Abstract
Urine is a proven source of metabolite biomarkers and has the potential to be a rapid, noninvasive, inexpensive, and efficient diagnostic tool for various human diseases. Despite these advantages, urine is an under-investigated source of biomarkers for multiple sclerosis (MS). The objective was to investigate the level of some urinary metabolites (urea, uric acid and hippuric acid) in patients with MS and correlate their levels to the severity of the disease, MS subtypes and MS treatment. The urine samples were collected from 73 MS patients-48 with RRMS and 25 with SPMS- and age matched 75 healthy controls. The values of urinary urea, uric acid and hippuric acid in MS patients were significantly decreased, and these metabolites in SPMS pattern showed significantly decrease than RRMS pattern. Also showed significant inverse correlation with expanded disability status scale and number of relapses. Accordingly, they may act as a potential urinary biomarkers for MS, and correlate to disease progression.
Keywords: Urinary, Urea, Uric acid, Hippuric acid, Multiple sclerosis
Introduction
Multiple sclerosis (MS) is a demyelinating disease of the central nervous system (CNS) characterized by selective loss of myelin sheath encapsulating the neuronal axons [1]. It is a complex neurological disease with a variable clinical course and several pathophysiological mechanisms, such as axonal/neuronal damage, demyelination, inflammation, gliosis, remyelination and oxidative injury. Alterations of the immune system together with biochemical disturbances and disruption of blood–brain barrier are also involved in pathomechanisms of MS [2].
Patients with MS are classified according to their clinical phenotype, with ~85% following a relapsing-remitting course (relapsing-remitting multiple sclerosis RRMS). Approximately 15% of patients follow a primary progressive course from disease onset, and half of relapsing-remitting patients develop a secondary progressive course within 20 years of disease onset (secondary progressive multiple sclerosis SPMS) [3].
Treatment of MS is more effective during the early course of the disease when symptoms are mild [4]. Thus, the early diagnosis of MS is critical in order to quickly initiate treatments that slow the progression of the disease and improve the quality of a patient’s life [5].
The biomarker research field is very active in MS. However, despite the large numbers of candidate molecular biomarkers proposed, very few biomarkers have been validated and used in clinical practice. A definitive diagnostic test for MS does not exist; instead physicians use a combination of medical history, magnetic resonance imaging, and cerebrospinal fluid (CSF) analysis. Significant effort has been employed to identify biomarkers from CSF to facilitate MS diagnosis; however, none of the proposed biomarkers have been successful [6].
Cerebrospinal fluid collection is an invasive procedure; therefore it is sampled only for a limited number of time-points, usually for first diagnostic investigation. More easily and less invasively collected are blood and urine samples. The main disadvantage of blood is the diurnal variation of many soluble markers, affected by systemic infections and biological degradation in the liver or by excretion in the kidney [7].
Urine is a proven source of a many biomarkers and has the potential to be a rapid, noninvasive, inexpensive, and efficient diagnostic tool for various human diseases. Although holds significant promise, the analysis of urine samples for MS biomarkers has only been minimally investigated [8].
However, there are some urinary markers which were suggested to be of great value in diagnosis of MS like urinary melatonin which was found to be decreased in patients with multiple sclerosis [9], levels of urinary neopterin and nitric oxide metabolites [10] and free light chains [11]. So, urinary metabolites may present a potential valuable approach for diagnosing MS and evaluating the in vivo efficacy of MS drug candidates [12].
Previously, it was reported that metabolomics analysis of urinary markers of MS using animal model of MS (EAE) demonstrate the potential of using some urinary metabolites as a source of biomarkers for MS (Ex. hippurate, citrate, urea, taurine and fructose) [5]. Herein, we report some metabolites analysis (urea, uric acid and hippuric acid) of human urine samples collected from MS patients and healthy controls.
Urea is critical for maintaining ammonia and amine nitrogen homeostasis through its role in amino-acid metabolism, so impaired urea-cycle activity can lead to hyperammonemia which is a major component of certain classes of acute neurological disturbances [13, 14].
A number of studies showed that neuroinflammation that occurs in several neurologic disorders, including MS, directly induce the production of nitric oxide and superoxide, leading to a vast increase in peroxynitrite formation, which induces demyelination through its ability to induce lipid peroxidation of the highly fatty myelin sheaths, and make axonal damage through inducing oxidative stress and DNA damage [15].
Uric acid is a potent antioxidant found throughout extracellular fluid, as sodium urate, and is thought to account for more than half of the antioxidant capacity of plasma. It has been suggested that uric acid may have neuroprotective influence as a scavenger of reactive nitrogen and oxygen radicals as peroxynitrite. A powerful antioxidant effect of uric acid on neurons has been demonstrated through in vivo and in vitro studies [16].
