Abstract
The role of bisphenol A (BPA) in autism was investigated in 49 children (mean age = 5.950 ± 1.911 years) with autism spectrum disorders (ASDs) and 40 comparable age and sex matched children used as controls (mean age = 5.333 ± 2.279 years). In addition, 8-Hydroxydeoxyguanosine (8-oxodG) was also studied as a biomarker of oxidative stress in the same set of two selected groups. The results showed that both BPA and 8-oxodG were significantly higher in children with autism than those of control children (p values = 0.025 and 0.0001, respectively). There were positive correlations between both BPA and 8-oxodG with ASDs severity (r = 0.400 and 0.805, respectively), these correlations were highly significant (p values = 0.004 and 0.001, respectively). There was a significance positive correlation between BMI and BPA, but the correlation between BMI and 8-oxodG was not significant in children with autism. The observed results revealed that BPA may increase oxidative stress resulting in mitochondrial dysfunction that affecting the behavior and functioning of ASDs children.
Keywords: Autism, Bisphenol A, 8-Hydroxydeoxyguanosine, Oxidative stress
Introduction
BPA (2,2 bis(4-hydroxyphenyl) propane) is a monomer used in the manufacture polycarbonate plastics, as an antioxidant in some plasticizers, in polyvinyl chloride (PVC) manufacture, and the epoxy resins that used to coat the inside of many food and beverage cans [1]. BPA has been shown to leach out of products, and high levels of the monomer have been identified in human and animal samples [2]. Human exposure of BPA can be attained through oral route (viz. food wrapped in plastics, drinking water, food and beverage containers), as well as through trans-dermal route (viz. flooring, direct contact with plastic products, bathing in water contaminated by BPA), and also via inhalation of contaminated indoor air [1].
The effect of BPA on endocrine disruption has been extensively studied on perinatal, childhood and adult health [3]. On the other hand, some recent studies introduced indirect evidence linking exposure to BPA and ASDs [4, 5]. Epidemiological studies found that increased maternal prenatal exposure to BPA was associated with behavioral problems including ASDs in their children later [6]. A Swedish study found that using PVC flooring material at home was linked with ASDs [7]. It was reported that BPA caused neurological effects with respect to fetal brain development that promoted neurodegenerative diseases [8]. Synaptogenesis inhibition and synaptic structural modification were recorded in mice models that exposed perinatal to BPA [9].
ASDs are a group of neurodevelopmental disorders that are characterized by communication and social deficits that accompanied with repetitive behaviors [10]. The etiology of ASDs is believed to be multifactorial including genetically and environmental origin [11]. Introduction of chemicals produced by human activity into the environment can have adverse effects on human health [11]. Several studies suggested that exposure of genetically sensitive individuals to an environmental toxicant is responsible for ASD manifestation [12].
On the other hand, ASD are characterized by oxidative stress, mitochondrial dysfunction, deficits in antioxidant and detoxification capacity as well as immunological disturbance [13]. Oxidative stress may be a key link between mitochondrial dysfunction and ASD, where reactive oxygen species (ROS) generated from pro-oxidant environmental toxicants and activated immune cells can cause mitochondrial dysfunction [14]. There are several lines of evidence suggesting that environmental exposure to plasticizers including BPA is associated with oxidative stress [15]. It was reported that BPA increases the generation of ROS and induced hepatic damage and mitochondrial dysfunction [16]. Recently, Eid et al. [17] found that early life exposure to BPA significantly increased oxidative/nitrosative stress, decreased antioxidant enzyme activities, inducing DNA damage and chronic severe inflammation in the hepatic tissue of female rat offspring in a time dependent manner.
8-oxodG was used extensively as biomarker for oxidative stress-derived DNA damage, mitochondrial dysfunction and afflicted metabolism [18]. Its level in the blood and urine is associated with the severity of internal DNA damage [19]. For the above reasons, the concentration of both BPA and 8-oxodG in the serum of the two selected groups of children were measured in the present study.
