMAD signalling, dissociated animal cap cells were fixed in MEMFA (0.1 M MOPS pH 7.4, 2 mM EGTA, 1 mM MgSO4, 3.7% formaldehyde) for 10 min at room temperature and then washed 3 × 5 min in PBS. Cells were permeabilised with 0.5% Triton X‐100 for 5 min followed by 3 × 5 min washes with PBS and then blocked in blocking buffer (PBS, 0.1% Tween‐20, 1% BSA, 10% goat serum) for 1 h at room temperature. Primary (anti‐P‐Smad1, CST #9511) and secondary (anti‐rabbit Alexa568, Invitrogen #A11031) were used at 1:1,000 in blocking buffer, each for 1 h, and then washed 3 × 5 min with PBS‐T. Cells were mounted using Prolong Gold Antifade Mountant with DAPI (ThermoFisher, #P36935). Images were captured on Zeiss LSM710 confocal microscope as described above.
For PAWS1 and CK1α IF, cells were fixed with 4% paraformaldehyde in PBS for 10 min, and permeabilised with 0.5% Triton X‐100 in PBS for 5 min. Coverslips were incubated in blocking buffer (3% BSA, 0.1% Triton X‐100 in PBS) for 30 min, followed by anti‐PAWS1 (1:250, Abcam, ab121750) and anti‐CK1α (1:100, Santa Cruz, sc6477) antibodies in blocking buffer for 1 h. Cells were washed with 0.1% Triton X‐100 in PBS and incubated with Alexa Fluor‐conjugated secondary antibodies (ThermoFisher Scientific) and DAPI (1 μg/ml). Images were captured using a DeltaVision system (Applied Precision). Z‐series were collected at 0.2 μm intervals and deconvolved using SoftWoRx (Applied Precision).
Cell culture
U2OS osteosarcoma cells, HEK293 cells, mouse fibroblast L‐cells that stably overexpress Wnt3A (ATCC, CRL‐2647) or L cells (ATCC, CRL‐2648) were grown in Dulbecco's Modified Eagle's Medium (DMEM; Gibco) supplemented with 10% (v/v) FBS (Hyclone), 2 mM l‐glutamine, 100 units/ml Penicillin and 100 μg/ml Streptomycin (Lonza). Flp‐IN TRex U2OS cells were maintained in growth media supplemented with 15 μg/ml blasticidin and (100 μg/ml) zeocin. Cells were grown until confluent at 37°C in a humidified incubator at 5% CO2. Prior to lysis, cells were stimulated as described in the figure legends. For transient transfections, cells were transfected with cDNA plasmids (2.5 μg per 10‐cm dish unless indicated otherwise) using polyethylenimine (PEI) (Polysciences Inc.) in OptiMem (Gibco) medium as described previously 59.
Preparation of cell extracts
Before being harvested, cells were rinsed in ice‐cold PBS and extracted in lysis buffer containing 50 mM Tris–HCl pH 7.5, 1 mM EGTA pH 8, 1 mM EDTA pH 8, 1 mM activated Na3VO4, 10 mM Na β‐glycerophosphate, 50 mM NaF, 5 mM Na Pyrophosphate, 270 mM Sucrose, 1% (v/v) Nonidet P‐40 and a protease inhibitor cocktail (1 tablet per 25 ml buffer) (Roche). Cell lysates were clarified by centrifugation at 13,000 × g for 20 min at 4°C. Supernatant was recovered (cell extract), and protein concentration was determined in a 96‐well format using Bradford protein assay reagent (Pierce). For nuclear and cytoplasmic extraction, a NE‐PER kit from Thermo Scientific (#78833) was used in accordance with the manufacturer's instructions. For membrane and cytoskeletal protein extraction, a compartment protein extraction kit from Merck (#2145) was used according to the manufacturer's instructions.
Xenopus extracts were prepared by titrating either embryos or animal caps with 10 μl/embryo or 2 μl/animal cap of embryo lysis buffer (1% Triton X‐100, 150 mM NaCl, 10 mM K‐HEPES pH 7.5, 2 mM EDTA, Roche protease inhibitor cocktail). Lipids and yolk were removed by extracting the lysate with an equal volume of 1,1,2‐Trichloro‐1,2,2‐trifluoroethane (FREON, Sigma Aldrich).