Hippuric acid, the glycine conjugate of benzoic acid, is a normal component of urine with a strong association with diet and the intestinal microbiota. As well as being part of the endogenous urinary metabolite profile, hippurate has other specific uses; it has been identified as a biomarker for high dose exposure to certain toxic compounds such as toluene, and is also commonly used as a measure of renal clearance [17].
Although hippuric acid (mammalian-microbial co metabolite) does not have causal relationship to the MS disease but it reflect the pathogenesis to some degree since it was described a difference in specific operational taxonomic units of gut microbiota in MS patients, also indicated that immunomodulatory medications cause alterations in the gut microbiota of MS patients. So, there is a potential role of gut microbiota in the pathogenesis and treatment of MS [18].
The aim of this study was to evaluate the urinary levels of urea, uric acid and hippuric acid and their potential use as preliminary diagnostic biomarkers in MS disease.
Patients and Methods
In this study 148 subjects were included (62 males, 86 females), categorized into two main groups; the first is MS patients group (n = 73) (20 males and 53 females), the second group comprised of 75 healthy control subjects (42 males and 33 females). All healthy controls were screened for neurological and other major medical illnesses and were age matched to MS group.
The MS patients were recruited from the Neurology department of Kasr Al Ainy hospital, Cairo, Egypt. The diagnosis of MS was based on the revised McDonald criteria [19], the patients group were further sub classified into relapsing–remitting (RRMS) (n = 48, 35 females and 13 males) and secondary progressive (SPMS) (n = 25, 18 females and 7 males.). Clinical severity, neurological disability were quantified using Kurtzke’s Expanded Disability Status Scale (EDSS) [20] and Progression Index (PI; calculated as disability grade divided by the duration of the disease) [21]. The clinical and demographic characteristics of all subjects included in the study were shown in Table 1. All subjects also were screened for any urinary tract infection and other urological or renal abnormalities. Urine samples were assessed for the presence of albumin (in order to exclude renal abnormalities) at the time of sampling using commercial dipstick analysis for albuminuria).
Table 1.
Demographic and clinical characteristics of MS patients and healthy control group
| Parameters | Healthy controls | MS patients |
|---|---|---|
| Age (Mean ± SEM) |
(30.44 ± 1.03) Range (20–50 years) |
(33.01 ± 0.82) Range (20–47 years) |
| Sex | 33 (44%) female 42 (56%) male |
53 (72.6%) female 20 (27.4%) male |
| EDSS (Mean ± SEM) |
– | (3.86 ± 0.22) |
| VEP | – | 52 abnormal 21 normal |
| Oligoclonal band | – | 20 positive 14 negative 39 not found |
| Total no. of attacks (Mean ± SEM) |
– | (4.87 ± 0.33) |
| Duration of disease (Mean ± SEM) |
(6.4 ± 0.53) years | |
| Age at disease onset (Mean ± SEM) |
(27.29 ± 0.8) years | |
| Type of treatment | Interferon beta-1a (9 patients) Interferon beta-1b (11 patients) Azathioprine (3 patients) Cyclophosphamide (8 patients) Monthly methylprednisolone (42 patients) |
EDSS expanded disability status scale; VEP visual evoked potential
All subjects gave written informed consent prior to their participation in the study. The study was performed according to the regulations and recommendations of declaration of Helsinki with approval number (002H-16).
Urine samples from all groups were collected as spot urine according to the suggested method of sampling provided by the kit manufacturer. The patient was asked to empty his/her bladder at the morning into a sterile polyethylene cup before breakfast and administration of drug treatment and before the monthly infusion of methyl prednisolone treatment. Urine samples were a liquated into three epindorf tubes for determination of urinary urea, uric acid and hippuric acid levels and processed without any additives within 2 h after collection.
Uric Acid Determination
The concentration of uric acid in urine was determined using colorimetric enzymatic assay according to the Trinder method [22] using the Spectrophotometer. Urine was diluted 1:10 with physiological solution (saline 0.9% NaCl) and the analysis was done according to the kit procedure (Bio Med-Uric acid-Egypt). Results were expressed as mg/dl of uric acid.
Urinary Urea Determination
The concentration of urea in urine was determined using colorimetric enzymatic assay [23] using the Spectrophotometers. Urine was diluted 1:50 with physiological solution (saline 0.9% NaCl) and the analysis was done according to the kit procedure (Bio Med-Urea-Egypt). Results were expressed as g/dl of urea.
