Abstract
Diabetes mellitus (DM) is one of the most prevalent diseases in the world, which is strongly associated with liver dysfunction. Hyperglycemia, through an oxidative stress pathway, damages various tissues. Herbal medicine is a good candidate to ameliorate hyperglycemia and oxidative stress. In this study, the effects of aqueous Allium sativum (garlic) extract (AGE) on gene expression of inducible nitric oxide synthases (iNOS) and production of nitric oxide (NO) were evaluated in the liver tissue of diabetic rats. Four groups of rats contained normal control rats, garlic control rats (AGE), Streptozotocin (STZ) + nicotinamide-induced diabetic rats (DM), and diabetic rats treated with garlic (DM + AGE). Glucose levels and liver enzymes activities were determined by colorimetric assay in the serum. Gene expression of iNOS by real-time PCR, NO levels by Griess method, oxidative stress parameters by spectrophotometric method and histopathological examination by hematoxylin and eosin staining method were evaluated in the liver tissues. Glucose levels, activities of liver enzymes, oxidative stress markers, iNOS gene expression, and NO production increased significantly in diabetic rats in comparison with control rats, whereas after oral administration of garlic, these parameters decreased significantly, close to the normal levels. Hence, the beneficial effects of garlic on the liver injury of diabetes could be included in the hypoglycaemic and antioxidant properties of garlic via a decrease in gene expression of iNOS and subsequent NO production.
Electronic supplementary material
The online version of this article (doi:10.1007/s12291-017-0656-3) contains supplementary material, which is available to authorized users.
Keywords: Diabetes mellitus, Allium sativum, Inducible nitric oxide synthases, Nitric oxide
Introduction
Diabetes mellitus (DM) is a progressive metabolic disease characterized by hyperglycemia, resulting from defects in insulin secretion, insulin action, or both. Long-term hyperglycemia has been proposed as a risk factor for damage to various organs such as kidney, eye, heart, and liver. Many studies have showed that DM is associated with liver diseases such as non alcoholic fatty liver (NAFLD), nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and, subsequently, hepatocellular carcinoma (HCC) [1].
Elevated glucose levels in the blood of diabetic patients lead to increases in reactive oxygen species (ROS) production and induction of oxidative stress. ROS are capable to activate gene expression through nuclear factor (NF)-κB pathway and subsequently up-regulate different genes including inducible nitric oxide synthases (iNOS), tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1) and cyclooxygenase-2 (COX-2). This activated pathway can cause inflammations, apoptosis and other complications of diabetes in various tissues like liver. iNOS is expressed by hepatocytes and plays an important role in pathological conditions and liver injury [2].
Since diabetic complications result from hyperglycemia, any component that can reduce glucose levels leads to an improvement in tissue dysfunctions. Currently, the use of natural remedy has increased as these are low-cost, have only a few side effects, and are accessible [3]. In herbal medicine, Allium sativum (garlic) from the Liliaceae family is useful in the treatment of several diseases. Hypoglycemic and antioxidant effects of this plant have earlier been proven by researchers [4]. In this study, we confirmed hypoglycemic and antioxidant properties of an aqueous extract of garlic (AGE) and investigated its effects on iNOS gene expression and nitric oxide (NO) production in the liver tissue of streptozotocin (STZ) + nicotinamide-induced diabetic rats.
Materials and Methods
Preparation of AGE
Fresh garlic bulbs were purchased from local market in Hamadan, Iran. The plant was taxonomically identified by botanists in the herbarium department of biology, Bu-Ali Sina University, Hamadan, Iran. The cloves were peeled, washed and cut into small pieces. About 50 g was blended in 250 mL of distilled water, and homogenized in a mixing machine. The supernatant was filtered through Whatman No. 1 filter paper. Garlic extract was used freshly or quickly frozen until used. Daily 1 mL of this solution/100 g body weight (~2 g/kg) was given to the rats by gavage [5].
Experimental Design
Adult male Wistar rats weighing around 250–300 g (6–8 week old) were obtained from Hamadan University of Medical Sciences, Hamadan, Iran. The animals housed in standard cages with 12-h light–dark cycles, constant temperature of 25 ± 2 °C and free access to food and water. Investigations were performed conferring to the ethical norms of the Institutional Animal Ethics Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1395.27). The animals were acclimatized for at least 5 days under these conditions before the start of the experiments. The rats were divided randomly into four groups each comprising of six animals. For induction of diabetes combination of STZ (Sigma, USA) and nicotinamide (Sigma, USA) were used.