Methods
Participants
Forty-nine children with autism and another forty children matched for age and sex as controls were included in the present study. Control children are free from any developmental, neurological, chronic or recurrent medical impairment. Subjects were selected from the out-patients clinic of Learning Disability and Neuro-Rehabilitation at Medical Excellence Centre, National Research Centre, Dokki, Egypt. ASD clinical diagnosis has been confirmed according to the criteria of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) [20]. The severity of autism was assessed using the Childhood Autism Rating Scale (CARS) [21]. All subjects with other causes of mental subnormality and delayed language were excluded. A comprehensive history was taken from the parents of the selected patients. Medical history was including: pregnancy history, perinatal history, developmental history, family history, and child’s medical and behavioral history, the age of onset at which manifestation of the disorder was recorded. Head circumference, body weight and height were measured followed by BMI calculation for each participant. All measurements followed the recommendations of the International Biological Program (IBP) [22].
Ethical Approval
All procedures performed in the current study involving human participants were in accordance with the ethical standards of the Medical Research Ethics Committee of the National Research Centre- Egypt, and with 1964 Helsinki declaration and its later amendments. Informed consent was obtained from parents of each individual participant included in the study.
Sampling
Blood samples were collected in 5-ml vaccutainer plain tubes followed by serum isolation by centrifugation at 3000 rpm within 30 min after collection. Aliquots of serum samples were kept immediately at −40 °C until assayed.
Procedures
The concentration of total BPA was analyzed in the collected serum samples for each individual using Enzyme-Linked Immunosorbent Assay technique (ELISA). BPA concentrations were measured by using Human PBA ELISA kit (GSCIENCE, USA) according to supplier’s instructions. The serum levels of 8-oxodG were measured using Human 8-oxodG ELISA kit (GSCIENCE, USA) following the manufacturer’s instructions. Body mass index (BMI) for patients and control subjects were calculated according to the following equation:
BMI for ASD patients and controls were compared to World Health Organization growth charts to check that they are normal or overweight [23].
Statistical Analyses