Urinary Hippuric Acid Determination
The concentration of hippuric acid in urine was determined using colorimetric assay [24]. Urinary Hippuric acid (HA) dissolved in pyridine (1:1) produces an orange colour when benzenesulfonyl chloride (BSC) is added, according to the following procedure: HA standard solution (0.4 mg/ml) prepared by dissolving 100 mg of hippuric acid (Merck Chemical, Rahway, N. J. 07065) in water and diluted to 100 ml then 2 ml of the stock solution was diluted to 5 ml with water on the day of use.
0.5 ml of urine + 0.5 ml of anhydrous pyridine were mixed, and then 0.2 ml of BSC was added and mixed. Then the colored solution was allowed to stand for 30 min at room temperature (20–25 °C), diluted to 5 ml with ethanol and well mixed. Then measure at 410 nm. Results were expressed as mg/ml of HA.
Statistical Analysis
Differences in variables were analyzed using Student t, ANOVA tests using SPSS package version 22 of windows (Chicago, IL, USA 2013). Probability values <0.05 were considered statistically significant. Linear regression analysis and graphs were plotted using Graphpad Prism 5 (For Windows, 1992–2007 Graphpad software Inc., V 5.01, USA).
Results
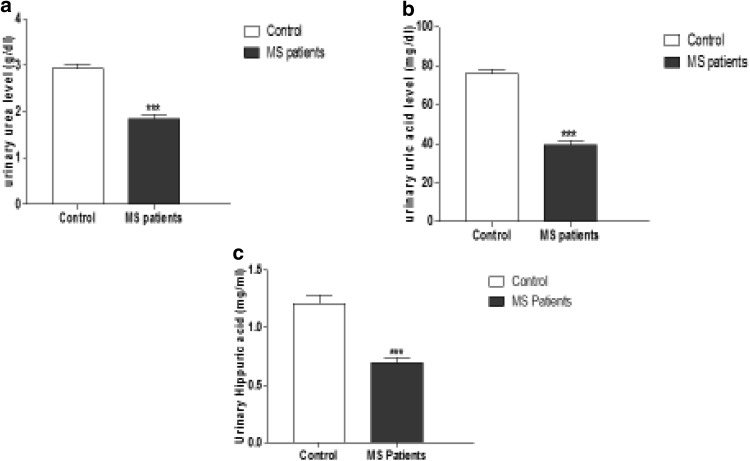
The mean urinary values of urea, uric acid and hippuric acid in MS patients (1.85 ± 0.83 g/dl), (39.59 ± 2.1 mg/dl) and (0.69 ± 0.05 mg/ml) respectively were significantly lower than those of control group (2.94 ± 0.83 g/dl), (75.87 ± 2.29 mg/dl) and (1.21 ± 0.075 mg/ml) respectively (P < 0.0001) as shown in (Fig. 1a–c).
Fig. 1.
The mean urinary value of urea, uric and hippuric acid in MSpatients and control groups. a Urinary area level (g/dl), b urinary uric acid level (mg/dl) and c urinary hippuric acid level (mg/ml) of control and MS patients, expressed as mean ± SEM. *** P < 0.0001 using unpaired t-student test followed by Welch’s correction
It was found that mean values of urinary urea, uric acid and hippuric acid in SPMS pattern were significantly lower than RRMS pattern as in (Table 2).
Table 2.