Control group: rats received neither STZ nor AGE, received only single dose of citrate buffer (0.1 M, pH 4.5, intraperitoneally).
DM group: rats received single dose of STZ (65 mg/kg body weight, in citrate buffer, 0.1 M, pH 4.5, intraperitoneally) 15 min after the injection of nicotinamide (110 mg/kg, intraperitoneally) for induction of diabetes. STZ-nicotinamide-exposed rats with blood glucose level in excess of 250 mg/mL, 7 days after exposure were considered as diabetic.
DM + AGE group (post-treatment group): rats received AGE (2 g/kg body weight/day, gavage, 33 days) after 7 days from the day on which STZ injected.
AGE group: normal rats received only AGE (2 g/kg body weight/day, gavage, 33 days).
At the end of treatment period, for fasting overnight, the food and water were removed from cages 12 h before testing. Serum samples were collected from all groups and stored at −20 °C for measuring biochemical parameters. The livers were snap-frozen in liquid nitrogen and stored at −80 °C for protein and RNA extraction and a portion of their fixed in 10% formalin for histological evaluation.
Determination of Biochemical Parameters
The glucose level and the activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were measured in the serum of rats using commercially available kits (Pars Azmoon diagnostics, Iran).
Preparation of Liver Tissue Homogenate
Liver tissue homogenates were prepared on ice using lysis buffer (10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 0.1% triton ×100, 10 mM HEPES, 0.5 mM DTT, protease inhibitor cocktail, pH 7.9) and incubated on ice for 20 min. The protein concentrations of liver homogenate samples were determined by using Bradford method [6].
Determination of Oxidative Stress Parameters
Lipid peroxidation in liver tissue homogenates was determined by malondialdehyde (MDA) assay according to Yagi method. The results were expressed as μmol MDA/mg protein content of the samples [7].
Total oxidative status (TOS) in liver tissue homogenates was determined using the oxidation of ferrous ion to ferric ion. The ferric ions form a colored complex with xylenol orange in an acidic medium. Therefore, the color intensity is related to the total number of oxidant molecules present in the sample. The results were expressed as μmol TOS/mg protein content of the samples [8].
Total antioxidant capacity (TAC) in liver tissue homogenates was assessed using ferric reducing antioxidant potential (FRAP) assay according to Benzie and Strain methods. This method is based on the reduction of the ferric tripyridyltriazine (TPTZ) to the blue colored ferrous form at low pH. This reduction is monitored by measuring the absorption change at 593 nm [9].
Determination of NO
The liver NO level was determined by measuring the nitrite concentration in the liver tissue homogenates using Griess method. The results were expressed as μmol nitrite/mg protein content of the samples [10].
Determination of iNOS Gene Expression
Total RNA was extracted from the liver tissues of samples using the RNX-Plus solution (Sinaclon, Iran) according to the manufacturer’s instructions. The purified RNA was quantified by spectrophotometer (A260) and its quality was examined by electrophoresis. Complementary DNA (cDNA) was prepared from purified RNA using RevertAid First Strand cDNA Synthesis kit (Thermo Scientific, Lithuania) according to the manufacturer’s instructions. Quantitative Real-time PCR was performed on cDNA samples using the SYBR Premix ExTaq real-time PCR kit (Takara Bio Inc, Japan), according to the manufacturer’s protocols. Gene specific primers were designed using the AlleleID7 software (Premier Biosoft Corporation, USA). The primer constructs (5′-3′ sequence) were: iNOS (forward) AGAGACGCTTCTGAGGTTC, iNOS (reverse) TTGATGCTTGTGACTCTTAGG, 18S rRNA (reference gene) (forward), GTAACCCGTTGAACCCCATT and 18S rRNA (reverse), CCATCCAATCGGTAGTAGCG. The relative changes in gene expression were determined using 2−∆∆CT method [11].
Histological Study
After scarification of rats, the liver tissues were quickly removed and cut into about 2-mm thick slices and fixed in 10% formalin for a week at room temperature. After embedded in paraffin, several sections were obtained from the livers and stained with hematoxylin and eosin (H&E).