Immunoprecipitation and immunoblotting
0.5–1 mg of total cell lysate protein was incubated with 10 μl beads (GFP‐Trap® or anti‐FLAG‐M2 or 10 μg anti‐CK1α antibody conjugated to 10 μl Protein G‐agarose beads (Abcam)) for 2 h at 4°C on a rotating platform. IPs were washed twice in lysis buffer supplemented with 250 mM NaCl and once in Buffer A (50 mM Tris–HCl pH 7.5, 0.1 mM EGTA). Immunoprecipitated proteins were denatured in SDS sample buffer supplemented with 1% β‐mercaptoethanol and then subjected to SDS–PAGE. After SDS–PAGE, the proteins were transferred to PVDF membrane. The membrane was blocked with 5% non‐fat dry milk in TBS‐T buffer (50 mM Tris–HCl, pH 7.5, 0.15 M NaCl and 0.1% Tween‐20) for 30 min and then with primary antibody (1:1,000 in TBS‐T with 5% BSA) overnight at 4°C. Blots were incubated for 1 h at room temperature with HRP‐conjugated secondary antibodies (1:5,000 in 5% non‐fat dry milk). Proteins were visualised using the ECL system (GE Healthcare).
Xenopus extracts were denatured in 4× loading buffer (LiCor, #928‐40004) supplemented with 10% v/v β‐mercaptoethanol, and either 1 embryo equivalent, or 10 animal cap equivalents were loaded per well of a 7.5% mini‐Protean TGX gel (Bio‐Rad #456‐1025). Following electrophoresis, proteins were transferred using Trans‐Blot Transfer System onto LF PVDF membranes. Blots were blocked with a 1:1 mixture of TBS and Odyssey Blocking Buffer (LiCor #92750000) for 1 h at room temperature and then incubated in primary antibodies diluted 1:1,000 in blocking buffer overnight at 4°C. Blots were washed 3 × 5 min in TBS‐T (TBS, 0.1% Tween‐20). Secondary antibodies were added (goat anti‐rabbit and anti‐mouse Abs labelled with either IRDye 680LT or IRDye 800CW) diluted in TBS‐T supplemented with 0.02% SDS for 1 h at room temperature. Blots were washed 3 × 5 min in TBS‐T and then imaged on a LiCor Odyssey scanner using ImageStudio software (LiCor). The 700‐ and 800‐nm channels were captured simultaneously at the setting of 5, the μ‐setting was at 165 μm with the quality setting set at lowest.
Generation of PAWS1−/− U2OS rescue cells stably expressing PAWS1WT or PAWS1 mutants
Retroviral pBabe‐puromycin empty vector or vectors encoding PAWS1WT or indicated PAWS1 mutants (6 μg) were co‐transfected with pCMV5‐gag‐pol (3.2 μg) and pCMV5‐VSV‐G (2.8 μg) into a 10‐cm‐diameter dish of ~70% confluent HEK293‐FT cells. Briefly, cDNAs were added to 1 ml Opti‐MEM medium to which 24 μl of 1 mg/ml PEI (diluted in 25 mM HEPES pH 7.5) was added. Following a brief vortexing (10 s) and incubation at room temperature for 20 min, the transfection mix was added dropwise to the HEK293‐FT cells. 16 h post‐transfection, fresh medium was added to the cells. 24 h later, the retroviral medium was collected and filtered through 0.45‐μm filters. Target PAWS1−/− U2OS cells (~60% confluent) were infected with the optimised titre of the retroviral medium (1:100) diluted in fresh medium containing 8 μg/ml polybrene (Sigma) for 4 h. The retroviral infection medium was then replaced with fresh medium, and 20 h later, the medium was again replaced with fresh medium containing 2 μg/ml puromycin for selection of cells which had integrated the rescue constructs. The rescue cells were analysed by Western blotting as indicated.