Mean urinary levels of urea, uric acid and hippuric acid in different disease patterns (RRMS and SPMS)
| RRMS n = 48 |
SPMS n = 25 |
||
|---|---|---|---|
| Urea (g/dl) | 1.97 ± 0.11 | 1.62 ± 0.13* | P < 0.05 |
| Uric acid (mg/dl) | 43.27 ± 2.68 | 32.5 ± 2.87** | P < 0.01 |
| Hippuric acid (mg/ml) | 0.81 ± 0.07 | 0.47 ± 0.04*** | P < 0.001 |
Values expressed as mean ± SEM
* P < 0.05, ** P < 0.01, *** P < 0.001 using unpaired t-student test followed by Welch’s correction
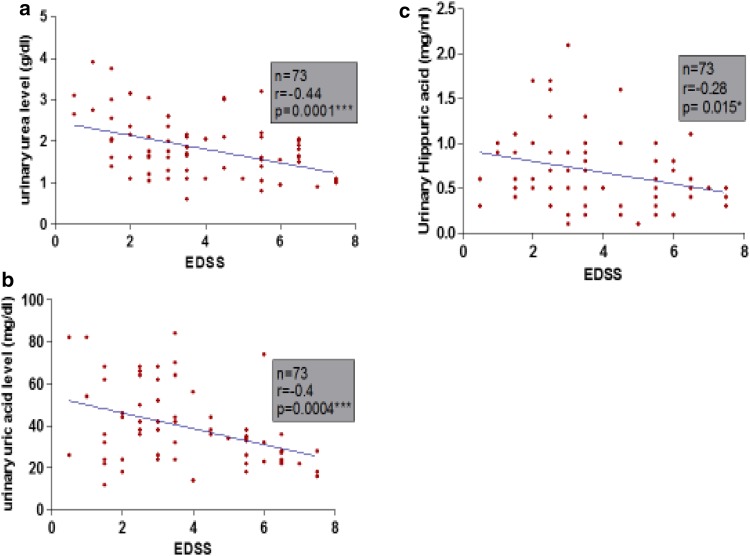
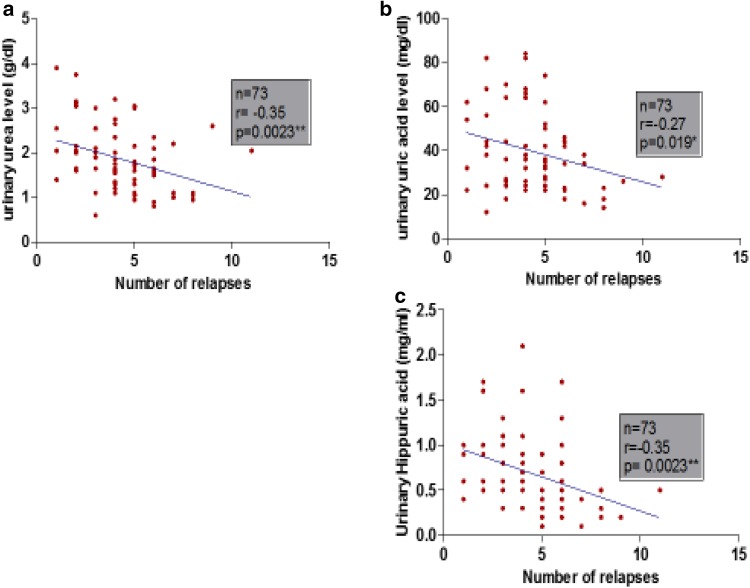
Pearson correlation revealed that urinary metabolites (urea, uric acid and hippuric acid) were negatively correlated significantly with EDSS and number of relapses as shown in Figs. 2a–c and 3a–c. Also there was a negative correlation with PI and duration of disease but did not reach significance as shown in (Table 3).
Fig. 2.
Correlation between urinary metabolites and Extended Disability Status Scale. Linear regression between expanded disability status scale (EDSS) and a urinary area level, b urinary uric acid level and c urinary hippuric acid level in MS group. n total number of patients, r Pearson rank correlation coefficient
Fig. 3.
Correlation between urinary metabolites and number of relapses. Linear regression between number of relapses and a urinary urea level, b urinary uric acid level and c urinary hippuric acid level in MS group. n total number of patients, r Pearson rank correlation coefficient
Table 3.
Simple linear regression analysis using urinary urea, uric acid and hippuric acid levels as dependent variable in MS patient group
| Variable | Urea | Uric acid | Hippuric acid | |||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| EDSS | −0.44 | P < 0.001*** | −0.4 | P < 0.001*** | −0.28 | P < 0.05* |
| Progression index (PI) | −0.23 | P > 0.05 | −0.21 | P > 0.05 | −0.016 | P > 0.05 |
| Duration of disease (year) | −0.044 | P > 0.05 | −0.062 | P > 0.05 | −0.093 | P > 0.05 |
| Number of relapses | −0.35 | P < 0.01** | −0.27 | P < 0.05* | −0.35 | P < 0.01** |
EDSS expanded disability status scale; r Pearson rank correlation coefficient
*P < 0.05, **P < 0.01, ***P < 0.001
Discussion
The use of effective biomarkers has great significance for the prognosis, diagnosis and treatment of many diseases. Urine is not subject to homeostatic mechanisms and accommodates many changes that may reflect status of the body [25]. These changes may be used as promising biomarkers [26]. Currently, most studies on urinary biomarkers have focused on kidney diseases due to the close relationship between the kidneys and urine [27]. The lack of attention to urinary biomarkers in other diseases, like MS which is considered as one of the brain diseases, may be due to the fact that anatomically, the brain and urine are not closely related. Most brain disease studies have focused on cerebrospinal fluid (CSF) and blood [28, 29]. However, there are only a few urinary biomarker studies on brain diseases.
