Abstract
The documentation of lead toxicity (plumbism) dates back to the times when man learnt its various applications. This versatile heavy metal is non-degradable and its ability to get accumulated in the body that goes undiagnosed, makes it a serious environmental health hazard. Lead is now known to affect almost every organ/tissue of the human body. With irreversible effects on neurobiological development of young children and foetus, its toxicity has lasting implications on the human life. Outlining the symptoms, diagnosis and treatment therapy for lead poisoning, the present review elaborates the pathophysiological effects of lead on various organs. This will be of immense help to the health professionals so as to inculcate a better understanding of the lead poisoning which otherwise is asymptomatic. With chelation therapy being the classic path of treatment, new strategies are being explored as additive/adjunct therapy. It is now understood that lead toxicity is completely preventable. In this regard significant efforts are in place in the developed countries whereas much needs to be done in the developing countries. Spreading the awareness amongst the masses by educating them and reducing the usage of lead following stricter industry norms appears to be the only roadmap to prevent lead poisoning. Efforts being undertaken by the Government of India and other organisations are also mentioned.
Keywords: BLL, Chelation therapy, Lead poisoning, Pathophysiology, Prevention
Introduction
The versatile properties of lead such as malleability, ductility, poor conductibility, softness and resistance to corrosion have led to its usage by man for over 6000 years, resulting in its wide distribution in environment [1]. Further, its wide industrial and household applicability is dependent on obvious physico-chemical properties, low cost and a visibly easy workability [1]. These factors have been instrumental in involvement of lead in over 900 occupations including smelting, mining and battery manufacturing to name a few [2]. Lead from various sources finds its way to the inside of the human body. Because it is non-biodegradable [3] and has a slow rate of elimination [4], it tends to accumulate in body and this chronic or acute accumulation of even small quantities of lead leads to the condition termed as lead poisoning (plumbism). It is a hazardous occupational and environmental disease that affects millions of adults and children worldwide.
The history of lead toxicity dates back to the era before Christ which is beautifully chronicled by Gidlow in the 2015 review citing one of the initial reports by Hippocrates in 370 BC [5]. Having such ancient history, one may assume that lead poisoning should effectively have been controlled by now and the urgent need to study this might not have been there. Instead, the truth is that lead poisoning is still a major health hazard around the world and important steps in this direction are still to be taken, particularly by the developing countries. With this in view, the seriousness of lead poisoning was realized and the present review was framed to develop a better understanding of the subject and to contribute towards arising the awareness on the lead associated diseases.
Exposure Routes
Lead is mainly considered as an environmental pollutant. It finds its entry into the human body through food, water, soil and air. Besides this, adults also face the occupational exposure to lead which also accounts to the ‘take home lead’ with their clothes. Lead has been a metal in the earth’s crust but became a hazardous health concern due to mining and other applications. Further, lead pollution was at peak during the twentieth century, around 1970’s when it was used as octane number enhancer in the form of tetraethyl lead in gasoline. At that time, the worldwide production of tetraethyl lead was of the order of 500,000 tonnes. Since the ban of usage of tetraethyl lead in gasoline, the current production of tetraethyl lead has reduced to ~4500 tonnes [5]. Experts consider that leaded gasoline alone deposited close to 4–5 million tonnes of lead in soil in the United States [6]. Earlier lead was used in the paints. Thus older buildings with paint chipping off or under renovation contribute to lead dust and expose the inhabitants. PVC pipes used for sanitation and in the potable water supplies also pose a threat of lead toxicity due to leaching as these contain high amounts of lead as impurity [7]. Besides, occupations like refining, smelting, battery manufacturing and recycling and car repair etc. also expose the workers to lead [3]. A significant source of lead toxicity remains the folk medicine such as Ayurveda and Chinese medicines, cosmetics such as kohl and surma and painted toys etc. In their review, Kianoush et al. [8] have provided detailed sources of lead exposure through environment, occupations and other sources. A concise list of the lead exposure is provided in the Table 1.
Table 1.