Generation of U2OS PAWS1‐GFP knock‐in cells (PAWS1GFP/GFP) by CRISPR/Cas9
U2OS cells were transfected with vectors encoding a pair of guide RNAs (pBABED‐Puro‐sgRNA1 and pX335‐CAS9‐D10A‐sgRNA2) targeting the last exon of PAWS1 (1 μg each) and a donor vector carrying the GFP construct fused to the last 129 amino acids of the last exon of PAWS1 (3 μg). 24 and 48 h post‐transfection, cells were cultured in medium containing 2 μg/ml puromycin for selection of transfected cells. Cells were then cultured in normal medium, and transfection was repeated followed by 2 days of puromycin selection. GFP‐positive cells were then sorted by flow cytometry, and single GFP‐positive cells were plated on individual wells of two 96‐well plates as described previously 60. Viable clones were expanded, and integration of GFP at the target locus was verified by Western blotting and genomic sequencing.
Generation of doxycycline‐inducible Flp‐IN TRex U2OS stable cells
Flp‐IN TRex U2OS cells grown in 10‐cm dishes were transfected with 1 μg pcDNA5‐Frt/TO vector encoding PAWS1‐GFP or GFP only and 9 μg pOG44 (Invitrogen), diluted in 1 ml OPTIMEM and 20 μl of 1 mg/ml PEI. 24 h post‐transfection, cells were cultured in medium containing 15 μg/ml blasticidin and 50 μg/ml hygromycin B for 2–3 weeks (by replacing fresh medium every 2 days) until positive clones were selected, verified and expanded. Protein expression was induced with 20 ng/ml doxycycline for 16 h prior to lysis.
Mass spectrometry
PAWS1GFP/GFP cells were lysed in DSP lysis buffer (40 mM HEPES pH 7.4, 120 mM NaCl, 1 mM EDTA pH 8, 10 mM Na‐pyrophosphate, 50 mM NaF, 1.5 mM activated Na‐orthovanadate, 1% (v/v) Nonidet P‐40, protease inhibitor cocktail (1 tablet per 25 ml buffer) and 2.5 mg/ml DSP. The lysates were left on ice for 30 min, and cross‐linking reactions were quenched by adding 62.5 mM Tris–HCl pH 7.5. Clarified lysates were filtered through a 0.45‐μm filter and then subjected to immunoprecipitation using 10 μl of GFP‐Trap® beads at 4°C overnight. IPs were washed twice in Lysis buffer supplemented with 500 mM NaCl and once in Buffer A (50 mM Tris–HCl pH 7.5, 0.1 mM EGTA). IPs were denatured in LDS sample buffer (Invitrogen) supplemented with 0.1 M DTT, incubated for 1 h at 37°C and then heated for 5 min at 95°C. Samples were filtered through a Spin‐X column by centrifugation, resolved by 4–12% gradient SDS–PAGE, and stained with colloidal Coomassie blue overnight. Gels were destained in distilled H2O until background staining was minimal. Sections of the gel were excised, trypsin digested, and peptides prepared for HPLC gradient fractionation and elution into a Thermo ScientificVelos Orbitrap mass spectrometer as described previously 61, 62. Ion assignments were conducted by in silico Mascot scoring (http://www.matrixscience.com), and peptide protein assignments were reported in Scaffold 4.3 (http://www.proteomesoftware.com).
Dual luciferase reporter assays
Cells (2 × 105) were plated in 6‐well plates. 24 h later, 500 ng of Super TOPFlash or Super FOPFlash and 10 ng of Renilla‐luciferase 63 plasmids were co‐transfected using PEI and OPTI‐MEM. For transient PAWS1 expression, 500 ng of pBabe PAWS1 (DU33460) was included to the transfection mix. 24 h later, cells were treated with L‐conditioned medium or L‐Wnt3A‐conditioned medium or 20 mM LiCl for 16 h, washed twice with PBS and lysed in passive Lysis buffer (Promega, #E194A). Firefly and Renilla luciferase activities were measured as described 60, 64. The firefly luciferase counts were normalised to Renilla luciferase counts, which represented a measure of transfection efficiency in each sample.