Statistical Analysis
SPSS software version 16 (SPSS, Chicago, IL, USA) was performed for statistical analysis and to compare means in different groups, analysis of variance (ANOVA) was used. Results were expressed as mean ± standard deviation (SD) and P < 0.05 was considered significance.
Results
Effects of AGE on Glucose Level, AST, ALT, and ALP Activities in the Serum
In Table 1, glucose level and liver damage enzyme markers in four groups of rats are summarized. In DM rats, glucose level was increased significantly (P < 0.001) compared with control rats. Oral administration of AGE in DM + AGE rats caused significant decrease in glucose level (P < 0.05) compared with untreated diabetic rats (DM).
Table 1.
Effects of AGE on glucose level, AST, ALT, and ALP activities in the serum of control and experimental groups of rats at the end of treatment
| Groups | Glucose (mg/dL) | AST (U/L) | ALT (U/L) | ALP (U/L) |
|---|---|---|---|---|
| Control | 89.12 ± 10.8 | 63.2 ± 7.4 | 103.25 ± 5.67 | 320.75 ± 50.73 |
| DM | 327 ± 140.31a* | 158.5 ± 37.74a# | 173.75 ± 50.8a† | 1234.6 ± 473.69a† |
| DM + AGE | 200 ± 74.3b# | 60.08 ± 8.62b# | 92 ± 15.81b† | 610.8 ± 346.09b# |
| AGE | 90.29 ± 10.1 | 73 ± 16.35 | 109 ± 23.76 | 351 ± 90.43 |
Results are mean ± SD (n = 6). a Compare with control rats, b compare with diabetic rats (DM); # P < 0.05, † P < 0.01, * P < 0.001; AGE aqueous garlic extract, AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatase
In DM rats, the activity of liver enzymes in serum were increased significantly (AST; P < 0.05, ALT; P < 0.01, ALP; P < 0.01) compared with control rats. Oral administration of AGE in DM + AGE rats caused significant decrease in enzymes activities (AST; P < 0.05, ALT; P < 0.01, ALP; P < 0.05) compared with DM.
Effects of AGE on Oxidative Stress Status in Liver Tissue
The levels of MDA, TOS and TAC were shown in Table 2. The levels of MDA in liver tissues of DM rats were increased significantly (P < 0.001) compared with control rats and decreased significantly (P < 0.05) in DM + AGE rats compared with DM rats.
Table 2.
Effects of AGE on oxidative stress parameters of liver tissues in control and experimental groups of rats
| Groups | MDA (μmol/mg protein) | TOS (μmol/mg protein) | TAC (μmol/mg protein) |
|---|---|---|---|
| Control | 0.026 ± 0.016 | 1.736 ± 0.196 | 0.045 ± 0.014 |
| DM | 0.084 ± 0.025a* | 2.811 ± 0.123a* | 0.016 ± 0.004a* |
| DM + AGE | 0.054 ± 0.002b# | 2.252 ± 0.142a*,b* | 0.034 ± 0.009b# |
| AGE | 0.042 ± 0.037 | 1.773 ± 0.127 | 0.05 ± 0.009 |
Results are mean ± SD (n = 6). a Compare with control rats, b compare with diabetic rats (DM); # P < 0.05, * P < 0.001; AGE aqueous garlic extract, MDA malondialdehyde, TOS total oxidative status, TAC total antioxidant capacity
The levels of TOS in liver tissues of DM rats were increased significantly (P < 0.001) compared with control rats and decreased significantly (P < 0.001) in DM + AGE rats compared with DM rats. However, it was still high level when compared with control (P < 0.001).
The levels of TAC in liver tissues of DM rats were decreased significantly (P < 0.001) compared with control rats and increased significantly (P < 0.05) in DM + AGE rats compared with DM rats.