Common sources of lead exposure.*
|
Occupational
Lead mining and refining, plumbing and pipe fitting, auto repair, glass manufacturing, printers, battery manufacturing and recycling Construction work, firing-range instruction, plastic manufacturing and gas station attendant |
|
Environmental
Lead paint, soil or dust near roadways or lead-painted homes, plastic window blinds, plumbing leachate (from pipes or solder), ceramic ware and lead-core candle wicks |
|
Hobbies
Glazed pottery making, target shooting at firing ranges, lead soldering, preparing lead shot or fishing sinkers, stained-glass making, painting and car or boat repair |
|
Other
Folk remedies, gasolinesniffing, costume jewelry and cosmetics |
Adapted and modified from Sanborn et al. [9]
How Toxic is Lead
For a large period of time, the toxic lead levels remained undefined. Most of the times, lead toxicity remains asymptomatic. It was documented that in case of adults, 20–70% of the ingested lead enters the blood while almost 100% of the inhaled lead finds its way into the bloodstream [10]. Young children (9 months to 3 years) are most vulnerable because of their developmental stage and their exploratory behaviour and frequent hand-to-mouth activity with 5–10 times more effective absorption in them [11]. Other high-risk groups include pregnant women and their foetus [11, 12] and occupationally exposed workers and artisans and their families [11, 13]. The amount of absorption can be higher for patients having zinc, iron or calcium deficiency [14]. Of the various tissues, the rate of deposition of lead in bones and teeth is almost 95% in case of adults while 70% in case of children [15]. Generally, the lead thus deposited is not always toxic. But as the remodelling of bones takes place in children [15] and pregnant women and due to larger half-life of lead in these tissues, the lead from the bones and teeth is mobilised into the blood stream long after the initial exposure, manifesting its toxic effects [16]. Blood lead level (BLL) is one of the major criteria to measure the extent of the lead poisoning. Another criterion of defining lead toxicity is ‘body burden of lead’, i.e. the amount of lead which is deposited in the soft tissues and bones of the body. Between 1960 and 1990, the Centres for Disease Control and Prevention (CDC) described ‘blood lead level of concern’ were lowered incrementally from 60 to 25 μg/dl. In 1991, it was lowered for individual intervention to 15 μg/dl. By this time, CDC also implemented community-wide primary lead-poisoning prevention activities in areas where many children had BLL > 10 μg/dl [17]. Until 2012, CDC, USA had set toxic BLL to be above 10 μg/dl including children [18]. But in 2012 CDC redefined the standards with BLL > 10 μg/dl for adults and >5 μg/dl for children as a matter of concern [19].
Lead presents its toxicity by two ways: inorganic lead and organic lead. Inorganic lead is defined as lead oxides, metallic lead, and lead salts [20]. The organic lead compounds include the tetra-alkyl lead compounds mainly tetraethyl lead and tetramethyl lead which have been incorporated into gasoline as anti-knock additives [21]. Lead toxicity leads to various pathophysiological conditions. Based on the BLL, the different effects of lead on individuals have been observed that are presented in the Table 2.
Table 2.
Blood lead levels and their putative impact on health of adults
| Blood lead levels (µg) | Males | Females |
|---|---|---|
| <5 | Nil | Nil |
| 5–10 | Possible hypertension and kidney dysfunction | Possible hypertension and kidney dysfunction Possible spontaneous abortion |
| 11–20 | Possible hypertension and kidney dysfunction Possible subclinical neurocognitive defects |
Possible hypertension and kidney dysfunction Possible subclinical neurocognitive defects Reduced birth weight Possible postnatal developmental delay |
| 21–29 | Hypertension and kidney dysfunction Possible subclinical neurocognitive defects |
Hypertension and kidney dysfunction Possible subclinical neurocognitive defects Possible spontaneous abortion Reduced birth weight Possible postnatal developmental delay |
| 30–39 | Hypertension and kidney dysfunction Possible neurocognitive defects |
Hypertension and kidney dysfunction Possible neurocognitive defects Spontaneous abortion Reduced birth weight Possible postnatal developmental delay |
| 40–79 | Hypertension and kidney dysfunction Subclinical peripheral neuropathy Neurocognitive defects Anaemia Sperm abnormalities Colic Possible gout |
Hypertension and kidney dysfunction Subclinical peripheral neuropathy Neurocognitive defects Anaemia Colic Possible gout Spontaneous abortion Reduced birth weight Possible postnatal developmental delay |
| >80 | Hypertension Nephropathy Peripheral neuropathy Neurocognitive defects Anaemia Sperm abnormalities Colic Gout Encephalopathy |
Hypertension Nephropathy Peripheral neuropathy Neurocognitive defects Anaemia Colic Gout Encephalopathy Spontaneous abortion Reduced birth weight Possible postnatal developmental delay |
Adapted from Wani et al. [3]
Diagnosis
A proper diagnosis of lead poisoning is a critical step in the starting of the treatment. Since lead poisoning is asymptomatic and often most of the symptoms are similar to other disorders, most of the times it goes undiagnosed and untreated [1]. General awareness and education regarding lead toxicity can be of significant help in early diagnosis and possible prevention. A proper diagnosis will start with a detailed enquiry into the possible routes of exposure, medical history and clinical signs. Appropriate staff including clinical toxicologists and medicinal specialists should be involved [22]. In case of children, the BLL of concern be investigated along with possible growth retardation, language or speech dysfunction, anaemia and behavioural or attentional disorders [23].