Quantitative PCR and primers
Total RNA was isolated from cells using the QIAGEN RNeasy kit. Real‐time quantitative reverse transcription PCR (qRT–PCR) was performed using 1 μg of isolated RNA and the SuperScript cDNA kit (Bio‐Rad) according to the manufacturer's protocol. qRT–PCRs were performed in triplicate in a 12 μl reaction volume. Each reaction included 0.5 μM forward primer, 0.5 μM reverse primer, 50% SYBR green master mix (Bio‐Rad), and cDNA equivalent to 1 ng/μl of RNA in a CFX96 real‐time system qRT–PCR machine (Bio‐Rad). The data were normalised to the geometrical mean of the β‐actin gene and analysed as described previously 62. Primers were designed using PerlPrimer and purchased from Invitrogen: AXIN2 forward: TACACTCCTTATTGGGCGATCA; reverse: TTGGCTACTCGTAAAGTTTTGGT. CYCLIN D1 forward: GCTGCGAAGTGGAAACCATC; reverse: CCTCCTTCTGCACACATTTGAA. CK1α forward: GAGGCAGCTATTCCGCATTC; reverse: TGGGGAGAAACAAATGCTGC. Actin forward: CCAACCGCGAGAAATGACC; reverse: GGAGTCCATCACGATGCCAG. For RT–PCR in Xenopus laevis, total RNA was extracted from either embryos or animal caps using TRIzol reagent (Ambion) according to the manufacturer's instructions. 1 μg of RNA was used in the cDNA synthesis reaction using random hexamers and MMLV‐RT (Promega). Quantitative PCR was performed using LightCycler 480 SYBR Green Master I (Roche) using standard run template conditions on a LightCycler 480 machine (Roche). Primers used are as follows: Histone H4 forward: CGGGATAACATTCAGGGTATCACT; Histone H4 reverse: ATCCATGGCGGTAACTGTCTTCCT; Siamois forward: AACCACTTTCCACTCTCCCC; Siamois reverse: AGAAGTCAGTTTGGGTAGGGC; Msx1 forward: GCAGGAACATCACACAGTCC; Msx1 reverse: GGGTGGGCTCATCCTTCT; Vent1 forward: GCATCTCCTTGGCATATTTGG; Vent1 reverse: TTCCCTTCAGCATGGTTCACC; Vent2 forward: TGAGACTTGGGCACTGTCTG; Vent2 reverse: CCTCTGTTGAATGGCTTGCT; Chordin forward: AACTGCCAGGACTGGATGGT; Chordin reverse: GGCAGGATTTAGAGTTGCTTC. Data were normalised to Histone H4.
In vitro kinase assays and phospho‐mapping
25 μl reactions were set up using 200 ng of kinase, 2 μg of substrate in buffer containing 50 mM Tris pH 7.5, 0.1 mM EGTA, 10 mM MgAc2, 2 mM DTT and 0.1 mM [γ32P]‐ATP (~500 cpm/pmol). Reactions were performed at 30°C for 30 min and stopped by adding 10 μl LDS buffer with 5% β‐mercaptoethanol and heating at 95°C for 5 min. Samples were resolved by SDS–PAGE, and gels were stained with Instant blue (Expedeon) and dried. Radioactivity was analysed by autoradiography. Kinase assays against substrate peptide (CK1tide, DSTT EP5630) were performed as described previously 33.
For phospho‐mapping, [γ32P]‐phosphorylated PAWS1 was subjected to trypsin digestion and peptides were reconstituted to a final concentration of 5% ACN/0.1% TFA. The peptides were injected into a C18 column equilibrated with 0.1% TFA with a linear ACN/0.1% gradient at a flow rate of 0.2 ml/min, and 100 μl fractions were collected. The major eluted peptides were analysed on OrbiTrap Velos LCMS system. To determine the phosphorylated residue in each [γ32P]‐labelled peptide, the peptides were immobilised on a Sequelon‐AA membrane and subjected to solid‐phase Edman degradation as described previously 65.