Effects of AGE on NO Level of Liver Tissue
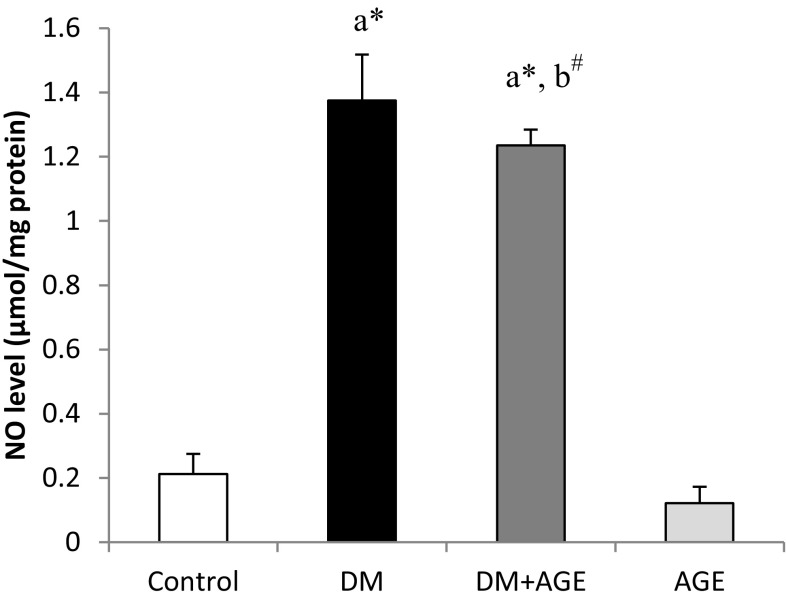
The levels of NO in the liver tissues of DM rats were elevated significantly (P < 0.001) compared to control rats and decreased significantly (P < 0.05) in diabetic rats treated with AGE in comparison with DM rats. However, it was still high level when compared with control (P < 0.001) (Fig. 1).
Fig. 1.
Effects of AGE on nitrite level of liver tissues in control and experimental groups of rats. Results are mean ± SD (n = 6). a Compare with control rats, b compare with diabetic rats (DM); # P < 0.05, * P < 0.001; AGE aqueous garlic extract, NO nitric oxide
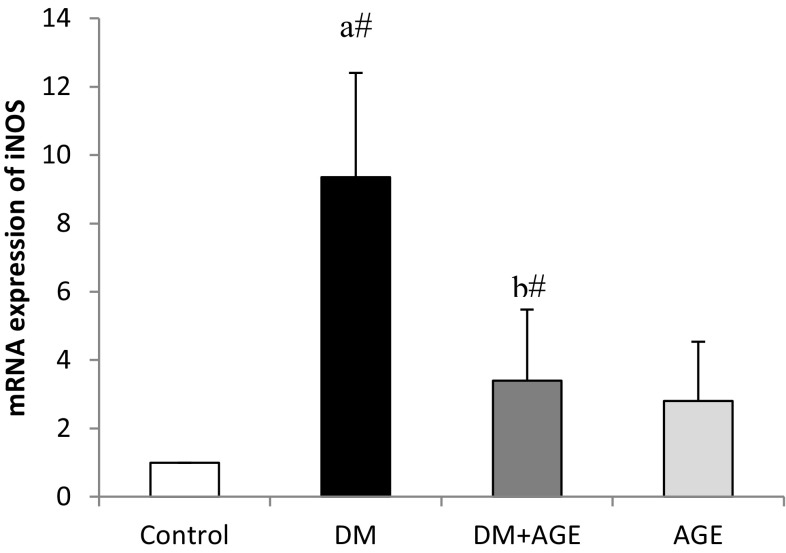
Effects of AGE on the iNOS mRNA Folding Changes in Liver Tissue
The mRNA expression of iNOS increased significantly (P < 0.05) in diabetic rats compared with normal control rats, while treatment with AGE significantly (P < 0.05) down-regulated the mRNA expression of iNOS in DM + AGE rats compared with DM rats (Fig. 2).
Fig. 2.