Data analysis was performed using Mann–Whitney test for comparing two nonparametric groups. Quantitative data were statistically represented in terms minimum, maximum, and median. Qualitative data were statistically represented in terms number and percent. A probability value was considered significant at p ≤ 0.05. Correlations between various variables were done using Spearman rank correlation coefficient (r). All statistical calculations were done using computer program Statistical Package for Social Science (SPSS) statistical program version (16.0). Graphs were done using Microsoft Excel program version 2010.
Results
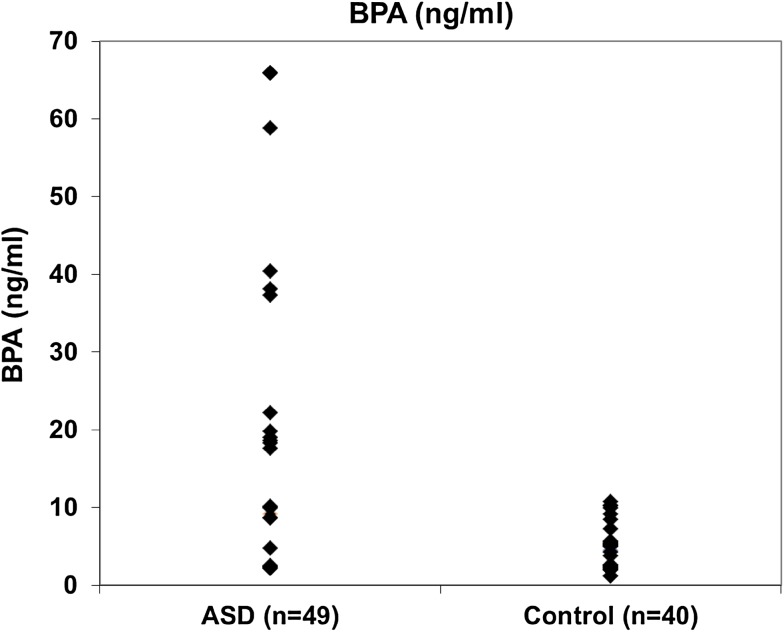
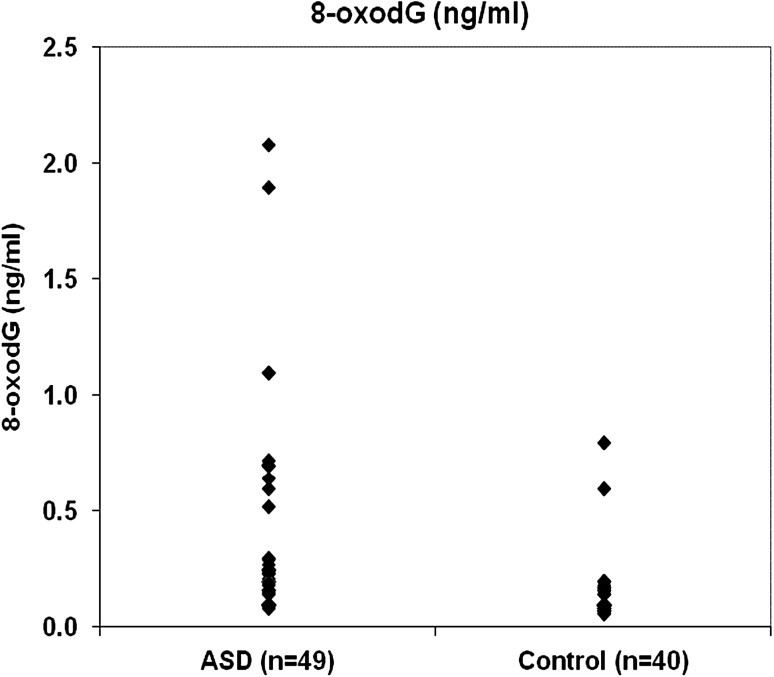
Table 1 shows general and clinical characteristics of ASDs and control subjects. As shown in Table 1, there were no significance differences between age and sex in the two studied groups. Statistical significant difference was observed in the BMI between the two studied groups (p = 0.007). ASD severity was classified into severe (6.1%), moderate (34.7%) and mild (59.2%) in the studied children with autism. Table 2 represents BPA and 8-oxodG values among ASD and control groups. As seen in Table 2, levels of both BPA and 8-oxodG were high in ASD group than those of control group and the difference was statistically significant for BPA (p = 0.025) and highly significant for 8-oxodG (p = 0.0001). In addition, Figs. 1 and 2 illustrates the distribution of BPA and 8-oxodG values, respectively in ASD and control children. As illustrated in Fig. 1 and 2 the median and maximum values are higher in ASD group than those of control group. On the other hand, Table 3 demonstrates the correlation between BPA and 8-oxodG among the studied ASD children. As shown in Table 3, a significant positive correlation was found between BPA and 8-oxodG in both autistic males and females (r = 0.390* and 0.735*; p values = 0.013 and 0.024, respectively). Moreover, the correlation between BMI and BPA was significant (r = 0.299* and p value = 0.037) in autistic children but not significant in control group. However, the positive correlation between BMI and 8-oxodG was not significant in the two studied groups (Table 4). ASD severity was found to be positively correlated with both BPA and 8-oxodG values (r = 0.400** and 0.805**, respectively) and the correlation was found to be highly significant (p < 0.01) (Table 5).
Table 1.
General and clinical characteristics of the study groups
| Characteristics | ASD | Control | χ2 | p value |
|---|---|---|---|---|
| Number | 49 | 40 | ||
| Gender | ||||
| Males | 81.6% | 72.5% | 1.054 | 0.305 |
| Females | 18.4% | 27.5% |
| Mean ± SD | t test | p value | ||
|---|---|---|---|---|
| Age (years) | 5.950 ± 1.911 | 5.333 ± 2.279 | 1.350 | 0.180 |
| BMI | 17.713 ± 4.228 | 15.994 ± 0.691 | 2.541* | 0.007 |
| ASD severity | ||||
| Severe | 6.1% | |||
| Moderate | 34.7% | |||
| Mild | 59.2% |
* The value is significant at p ≤ 0.01 level
Table 2.