The study of the biomarkers in MS disease is very limited especially urinary level of urea, uric acid and hippuric acid so this study is one of a few studies that investigate the urinary levels of these metabolites in MS patients. In this study, it was found that urinary urea level in MS patients was significantly lower than healthy control. This result come in accordance with results obtained from Gebregiworgis et al. [5], who found that EAE (animal model of MS) have lower urinary urea level. Urea cycle activity takes place primarily in the liver. Mangalam et al. [30], on his study on EAE found that several major pathways affected in the liver include bile acid biosynthesis, taurine metabolism, tryptophan and histidine metabolism, and also citrulline was down regulated which affect urea cycle and arginine metabolism.
In autoimmune diseases like MS, citrulline down regulation may be due to its consumption in excessive protein citrullination including myelin basic protein that triggering autoimmunity [31]. So the consumption of citrulline may be the potential reason of decreasing the urea concentration in the present study.
Malnutrition is often identified in patients affected by acute or chronic diseases. Dysphagia, a potential contributing factor to malnutrition, is a frequent symptom in degenerative chronic illness such as in MS [32]. Also, Sorgun et al. [33] found malnutrition was more prevalent in MS patients than in other chronic diseases. This malnutrition may affect protein intake and subsequently may decrease urea level in body fluids since it is the product of protein catabolism.
The current study also demonstrate that, urea level was negatively correlated to severity of MS disease markers (EDSS and total number of relapses), it was found that MS patients with secondary progressive form had lower urinary urea level than relapsing remitting form, this might indicate that urinary urea level may have a relation with disease progression.
Uric acid is a natural anti-oxidant and a peroxynitrite scavenger, it accounts for up to 60% of the free radical scavenging activity in human blood. In vitro studies demonstrated that urate levels may reduce the neurons damage caused by reactive oxygen species, peroxynitrite and glutamate excitotoxicity [15].
However, several reports investigated serum urate levels in patients with MS compared to neurological and healthy controls, with conflicting results. Rentzos et al. [34] indicated no change in serum uric acid levels in MS patients compared to controls and Amorini et al. [35] found that there was an increase in plasma/serum uric acid of MS patients. But Zoccolella et al. [36] indicated that serum urate levels were lower in female-MS, compared to those in female of healthy control. But the studies on urinary level are very limited, so this study is unique in investigating the urinary level of uric acid in MS patients.
In the present study, we found a significant decrease in urinary concentrations of uric acid in MS patients. The probable explanation for this finding is that brain uric acid, acting as a potent peroxynitrite scavenger, is oxidized in consequence of the increased nitric oxide generation. The final result would be a significant decrease in uric acid circulating level. In contrast to these results,
On the other hand, several studies agree with our results favoring the view that reduced uric acid in MS is secondary to its peroxynitrite scavenging activity during inflammatory disease activity, rather than a primary deficiency [16, 37, 38]. Also, in this study it was found that urinary uric acid level was lower in SPMS than RRMS and inversely correlated with disability score (EDSS and number of relapses) which agree with Markowitz et al. [39] who found that inosine treatment (precursor of uric acid) increased serum uric acid levels and was associated with a significant decrease in the number of gadolinium enhanced lesions and improved EDSS. Also, Guerrero et al. [40] observed that lower uric acid levels in MS patients are connected with clinical relapse of MS and correlated with disability of MS patients assessed by EDSS score which is consistent with our results. In contrary, Peng et al. showed that UA levels do not correlate with MRI activity, disability or subtype of disease in MS patients and NMO [41, 42].
Hippuric acid, a conjugate of benzoic acid with glycine has been a major human metabolite for years. However, hippurate has other specific uses; it has been identified as a biomarker for high dose exposure to certain toxic compounds such as toluene [43] and is also commonly used as a measure of renal clearance [44]. Benzoic acid (sodium benzoate), a metabolite of cinnamon which found also in polyphenol-rich components of the diet such as vegetables and fruit. Is a widely used food additive, which is long known for its antimicrobial effect [45, 46].
Studies in the urinary level of hippuric acid in MS patients are very limited; the current study demonstrated a significant decrease in urinary hippuric acid concentration in MS patients, this lower level of hippuric acid may be due to an intrinsic deficiency in glycine conjugation or benzoic acid deficiency.