Effects of AGE on mRNA folding changes of iNOS in liver tissues of control and experimental groups of rats. Results are mean ± SD (n = 6). a Compare with control rats, b compare with diabetic rats (DM); # P < 0.05; AGE aqueous garlic extract, iNOS inducible nitric oxide synthase, DM diabetes mellitus
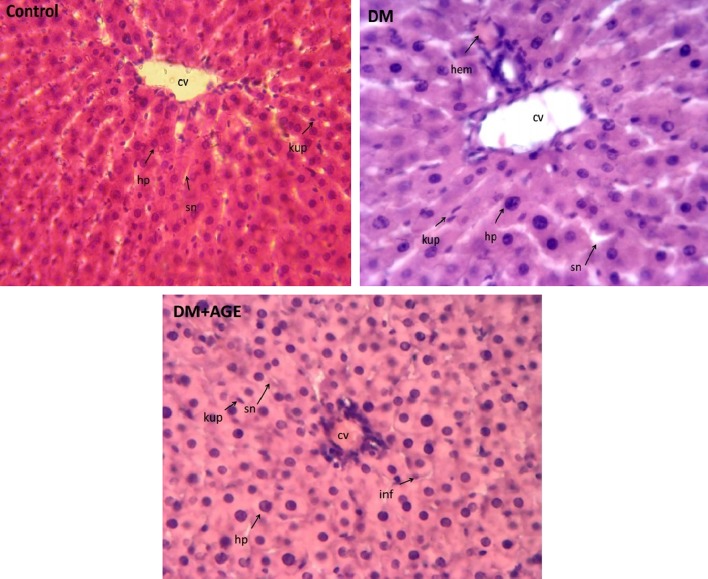
Effects of AGE on Histopathology of Liver Tissue
In control rats, central vein (CV) completely was clear. Basophilic hepatocytes (hp) were arranged radial around CV. Sinusoids (sn) had normal figures with Kupffer cells (kup) and there were not any hemorrhage (hem), foam cells (fc), and infiltration (inf). In DM rats, there was a decrease in hepatocyte numbers with loss of radial arrangement. There were hemorrhage, infiltration, foam cells, and increased Kupffer cells. In DM + AGE rats, the number of hepatocyte was increased near to normal and radial arrangement was better than untreated diabetic rats. There was a decrease in Kupffer cells and infiltration and foam cells were not seen (Fig. 3).
Fig. 3.
Histological staining with H&E (×400) in livers of control and experimental groups of rats. DM Diabetes mellitus, AGE aqueous garlic extract, CV central vein, hp hepatocyte, sn sinusoid, kup Kupffer cell, hem hemorrhage, fc foam cell, inf infiltration
Discussion
DM is a most common metabolic disease, which is rising with modern living worldwide. Though there are various chemical drugs for the treatment and control of diabetes, particular attention is paid to medicinal plants as they have low cost, a few side effects and greater accessibility. Many common medicines that have therapeutic effects on diseases are materials extracted from plants, such as Metformin from Galega officinalis [12], and Silymarin from milk thistle [13]. Garlic is a medicinal herb used in traditional nutrition in many cultures, including Iran [14].
Appropriate experimental models are essential tools for evaluating the mechanism involved in pathogenesis, complications and genetics of diabetes, and investigation into the therapeutic or protective agents on this disease. Several models can be used in experimental designs, such as nutritional, surgical and chemical-induced diabetes. Alloxan and STZ are two components that are generally used to induce diabetes in animals. STZ alone induced type 1 diabetes while combination of STZ and nicotinamide can produce a milder form of diabetes, namely late stage or type 2 diabetes [15]. STZ causes alkylation and degradation of DNA in β cells of the pancreas and impairs insulin secretion, whereas nicotinamide partly protects β cells from toxicity of STZ and causes induction of a milder form of diabetes mellitus [16]. In this study, we used STZ + nicotinamide-induced diabetic rats to evaluate the effects of garlic extract on liver function in this situation. Hyperglycemia is an important problem in diabetes mellitus, which has been shown in many researches [17]. The high glucose levels after receiving single dose of STZ and nicotinamide confirmed the induction of diabetes in these rats. Hyperglycemia can damage the functions of several tissues, including kidney, eye, testis and liver [18]. To evaluate hepatic disorders, the measurement of liver enzymes activities, including AST, ALT, and ALP in the serum, is common. An elevation in the activities of these enzymes reflects liver injury [14]. In the current study, the levels of these enzymes’ activities significantly increased in diabetic rats in comparison with normal control rats. Also, the histopathological changes—such as the presence of foam cells and infiltration—were seen. It seems that hyperglycaemia damages hepatocytes membrane and causes the release of liver enzymes into the bloodstream. Also, in conditions of diabetes, the elevation of protein catabolism, gluconeogenesis and urea synthesis lead to increases in the liver enzymes in serum [19].
Many complication of diabetes can be caused by oxidative stress as a result of hyperglycaemia, which increases electron transport in mitochondria, inhibits complex III, induces superoxide overproduction, and activates hexosamine pathway [20]. Elevation of free radicals can damage several tissues like liver. In this study, increases in the levels of TOS and MDA and decreases in the level of TAC in liver tissues demonstrated oxidative stress condition in diabetic rats compared to control rats. Similar to our results, the oxidative stress induction in diabetes—with increase in oxidant agents and decrease in antioxidant defence—were shown in several studies [21, 22]. Therefore, one of the best strategies for protection or treatment of diabetic complications is the control of oxidative stress. Most herbal extracts have antioxidant capacity because they have polyphenols as components. Garlic is a traditional plant that it used to provide protection from and treat many diseases.