Values of BPA and 8-oxodG in the study groups
| ASD | Control | |
|---|---|---|
| Number | 49 | 40 |
| BPA (ng/ml) | ||
| Minimum | 2.15 | 1.19 |
| Maximum | 65.9 | 10.71 |
| Median | 8.67 | 4.73 |
| Mann–Whitney | 708.00* | |
| p value | 0.025 | |
| 8-oxodG (ng/ml) | ||
| Minimum | 0.08 | 0.06 |
| Maximum | 2.08 | 0.8 |
| Median | 0.2 | 0.09 |
| Mann–Whitney | 312.50** | |
| p value | 0.0001 | |
* The value is significant at p ≤ 0.05
** The value is highly significant at the p ≤ 0.01 level
Fig. 1.
Distribution of BPA between the study groups (no. = 89)
Fig. 2.
Distribution of 8-oxod G between the study groups (no. = 89)
Table 3.
Correlation coefficient between BPA and 8-oxodG in ASD children
| Sex | Spearman correlation (r) | p value | |
|---|---|---|---|
| BPA (ng/ml) with 8-oxodG (ng/ml) | Male | 0.390* | 0.013 |
| Female | 0.735* | 0.024 |
* The value is significant at the p ≤ 0.05 level
Table 4.
Correlation coefficient between BMI with BPA and 8-oxodG values in the studied groups
| BMI with BPA (ng/ml) | Spearman correlation (r) | p value |
|---|---|---|
| ASD | 0.299* | 0.037 |
| Control | 0.029 | 0.859 |
| BMI with 8-oxodG (ng/ml) | ||
| ASD | 0.024 | 0.872 |
| Control | 0.057 | 0.725 |
* The value is significant at the p ≤ 0.05 level
Table 5.
Correlation between severity of ASD with BPA and 8-oxodG values
| Spearman Correlation (r) | p value | |
|---|---|---|
| ASD Severity with BPA (ng/ml) | 0.400** | 0.004 |
| ASD Severity with 8-oxodG (ng/ml) | 0.805** | 0.001 |
** Correlation is significant at the p ≤ 0.01 level
Discussion
The prevalence of autism has been exponentional increased in the past few years. This increasing couldn’t be attributed only to improved diagnostic programs and awareness but also to the interaction between genetic, epigenetic, and environmental factors [13, 24]. ASD has traditionally been considered as behavioral disorder. However, research results are accumulating to support an evidence that it is characterized by specific physiological abnormalities including oxidative stress; mitochondrial dysfunction; immune dysregulation and inflammation [25, 26]. It has been reported that reactive oxygen species (ROS) are induced in response to BPA exposure [15]. Unfortunately, limited numbers of studies were done to investigate the effect of BPA on oxidative stress in children with autism [24].
The results of the present study showed that there was no statistical significance between age and sex of the two study groups (Table 1). However, a significant difference was observed in the BMI of autistic and control children (Table 1). In addition, statistically significant correlation was observed between BMI and BPA in children with autism but no significance was found in control group (Table 4). The correlation between BMI and 8-oxodG was not significant in the study groups (Table 4). These results are in agreement with other studies suggested that BPA could be a potential new environmental obesogen [27].
Referring to Table 2 and Fig. 1 the values of BPA in ASD group are higher than that of control group. In addition, a significant positive correlation was found between ASD severity and BPA (r: 0.400) (Table 5). The direct effect of BPA on the autism has been studied by different research groups [4, 5]. Recently, Kaur and his coworkers [24] give direct evidence linking the exposure to BPA and ASD incidence, namely, the effect of BPA on: cell viability, mitochondrial membrane potential, generation of reactive oxygen species (ROS), and mtDNA copy number in lymphoblas from ASD children and unaffected siblings. Their results confirm that BPA may act as risk factor for autism in genetically susceptible children. Furthermore, Kaur and his coauthors, proposed that BPA increased oxidative stress and mitochondrial dysfunction in autism. Table 2 and Figs. 1 and 2, indicated that the values of BPA and 8-oxodG in ASD group are higher than those of control group. These results run in a good harmony with the results of Kaur et al., In conclusion, the obtained results can be attributed to the effect of BPA which increased the oxidative stress in ASD children.