Gut microbiota is one of the most significant factors in determining the rate of hippurate production. Authors found a lot of similarities between inflammatory bowel disease (IBD) as (Crohn’s disease and ulcerative colitis) and MS; MS is one of the diseases that affect gut microbiota [47–49]. It was found that hippurate excretion was significantly lower in patients with ulcerative colitis and Crohn’s disease. It was reported that the difference in hippurate excretion was a reflection of differences in the intestinal microbiota of the different disease groups [50]. Also, Williams et al. [51] supported this theory in Crohn’s disease patients as urinary hippurate concentrations were found to be significantly lower in patients with Crohn’s disease, compared to controls. This might explain the lower urinary level of hippurate in MS patients. Furthermore, many studies showed decreased excretion of hippurate in patients with other neurological disorders such as schizophrenia and depression [17].
In conclusion, this study gives some promising results, indicating that lower levels of urinary urea, uric acid and hippuric acid in MS patients may be potential applicable biomarkers of clinical value. Also their precursors (inosine) may have some therapeutic application in MS.
These metabolites could be used as biomarkers for monitoring disease activity in MS as they correlated to EDSS and number of relapses. Based on this study, searching in MS biomarkers in urine would open new way to find more MS biomarkers for clinical application if proved to be reliable. Further clinical studies are needed to verify these findings.
Acknowledgement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interests related to the publication of this paper.
References
- 1.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 2.Miller E, Walczak A, Saluk J, Ponczek MB, Majsterek I. Oxidative modification of patient’s plasma proteins and its role in pathogenesis of multiple sclerosis. Clin Biochem. 2012;45(1–2):26–30. doi: 10.1016/j.clinbiochem.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Tremlett H, van der Mei IA, Pittas F, Blizzard L, Paley G, Mesaros D, et al. Monthly ambient sunlight, infections and relapse rates in multiple sclerosis. Neuroepidemiology. 2008;31(4):271–279. doi: 10.1159/000166602. [DOI] [PubMed] [Google Scholar]
- 4.Marrie RA, Cohen JA. Interferons in secondary progressive multiple sclerosis, in multiple sclerosis therapeutics. 3. London: Informa Healthcare; 2007. [Google Scholar]
- 5.Gebregiworgis T, Massilamany C, Gangaplara A, Thulasingam S, Kolli V, Werth MT, et al. The potential of urinary metabolites for diagnosing multiple sclerosis. ACS Chem Biol. 2013;8(4):684–690. doi: 10.1021/cb300673e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lourenco AS, Baldeiras I, Graos M, Duarte CB. Proteomics-based technologies in the discovery of biomarkers for Multiple Sclerosis in the cerebrospinal fluid. Curr Mol Med. 2011;11(4):326–349. doi: 10.2174/156652411795677981. [DOI] [PubMed] [Google Scholar]
- 7.Bielekova B, Martin R. Development of biomarkers in multiple sclerosis. Brain. 2004;127(Pt 7):1463–1478. doi: 10.1093/brain/awh176. [DOI] [PubMed] [Google Scholar]
- 8.Dobson R. Urine: an under-studied source of biomarkers in multiple sclerosis? Mult Scler Relat Disord. 2012;1(2):76–80. doi: 10.1016/j.msard.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Gholipour T, Ghazizadeh T, Babapour S, Mansouri B, Ghafarpour M, Siroos B, et al. Decreased urinary level of melatonin as a marker of disease severity in patients with multiple sclerosis. Iran J Allergy Asthma Immunol. 2015;14(1):91–97. [PubMed] [Google Scholar]
- 10.Rejdak K, Leary SM, Petzold A, Thompson AJ, Miller DH, Giovannoni G. Urinary neopterin and nitric oxide metabolites as markers of interferon beta-1a activity in primary progressive multiple sclerosis. Mult Scler. 2010;16(9):1066–1072. doi: 10.1177/1352458510375100. [DOI] [PubMed] [Google Scholar]