RNA interference (RNAi)
U2OS cells were seeded at ~1 × 105 cells per well in 6‐well cell culture plates. Cells were transfected with 5, 10 or 20 nM of the relevant small interfering RNA (siRNA) oligonucleotides using 4 μl Lipofectamine RNAiMAX (Invitrogen) or TransFectin (Bio‐Rad) per well in Opti‐MEM reduced serum medium (Gibco). After 16 h, the transfection media were removed and replaced with normal DMEM culture medium. Cells were lysed 48 h post‐transfection. All siRNA oligonucleotides were purchased from Dharmacon (Product refs: J‐003957‐13, J‐003957‐14, J‐003957‐15, J003957‐16).
Statistical analysis
Data are presented as mean ± standard error of the mean. Statistical significances of differences between experimental groups were assessed using two‐way ANOVA with post hoc Bonferroni correction, unless stated differently in figure legends, using Prism6 software. Differences in means were considered significant if P < 0.05.
Author contributions
PB performed most of the experiments in mammalian cells, collected and analysed data and contributed to the editing of the manuscript. KSD conceived and performed most of the experiments in Xenopus embryos and animal caps, wrote parts of the manuscript and collected and analysed data. KZLW, FC, TDC, LDH and JVo performed some of the experiments. TJM designed strategies and developed methodologies for and generated CRIPSR/Cas9‐knock‐out and knock‐in constructs. NTW performed cloning and mutagenesis for most of the constructs. JVa, RG and DGC collected and analysed mass spectrometry data. JCS and GPS conceived the project and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7
Source Data for Figure 8
Source Data for Figure 9
Acknowledgements
We thank L. Fin, J. Stark, and A. Muir for help with tissue culture, the staff at the Sequencing Service (School of Life Sciences, University of Dundee, UK) for DNA sequencing, and the protein & antibody production and cloning teams at the Division of Signal Transduction Therapy (DSTT; University of Dundee) coordinated by H. McLauchlan and J. Hastie. KW and TDC are supported by the UK MRC Career Development Fellowship. PB and LDH are supported by the U.K. MRC Prize PhD studentship. GPS is supported by the U.K. Medical Research Council (MC_UU_12016/3) and the pharmaceutical companies supporting the DSTT (Boehringer‐Ingelheim, GlaxoSmithKline and Merck‐Serono). JCS, KSD and FC are supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001157), the UK Medical Research Council (FC001157) and the Wellcome Trust (FC001157).
EMBO Reports (2018) 19: e44807 29514862
Contributor Information
James C Smith, Email: jim.smith@crick.ac.uk.
Gopal P Sapkota, Email: g.sapkota@dundee.ac.uk.
References
- 1. Vogt J, Dingwell KS, Herhaus L, Gourlay R, Macartney T, Campbell D, Smith JC, Sapkota GP (2014) Protein associated with SMAD1 (PAWS1/FAM83G) is a substrate for type I bone morphogenetic protein receptors and modulates bone morphogenetic protein signalling. Open Biol 4: 130210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cipriano R, Miskimen KL, Bryson BL, Foy CR, Bartel CA, Jackson MW (2014) Conserved oncogenic behavior of the FAM83 family regulates MAPK signaling in human cancer. Mol Cancer Res 12: 1156–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartel CA, Parameswaran N, Cipriano R, Jackson MW (2016) FAM83 proteins: fostering new interactions to drive oncogenic signaling and therapeutic resistance. Oncotarget 7: 52597–52612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Radden LA II, Child KM, Adkins EB, Spacek DV, Feliciano AM, King TR (2013) The wooly mutation (wly) on mouse chromosome 11 is associated with a genetic defect in Fam83 g. BMC Res Notes 6: 189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drogemuller M, Jagannathan V, Becker D, Drogemuller C, Schelling C, Plassais J, Kaerle C, Dufaure de Citres C, Thomas A, Muller EJ et al (2014) A mutation in the FAM83G gene in dogs with hereditary footpad hyperkeratosis (HFH). PLoS Genet 10: e1004370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones CM, Smith JC (2008) An overview of Xenopus development. Methods Mol Biol 461: 385–394 [DOI] [PubMed] [Google Scholar]