Oral administration of an aqueous extract of garlic significantly decreased glucose levels in the serum of garlic-treated diabetic rats as compared with untreated diabetic rats. Although, it had mild hypoglycemic effect due to short time treatment duration. If we treated animals with this extract for longer time, the results were better and closed to normal level. In agreement with the present results, other studies have shown the hypoglycemic effect of garlic. Hypoglycemic effects of garlic in animals were shown in different studies [23].
The results of the current study showed the improvement of histopathological changes in the liver tissues and AST, ALT and ALP levels in the serum of garlic-treated diabetic rats. Similar effects on levels of aminotransferases in the serum and liver histology were earlier shown [24].
Since garlic has cysteine-containing compounds like S-allyl cysteine (SAC), it is a good antioxidant. Yang et al. [25] showed that SAC treatment of diabetic rats can prevent ROS formation through modulation of NADPH oxidase subunit expression. Saravanan et al. [26] demonstrated that superoxide dismutase, catalase, glutathione peroxidase and reduced glutathione were increased in SAC-treated diabetic rats.
The results of the current study showed that the level of iNOS gene expression and NO production increased in diabetic rats and decreased close to normal levels after treatment with garlic extract. Dias et al. [27] demonstrated that hyperglycemia and oxidative stress enhance the level of TNF-α and activates NF-κB. Activation of NF-κB up-regulates transcription of several genes involved in inflammation and apoptosis pathways. One of the target genes for NF-κB is iNOS. Oxidative stress and hyperglycemia caused the activation of NF-κB and increased gene expression of iNOS and production of NO in conditions of diabetes [28]. Elevation of NO level resulted in the starting cascade of apoptosis pathway, which can impair the normal tissue functions and their histology [29]. Therefore, each component like garlic, which has antioxidant capacity, can reduce oxidative stress status and down-regulate iNOS gene expression and NO level. Consequently, damage to hepatocytes was decreased and, so, the levels of liver enzymes in serum were decreased and the histopathology of liver tissues was close to normal rats in diabetic rats after treatment with garlic extract.
Thus, it can be concluded that the garlic extract must be considered an excellent candidate for future studies on DM. In addition, further comprehensive pharmacological investigations, including experimental chronic studies, should be carried out.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The present study was funded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (No. 9504081809).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12291-017-0656-3) contains supplementary material, which is available to authorized users.
References
- 1.Ziamajidi N, Khaghani S, Hassanzadeh G, Vardasbi S, Ahmadian S, Nowrouzi A, et al. Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPAR alpha and SREBP-1. Food Chem Toxicol. 2013;58:198–209. doi: 10.1016/j.fct.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Madar Z, Kalet-Litman S, Stark AH. Inducible nitric oxide synthase activity and expression in liver and hepatocytes of diabetic rats. Pharmacology. 2005;73(2):106–112. doi: 10.1159/000081952. [DOI] [PubMed] [Google Scholar]
- 3.Ghamarian A, Abdollahi M, Su X, Amiri A, Ahadi A, Nowrouzi A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. DARU. 2012;20(1):56. doi: 10.1186/2008-2231-20-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh VK, Singh DK. Pharmacological effects of garlic (Allium sativum L.) Annu Rev Biomed Sci. 2008;10:6–26. doi: 10.5016/1806-8774.2008.v10p6. [DOI] [Google Scholar]
- 5.El-Demerdash FM, Yousef MI, El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43(1):57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 8.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Abbasalipourkabir R, Moradi H, Zarei S, Asadi S, Salehzadeh A, Ghafourikhosroshahi A, et al. Toxicity of zinc oxide nanoparticles on adult male Wistar rats. Food Chem Toxicol. 2015;84:154–160. doi: 10.1016/j.fct.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Bailey CJ, Day C. Metformin: its botanical background. Pract Diabet Int. 2004;21(3):115–117. doi: 10.1002/pdi.606. [DOI] [Google Scholar]