- 11.Dobson R, Miller RF, Palmer HE, Feldmann M, Thompson EJ, Thompson AJ, et al. Increased urinary free immunoglobulin light chain excretion in patients with multiple sclerosis. J Neuroimmunol. 2010;220(1–2):99–103. doi: 10.1016/j.jneuroim.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Gebregiworgis T, Powers R. Application of NMR metabolomics to search for human disease biomarkers. Comb Chem High Throughput Screen. 2012;15(8):595–610. doi: 10.2174/138620712802650522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patassini S, Begley P, Reid SJ, Xu J, Church SJ, Curtis M, et al. Identification of elevated urea as a severe, ubiquitous metabolic defect in the brain of patients with Huntington’s disease. Biochem Biophys Res Commun. 2015;468(1–2):161–166. doi: 10.1016/j.bbrc.2015.10.140. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Begley P, Church SJ, Patassini S, Hollywood KA, Jüllig M, et al. Graded perturbations of metabolism in multiple regions of human brain in Alzheimer’s disease: snapshot of a pervasive metabolic disorder. Biochim Biophys Acta. 2016;1862(6):1084–1092. doi: 10.1016/j.bbadis.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutzing MK, Firestein BL. Altered uric acid levels and disease states. J Pharmacol Exp Ther. 2008;324(1):1–7. doi: 10.1124/jpet.107.129031. [DOI] [PubMed] [Google Scholar]
- 16.Pakpoor J, Seminog OO, Ramagopalan SV, Goldacre MJ. Clinical associations between gout and multiple sclerosis, Parkinson’s disease and motor neuron disease: record-linkage studies. BMC Neurol. 2015;15:16. doi: 10.1186/s12883-015-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lees HJ, Swann JR, Wilson ID, Nicholson JK, Holmes E. Hippurate: the natural history of a mammalian-microbial cometabolite. J Proteome Res. 2013;12(4):1527–1546. doi: 10.1021/pr300900b. [DOI] [PubMed] [Google Scholar]
- 18.Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Invest Med. 2015;63(5):729–734. doi: 10.1097/JIM.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 21.Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, et al. Multiple sclerosis severity score: using disability and disease duration to rate disease severity. Neurology. 2005;64(7):1144–1151. doi: 10.1212/01.WNL.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- 22.Trinder P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J Clin Pathol. 1969;22(2):246. doi: 10.1136/jcp.22.2.246-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaney A, Marbach E. Modified reagents for determination of urea and ammonia. Clin Chem. 1962;8:130–132. [PubMed] [Google Scholar]
- 24.Tomokuni K, Ogata M. Direct colorimetric determination of hippuric acid in urine. Clin Chem. 1972;18(4):349–351. [PubMed] [Google Scholar]
- 25.Wu J, Gao Y. Physiological conditions can be reflected in human urine proteome and metabolome. Expert Rev Proteom. 2015;12(6):623–636. doi: 10.1586/14789450.2015.1094380. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y. Urine-an untapped goldmine for biomarker discovery. Sci China Life Sci. 2013;56(12):1145–1146. doi: 10.1007/s11427-013-4574-1. [DOI] [PubMed] [Google Scholar]
- 27.Schanstra JP, Mischak H. Proteomic urinary biomarker approach in renal disease: from discovery to implementation. Pediatr Nephrol. 2015;30(5):713–725. doi: 10.1007/s00467-014-2790-y. [DOI] [PubMed] [Google Scholar]
- 28.Jove M, Portero-Otin M, Naudi A, Ferrer I, Pamplona R. Metabolomics of human brain aging and age-related neurodegenerative diseases. J Neuropathol Exp Neurol. 2014;73(7):640–657. doi: 10.1097/NEN.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 29.Miao Y, Liao JK. Potential serum biomarkers in the pathophysiological processes of stroke. Expert Rev Neurother. 2014;14(2):173–185. doi: 10.1586/14737175.2014.875471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangalam A, Poisson L, Nemutlu E, Datta I, Denic A, Dzeja P, et al. Profile of circulatory metabolites in a relapsing-remitting animal model of multiple sclerosis using global metabolomics. J Clin Cell Immunol. 2013 doi: 10.4172/2155-9899.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradford CM, Ramos I, Cross AK, Haddock G, McQuaid S, Nicholas AP, et al. Localisation of citrullinated proteins in normal appearing white matter and lesions in the central nervous system in multiple sclerosis. J Neuroimmunol. 2014;273(1–2):85–95. doi: 10.1016/j.jneuroim.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Pasquinelli S, Solaro C. Nutritional assessment and malnutrition in multiple sclerosis. Neurol Sci. 2008;29(Suppl 4):S367–S369. doi: 10.1007/s10072-008-1046-7. [DOI] [PubMed] [Google Scholar]