- 7. De Robertis EM, Kuroda H (2004) Dorsal‐ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol 20: 285–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu J, Yang J, Klein PS (2005) Neural crest induction by the canonical Wnt pathway can be dissociated from anterior‐posterior neural patterning in Xenopus . Dev Biol 279: 220–232 [DOI] [PubMed] [Google Scholar]
- 9. Gerhart J, Danilchik M, Doniach T, Roberts S, Rowning B, Stewart R (1989) Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development 107(Suppl): 37–51 [DOI] [PubMed] [Google Scholar]
- 10. Guger KA, Gumbiner BM (1995) beta‐Catenin has Wnt‐like activity and mimics the Nieuwkoop signaling center in Xenopus dorsal‐ventral patterning. Dev Biol 172: 115–125 [DOI] [PubMed] [Google Scholar]
- 11. He X, Saint‐Jeannet JP, Woodgett JR, Varmus HE, Dawid IB (1995) Glycogen synthase kinase‐3 and dorsoventral patterning in Xenopus embryos. Nature 374: 617–622 [DOI] [PubMed] [Google Scholar]
- 12. Pierce SB, Kimelman D (1995) Regulation of Spemann organizer formation by the intracellular kinase Xgsk‐3. Development 121: 755–765 [DOI] [PubMed] [Google Scholar]
- 13. Smith WC, Harland RM (1991) Injected Xwnt‐8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell 67: 753–765 [DOI] [PubMed] [Google Scholar]
- 14. Funayama N, Fagotto F, McCrea P, Gumbiner BM (1995) Embryonic axis induction by the armadillo repeat domain of beta‐catenin: evidence for intracellular signaling. J Cell Biol 128: 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lemaire P, Garrett N, Gurdon JB (1995) Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal‐vegetal cells of blastulae and able to induce a complete secondary axis. Cell 81: 85–94 [DOI] [PubMed] [Google Scholar]
- 16. Sokol SY, Klingensmith J, Perrimon N, Itoh K (1995) Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development 121: 1637–1647 [DOI] [PubMed] [Google Scholar]
- 17. Yost C, Farr GH III, Pierce SB, Ferkey DM, Chen MM, Kimelman D (1998) GBP, an inhibitor of GSK‐3, is implicated in Xenopus development and oncogenesis. Cell 93: 1031–1041 [DOI] [PubMed] [Google Scholar]
- 18. Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer‐specific homeobox genes. Cell 79: 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cummins TD, Wu KZL, Bozatzi P, Dingwell KS, Macartney TJ, Wood NT, Varghese J, Gourlay R, Campbell DG, Prescott A et al (2018) PAWS1 controls cytoskeletal dynamics and cell migration through association with the SH3 adaptor CD2AP. J Cell Sci 131: jcs202390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D (1997) A beta‐catenin/XTcf‐3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus . Genes Dev 11: 2359–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fan MJ, Gruning W, Walz G, Sokol SY (1998) Wnt signaling and transcriptional control of Siamois in Xenopus embryos. Proc Natl Acad Sci USA 95: 5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nelson RW, Gumbiner BM (1998) Beta‐catenin directly induces expression of the Siamois gene, and can initiate signaling indirectly via a membrane‐tethered form. Ann N Y Acad Sci 857: 86–98 [DOI] [PubMed] [Google Scholar]
- 23. Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta‐catenin‐Tcf complex in APC‐/‐ colon carcinoma. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- 24. Fagotto F, Funayama N, Gluck U, Gumbiner BM (1996) Binding to cadherins antagonizes the signaling activity of beta‐catenin during axis formation in Xenopus . J Cell Biol 132: 1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clevers H (2006) Wnt/beta‐catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- 26. MacDonald BT, Tamai K, He X (2009) Wnt/beta‐catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stamos JL, Weis WI (2013) The beta‐catenin destruction complex. Cold Spring Harb Perspect Biol 5: a007898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X (2002) Control of beta‐catenin phosphorylation/degradation by a dual‐kinase mechanism. Cell 108: 837–847 [DOI] [PubMed] [Google Scholar]