On the other hand, it has been reported that BPA exposure to lympphoblastoid cells from autistic subjects and age-matched unaffected siblings controls showed a decrease in mitochondrial membrane potential (MMP) in a dose-dependent manner in both groups. The MMP is an important indicator of the energy status of the cell. Decreasing in MMP is an initial step in apoptosis. Continuous BPA exposure can lead to cell death [24]. The results of the present study showed a significant positive correlation between BPA and 8-oxodG in both males and females groups among ASD children as shown in Table 3. These results are in consistent with the results of Kaur et al. [24]. These observed results could be explained on the basis that elevated BPA in children with autism increased ROS generation that supported by elevation in 8-oxodG levels in autistic children compared to control group. According to Kaur et al. [24] BPA-induced increase in ROS production could result in the loss of cellular membrane integrity and fluidity.
A recent meta-analysis showed that the prevalence of mitochondrial disease in ASD children was 5% [28]. In addition, it was reported that BPA exposure increased ROS generation and decrease mitochondrial membrane potential (MMP), which is the initial step in apoptosis, and increased copy number variation (CNV) of the genes necessary for mitochondrial oxidative phosphorylation. These effects may cause defect in mitochondrial electron transport chain (ETC) and mtDNA damage [24, 29].
The results in this work can be further explained by adapting the above suggestion of Kaur et al. [24] and Rossignol and Frye [28] namely, the prenatal exposure to BPA may increase oxidative stress that might damage the mitochondrial function and over production of ROS can affect functioning and behavior of children with autism. These effects of prenatal exposure of BPA on the behavior and functioning of children were reported elsewhere [30]. In the present study a positive correlation was observed between severity of ASD with BPA and 8-oxodG as shown in Table 5. These results support the idea that BPA exposure increases oxidative stress that could affect the severity of ASD. Further studies are needed to investigate CNVs of mitochondrial ETC complexes I and III and MMP in the studied cases and controls.
Acknowledgements
We would like to thank all children participated in this study and their families.
Contributor Information
Fateheya M. Metwally, Email: fathyamm@yahoo.com
Hend Rashad, Email: hendgoma@gmail.com.
Hala M. Zeidan, Phone: +201208434272, Email: halazeidan@yahoo.com
Ayman Kilany, Phone: +201003842224, Email: elkilany7000@yahoo.com.
Ehab R. Abdol Raouf, Email: ehabragaa@gmail.com
References
- 1.Makris KC, Andra SS, Jia A, Herrick L, Christophi CA, Snyder SA, et al. Association between water consumption from polycarbonate containers and bisphenol A intake during harsh environmental conditions in summer. Environ Sci Technol. 2013;47:3333–3343. doi: 10.1021/es304038k. [DOI] [PubMed] [Google Scholar]
- 2.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Kardas F, Bayram AK, Demirci E, Akin L, Ozmen S, Kendirci M, et al. Increased serum phthalates (MEHP, DEHP) and bisphenol a concentrations in children with autism spectrum disorder: the role of endocrine disruptors in autism etiopathogenesis. J Child Neurol. 2016;31(5):629–635. doi: 10.1177/0883073815609150. [DOI] [PubMed] [Google Scholar]
- 5.Stein TP, Schluter MD, Steer RA, Guo L, Ming X. Bisphenol A exposure in children with autism spectrum disorders. Autism Res. 2015;8:272–283. doi: 10.1002/aur.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miodovnik A, Engel SM, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. Enodcrine disruptors and childhood social impairment. Neurotoxicology. 2011;32:261–267. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6–8 years of age. Neurotoxicology. 2009;30:822–831. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szychowski KA, W´ojtowicz AK. Components of plastic disrupt the function of the nervous system. Poste¸py Higieny iMedycyny Do´swiadczalnej. 2013; 67:499–506. [DOI] [PubMed]
- 9.Xu X, Xie L, Hong X, et al. Perinatal exposure to bisphenol-A inhibits synaptogenesis and affects the synaptic morphological development in offspring male mice. Chemosphere. 2013;91(8):1073–1081. doi: 10.1016/j.chemosphere.2012.12.065. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC; 1994.