- 33.Sorgun MH, Yucesan C, Tegin C. Is malnutrition a problem for multiple sclerosis patients? J Clin Neurosci. 2014;21(9):1603–1605. doi: 10.1016/j.jocn.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Rentzos M, Nikolaou C, Anagnostouli M, Rombos A, Tsakanikas K, Economou M, et al. Serum uric acid and multiple sclerosis. Clin Neurol Neurosurg. 2006;108(6):527–531. doi: 10.1016/j.clineuro.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Amorini AM, Petzold A, Tavazzi B, Eikelenboom J, Keir G, Belli A, et al. Increase of uric acid and purine compounds in biological fluids of multiple sclerosis patients. Clin Biochem. 2009;42(10–11):1001–1006. doi: 10.1016/j.clinbiochem.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Zoccolella S, Tortorella C, Iaffaldano P, Direnzo V, D’Onghia M, Luciannatelli E, et al. Low serum urate levels are associated to female gender in multiple sclerosis patients. PLoS ONE. 2012;7(7):e40608. doi: 10.1371/journal.pone.0040608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattle HP, Lienert C, Greeve I. Uric acid and multiple sclerosis. Ther Umsch. 2004;61(9):553–555. doi: 10.1024/0040-5930.61.9.553. [DOI] [PubMed] [Google Scholar]
- 38.Chittoor G, Kent JW, Jr, Almeida M, Puppala S, Farook VS, Cole SA, et al. GWAS and transcriptional analysis prioritize ITPR1 and CNTN4 for a serum uric acid 3p26 QTL in Mexican Americans. BMC Genom. 2016;17:276. doi: 10.1186/s12864-016-2594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markowitz CE, Spitsin S, Zimmerman V, Jacobs D, Udupa JK, Hooper DC, et al. The treatment of multiple sclerosis with inosine. J Altern Complement Med. 2009;15(6):619–625. doi: 10.1089/acm.2008.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerrero AL, Gutiérrez F, Iglesias F, Martín-Polo J, Merino S, Martín-Serradilla J, et al. Serum uric acid levels in multiple sclerosis patients inversely correlate with disability. Neurol Sci. 2011;32(2):347–350. doi: 10.1007/s10072-011-0488-5. [DOI] [PubMed] [Google Scholar]
- 41.Peng F, Zhang B, Zhong X, Li J, Xu G, Hu X, et al. Serum uric acid levels of patients with multiple sclerosis and other neurological diseases. Mult Scler. 2008;14:188–196. doi: 10.1177/1352458507082143. [DOI] [PubMed] [Google Scholar]
- 42.Peng F, Zhong X, Deng X, Qiu W, Wu A, Long Y, et al. Serum uric acid levels and neuromyelitis optica. J Neurol. 2010;257:1021–1026. doi: 10.1007/s00415-010-5455-1. [DOI] [PubMed] [Google Scholar]
- 43.Kawai T, Ukai H, Inoue O, Maejima Y, Fukui Y, Ohashi F, et al. Evaluation of biomarkers of occupational exposure to toluene at low levels. Int Arch Occup Environ Health. 2008;81(3):253–262. doi: 10.1007/s00420-007-0203-2. [DOI] [PubMed] [Google Scholar]
- 44.Castellani S, Ungar A, Cantini C, La Cava G, Di Serio C, Altobelli A, et al. Excessive vasoconstriction after stress by the aging kidney: inadequate prostaglandin modulation of increased endothelin activity. J Lab Clin Med. 1998;132(3):186–194. doi: 10.1016/S0022-2143(98)90167-6. [DOI] [PubMed] [Google Scholar]
- 45.Brahmachari S, Pahan K. Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol. 2007;179(1):275–283. doi: 10.4049/jimmunol.179.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pahan K. Immunomodulation of experimental allergic encephalomyelitis by cinnamon metabolite sodium benzoate. Immunopharmacol Immunotoxicol. 2011;33(4):586–593. doi: 10.3109/08923973.2011.561861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, et al. Role of gut commensal microflora in the development of experimental auto-immune encephalomyelitis. J Immunol. 2009;183(10):6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 48.Benzel F, Erdur H, Kohler S, Frentsch M, Thiel A, Harms L, et al. Immune monitoring of Trichuris suis egg therapy in multiple sclerosis patients. J Helminthol. 2012;86(3):339–347. doi: 10.1017/S0022149X11000460. [DOI] [PubMed] [Google Scholar]
- 49.Wilson JC, Furlano RI, Jick SS, Meier CR. Inflammatory bowel disease and the risk of autoimmune diseases. J Crohns Colitis. 2016;10(2):186–193. doi: 10.1093/ecco-jcc/jjv193. [DOI] [PubMed] [Google Scholar]
- 50.Schicho R, Shaykhutdinov R, Ngo J, Nazyrova A, Schneider C, Panaccione R, et al. Quantitative metabolomic profiling of serum, plasma, and urine by 1H NMR spectroscopy discriminates between patients with inflammatory bowel disease and healthy individuals. J Proteome Res. 2012;11(6):3344–3357. doi: 10.1021/pr300139q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams HR, Cox IJ, Walker DG, Cobbold JF, Taylor-Robinson SD, Marshall SE, et al. Differences in gut microbial metabolism are responsible for reduced hippurate synthesis in Crohn’s disease. BMC Gastroenterol. 2010;10:108. doi: 10.1186/1471-230X-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]