- 29. Cruciat CM (2014) Casein kinase 1 and Wnt/beta‐catenin signaling. Curr Opin Cell Biol 31: 46–55 [DOI] [PubMed] [Google Scholar]
- 30. Price MA (2006) CKI, there's more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev 20: 399–410 [DOI] [PubMed] [Google Scholar]
- 31. Fulcher L, Bozatzi P, Tachie‐Menson T, Wu KZL, Cummins TD, Bufton JC, Pinkas DM, Dunbar K, Shrestha S, Wood NT et al (2017) The FAM83 family of proteins are anchors for Casein Kinase 1 isoforms through the DUF1669 domain. Sci Signal in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okamura H, Garcia‐Rodriguez C, Martinson H, Qin J, Virshup DM, Rao A (2004) A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol Cell Biol 24: 4184–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hastie CJ, McLauchlan HJ, Cohen P (2006) Assay of protein kinases using radiolabeled ATP: a protocol. Nat Protoc 1: 968–971 [DOI] [PubMed] [Google Scholar]
- 34. Amit S, Hatzubai A, Birman Y, Andersen JS, Ben‐Shushan E, Mann M, Ben‐Neriah Y, Alkalay I (2002) Axin‐mediated CKI phosphorylation of beta‐catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev 16: 1066–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM (2002) Casein kinase I phosphorylates and destabilizes the beta‐catenin degradation complex. Proc Natl Acad Sci USA 99: 1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McKay RM, Peters JM, Graff JM (2001) The casein kinase I family in Wnt signaling. Dev Biol 235: 388–396 [DOI] [PubMed] [Google Scholar]
- 37. Wallingford JB, Mitchell B (2011) Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev 25: 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K (2002) The Wnt/calcium pathway activates NF‐AT and promotes ventral cell fate in Xenopus embryos. Nature 417: 295–299 [DOI] [PubMed] [Google Scholar]
- 39. Mackintosh C (2004) Dynamic interactions between 14‐3‐3 proteins and phosphoproteins regulate diverse cellular processes. Biochem J 381: 329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H (2002) Wnt signaling controls the phosphorylation status of beta‐catenin. J Biol Chem 277: 17901–17905 [DOI] [PubMed] [Google Scholar]
- 41. McCrea PD, Turck CW, Gumbiner B (1991) A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E‐cadherin. Science 254: 1359–1361 [DOI] [PubMed] [Google Scholar]
- 42. Tan CW, Gardiner BS, Hirokawa Y, Layton MJ, Smith DW, Burgess AW (2012) Wnt signalling pathway parameters for mammalian cells. PLoS ONE 7: e31882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bernatik O, Ganji RS, Dijksterhuis JP, Konik P, Cervenka I, Polonio T, Krejci P, Schulte G, Bryja V (2011) Sequential activation and inactivation of Dishevelled in the Wnt/beta‐catenin pathway by casein kinases. J Biol Chem 286: 10396–10410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klimowski LK, Garcia BA, Shabanowitz J, Hunt DF, Virshup DM (2006) Site‐specific casein kinase 1epsilon‐dependent phosphorylation of Dishevelled modulates beta‐catenin signaling. FEBS J 273: 4594–4602 [DOI] [PubMed] [Google Scholar]
- 45. Rubinfeld B, Tice DA, Polakis P (2001) Axin‐dependent phosphorylation of the adenomatous polyposis coli protein mediated by casein kinase 1epsilon. J Biol Chem 276: 39037–39045 [DOI] [PubMed] [Google Scholar]
- 46. Sakanaka C, Leong P, Xu L, Harrison SD, Williams LT (1999) Casein kinase iepsilon in the wnt pathway: regulation of beta‐catenin function. Proc Natl Acad Sci USA 96: 12548–12552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peters JM, McKay RM, McKay JP, Graff JM (1999) Casein kinase I transduces Wnt signals. Nature 401: 345–350 [DOI] [PubMed] [Google Scholar]
- 48. Del Valle‐Perez B, Arques O, Vinyoles M, de Herreros AG, Dunach M (2011) Coordinated action of CK1 isoforms in canonical Wnt signaling. Mol Cell Biol 31: 2877–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cegielska A, Gietzen KF, Rivers A, Virshup DM (1998) Autoinhibition of casein kinase I epsilon (CKI epsilon) is relieved by protein phosphatases and limited proteolysis. J Biol Chem 273: 1357–1364 [DOI] [PubMed] [Google Scholar]