- 13.Behrouj H, Ziamajidi N, Abbasalipourkabir R, Nasiri A, Soleimani Asl S. Therapeutic effect of Silybum marianum plant extract on tamoxifen-induced fatty liver inr. Avicenna J Med Biochem. 2015;3(1):e27160. doi: 10.17795/ajmb-27160. [DOI] [Google Scholar]
- 14.Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13(9):624–629. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Tripathi V, Verma J. Different models used to induce diabetes: a comprehensive review. Int J Pharm Pharm Sci. 2014;6(6):29–32. [Google Scholar]
- 16.Mojani MS, Sarmadi VH, Vellasamy S, Sandrasaigaran P, Rahmat A, Peng LS, et al. Evaluation of metabolic and immunological changes in streptozotocin-nicotinamide induced diabetic rats. Cell Immunol. 2014;289:145–149. doi: 10.1016/j.cellimm.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Rezagholizadeh L, Pourfarjam Y, Nowrouzi A, Nakhjavani M, Meysamie A, Ziamajidi N, et al. Effect of Cichorium intybus L. on the expression of hepatic NF-κB and IKKβ and serum TNF-α in STZ− and STZ+ niacinamide-induced diabetes in rats. Diabetol Metab Syndr. 2016;8:11. doi: 10.1186/s13098-016-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pourfarjam Y, Rezagholizadeh L, Nowrouzi A, Meysamie A, Ghaseminejad S, Ziamajidi N, et al. Effect of Cichorium intybus L. seed extract on renal parameters in experimentally induced early and late diabetes type 2 in rats. Ren Fail. 2017;39(1):211–221. doi: 10.1080/0886022X.2016.1256317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prakasam A, Sethupathy S, Pugalendi KV. Influence of Casearia esculenta root extract on protein metabolism and marker enzymes in streptozotocin-induced diabetic rats. Pol J Pharmacol. 2004;56(5):587–594. [PubMed] [Google Scholar]
- 20.Du X-L, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci. 2000;97(22):12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anwar MM, Meki AR. Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comp Biochem Physiol A: Mol Integr Physiol. 2003;135(4):539–547. doi: 10.1016/S1095-6433(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 22.Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol. 2003;14(suppl 3):S250–S253. doi: 10.1097/01.ASN.0000077412.07578.44. [DOI] [PubMed] [Google Scholar]
- 23.Raju TN, Kanth VR, Lavanya K. Effect of methanolic extract of Allium sativum (AS) in delaying cataract in STZ-induced diabetic rats. J Ocul Biol Dis Inform. 2008;1(1):46–54. doi: 10.1007/s12177-008-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masjedi F, Gol A, Dabiri S, Javadi A. Preventive effect of garlic on histopathology of liver and markers of hepatic injury in streptozotocin-induced diabetic rats. Iran J Endocrinol Metab. 2009;11(4):433–441. [Google Scholar]
- 25.Yang J, Wang T, Rao K, Zhan Y, Chen RB, Liu Z, et al. S-allyl cysteine restores erectile function through inhibition of reactive oxygen species generation in diabetic rats. Andrology. 2013;1(3):487–494. doi: 10.1111/j.2047-2927.2012.00060.x. [DOI] [PubMed] [Google Scholar]
- 26.Saravanan G, Ponmurugan P. S-allylcysteine improves streptozotocin-induced alterations of blood glucose, liver cytochrome P450 2E1, plasma antioxidant system, and adipocytes hormones in diabetic rats. Int J Endocrinol Metab. 2013;11(4):e10927. doi: 10.5812/ijem.10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, Gonzalez-Gallego J. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr. 2005;135(10):2299–2304. doi: 10.1093/jn/135.10.2299. [DOI] [PubMed] [Google Scholar]
- 28.Nasiri A, Ziamajidi N, Abbasalipourkabir R, Goodarzi MT, Saidijam M, Behrouj H, et al. Beneficial effect of aqueous garlic extract on inflammation and oxidative stress status in the kidneys of type 1 diabetic rats. Indian J Clin Biochem. 2016;1–8. doi:10.1007/s12291-016-0621-6 [DOI] [PMC free article] [PubMed]
- 29.Ingaramo PI, Ronco MT, Frances DE, Monti JA, Pisani GB, Ceballos MP, et al. Tumor necrosis factor alpha pathways develops liver apoptosis in type 1 diabetes mellitus. Mol Immunol. 2011;48(12–13):1397–1407. doi: 10.1016/j.molimm.2011.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.