- 11.Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 2010;23(2):103–110. doi: 10.1097/WCO.0b013e328336a01f. [DOI] [PubMed] [Google Scholar]
- 12.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faber S, Zinn GM, Boggess A, Fahrenholz T, Kern JC, Kingston HS. A cleanroom sleeping environment’s impact on markers of oxidative stress, immune dysregulation, and behavior in children with autism spectrum disorders BMC. Comple Alter Med. 2015;15(1):71. doi: 10.1186/s12906-015-0564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossignol DA, Frye RE. A review of research trends in physiological abnormalitiesin autism spectrum disorders: immune dysregulation, inflammation, oxidativestress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17:389–401. doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kourouma A, Peng D, Chao Q, Changjiang L, Chengmin W, Wenjuan F, et al. Bisphenol A induced reactive oxygen species (ROS) in the liver and affect epididymal semen quality in adults Sprague-Dawley rats. J Toxi Enviro Health Sci. 2014;6(4):103–112. doi: 10.5897/JTEHS2014.0309. [DOI] [Google Scholar]
- 16.Moon MK, Kim MJ, Jung IK, Koo YD, Ann HY, Lee KJ, Kim SH, Yoon YC, Cho BJ, Park KS, Jang HC, Park YJ. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Korean Med Sci. 2012;27:644–652. doi: 10.3346/jkms.2012.27.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eid JI, Eissa SM, El-Ghor AA. Bisphenol A induces oxidative stress and DNA damage in hepatic tissue of female rat offspring. J Basic Appl Zoology. 2015;71:10–19. doi: 10.1016/j.jobaz.2015.01.006. [DOI] [Google Scholar]
- 18.Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. Apmis. 2007;115(2):81–103. doi: 10.1111/j.1600-0463.2007.apm_514.x. [DOI] [PubMed] [Google Scholar]
- 19.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 20.DSM-IV-TR . A. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 21.Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: childhood autism rating scale (CARS) J Autism Dev Disord. 1980;10(1):91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 22.Tanner JM, Hiernaux J, Jarman S. Growth and physique studies. In: Weiner JS, Lourie JA, editors. Human biology: a guide to field methods, I.B.P. handbook No.9. Oxford and Edinburgh: Blackwell Scientific Publications; 1969. p. 1–29.
- 23.WHO| The WHO Child Growth Standards, 2007-2017. WHO.http://www.who.int/childgrowth/en/.
- 24.Kaur K, Chauhan V, Gu F, Chauhan A. Bisphenol A induces oxidative stress and mitochondrial dysfunction in lymphoblasts from children with autism and unaffected siblings. Free Radic Biol Med. 2014;76:25–33. doi: 10.1016/j.freeradbiomed.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Rossignol DA, Frye RE. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol. 2014; 5(150):1–15.http://journal.frontiersin.org/article/10.3389/fphys.2014.00150/full Doi: 10.3389/fphys.2014.00150 [DOI] [PMC free article] [PubMed]
- 26.Meguid NA, Dardir AA, Abdel-Raouf ER, Hashish A. Evaluation of oxidative stress in autism: defective antioxidant enzymes and increased lipid peroxidation. Biol Trace Elem Res. 2011;143:58–65. doi: 10.1007/s12011-010-8840-9. [DOI] [PubMed] [Google Scholar]
- 27.Vafeiadi M, Roumeliotaki T, Myridakis A, Chalkiadaki G, Eleni Fthenou E, et al. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ Res. 2016;146:379–387. doi: 10.1016/j.envres.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: asystematic review and meta-analysis. Mol Psychiatry. 2012 doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Napoli E, Wong S, Giulivi C. Evidence of reactive oxygen species-mediated damage to mitochondrial DNA in children with typical autism. Mol Autism. 2013;4:2. doi: 10.1186/2040-2392-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]