- 50. Rivers A, Gietzen KF, Vielhaber E, Virshup DM (1998) Regulation of casein kinase I epsilon and casein kinase I delta by an in vivo futile phosphorylation cycle. J Biol Chem 273: 15980–15984 [DOI] [PubMed] [Google Scholar]
- 51. Knippschild U, Kruger M, Richter J, Xu P, Garcia‐Reyes B, Peifer C, Halekotte J, Bakulev V, Bischof J (2014) The CK1 family: contribution to cellular stress response and its role in carcinogenesis. Front Oncol 4: 96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Good MC, Zalatan JG, Lim WA (2011) Scaffold proteins: hubs for controlling the flow of cellular information. Science 332: 680–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dajani R, Fraser E, Roe SM, Yeo M, Good VM, Thompson V, Dale TC, Pearl LH (2003) Structural basis for recruitment of glycogen synthase kinase 3beta to the axin‐APC scaffold complex. EMBO J 22: 494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ha NC, Tonozuka T, Stamos JL, Choi HJ, Weis WI (2004) Mechanism of phosphorylation‐dependent binding of APC to beta‐catenin and its role in beta‐catenin degradation. Mol Cell 15: 511–521 [DOI] [PubMed] [Google Scholar]
- 55. Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A (1998) Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK‐3beta and beta‐catenin and promotes GSK‐3beta‐dependent phosphorylation of beta‐catenin. EMBO J 17: 1371–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sobrado P, Jedlicki A, Bustos VH, Allende CC, Allende JE (2005) Basic region of residues 228‐231 of protein kinase CK1alpha is involved in its interaction with axin: binding to axin does not affect the kinase activity. J Cell Biochem 94: 217–224 [DOI] [PubMed] [Google Scholar]
- 57. Jones SD, Ho L, Smith JC, Yordan C, Stiles CD, Mercola M (1993) The Xenopus platelet‐derived growth factor alpha receptor: cDNA cloning and demonstration that mesoderm induction establishes the lineage‐specific pattern of ligand and receptor gene expression. Dev Genet 14: 185–193 [DOI] [PubMed] [Google Scholar]
- 58. Harland RM (1991) In situ hybridization: an improved whole‐mount method for Xenopus embryos. Methods Cell Biol 36: 685–695 [DOI] [PubMed] [Google Scholar]
- 59. Fulcher LJ, Hutchinson LD, Macartney TJ, Turnbull C, Sapkota GP (2017) Targeting endogenous proteins for degradation through the affinity‐directed protein missile system. Open Biol 7: 170066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rojas‐Fernandez A, Herhaus L, Macartney T, Lachaud C, Hay RT, Sapkota GP (2015) Rapid generation of endogenously driven transcriptional reporters in cells through CRISPR/Cas9. Sci Rep 5: 9811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Herhaus L, Al‐Salihi M, Macartney T, Weidlich S, Sapkota GP (2013) OTUB1 enhances TGFbeta signalling by inhibiting the ubiquitylation and degradation of active SMAD2/3. Nat Commun 4: 2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Herhaus L, Al‐Salihi MA, Dingwell KS, Cummins TD, Wasmus L, Vogt J, Ewan R, Bruce D, Macartney T, Weidlich S et al (2014) USP15 targets ALK3/BMPR1A for deubiquitylation to enhance bone morphogenetic protein signalling. Open Biol 4: 140065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sapkota G, Alarcon C, Spagnoli FM, Brivanlou AH, Massague J (2007) Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell 25: 441–454 [DOI] [PubMed] [Google Scholar]
- 64. Baker JM, Boyce FM (2014) High‐throughput functional screening using a homemade dual‐glow luciferase assay. J Vis Exp 88: e50282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Campbell DG, Morrice NA (2002) Identification of protein phosphorylation sites by a combination of mass spectrometry and solid phase Edman sequencing. J Biomol Tech 13: 119–130 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7
Source Data for Figure 8
Source Data for Figure 9