The primary test will be to estimate the BLL. Lead poisoning can also be diagnosed on the basis of haematological influences showing basophilic stipplings (products of ribonucleic acid degradation) of red blood cells. These stipplings are visible as red dots under microscope [16]. Other haematological influences are raised levels of urinary porphyrins, coproporphyrins, zinc protoporphyrin and erythrocyte protoporphyrin (EP). But EP levels also rise in iron deficiency and the use of this investigative method alone for diagnosing lead poisoning is being discouraged [3]. All these are due to the alterations in the three enzymes involved in the biosynthesis of heme [17]. δ-aminolevulinic acid (ALA) in urine is also an indicator of lead poisoning [24]. Saturinine gout has also been seen in case of lead poisoning as urate excretion was detected in the urine of suffering patients [25]. Lead toxicity has also been observed to contribute to reduced total haemoglobin, red blood cell count and plasma T3 and T4 without affecting the white blood cell count [26]. Acute high-level lead exposure has also been associated with haemolytic anaemia. It is to be noted that the anaemia of chronic lead intoxication is hypochromic and normocytic or microcytic with a reticulocytosis [5].
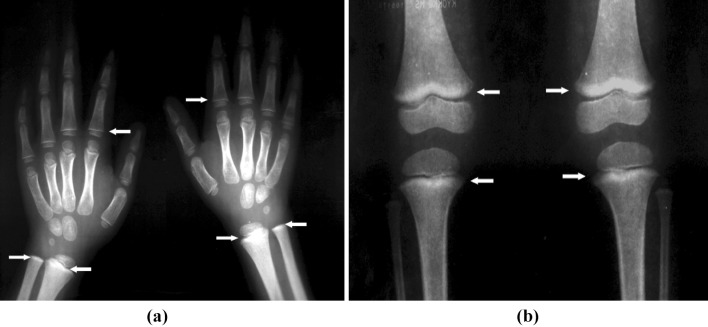
It is important to realize that BLL is just an indicator of recent lead exposure and does not reflect upon the total body burden. In order to determine the total body burden, X-ray fluorescence can be applied as a non-invasive method to measure whole body lead in bones [27]. Abdominal radiographs are also helpful in revealing lead containing foreign materials such as paint chips that have accumulated in the gastrointestinal tract [28, 29]. These are helpful only in cases of acute ingestion (e.g. lead sinkers, curtain weights, jewelry, or paint chips) or unusual persistence of high BLL. Longbone radiographs can show “lead lines”. These are lines of increased density on the metaphysis growth plate of the bone, showing growth retardation. This is not a routine procedure to identify lead poisoning, but a radiological finding of chronic exposure. Figure 1 provides the long bone radiographs of lead exposed children showing “lead lines”.
Fig. 1.
Long bone Radiograph showing “lead lines” shown as increased density on the metaphysis growth plate. a Hands—of the proximal segments of phalanges and distal segments of ulna and radius in a 5 year old male with radiological growth retardation and blood lead level of 37.7 µg/dl. b Knees—especially in the femur in 3 year 2 month old female with blood lead level of 10.6 µg/dl [10]
Pathophysiology
Despite the interesting fact that no normal physiological role for lead could be found, lead toxicology causes numerous serious pathophysiological manifestations in the patients [25].
Oxidative Stress
Oxidative stress understandably the major mechanism of lead poisoning is caused when generation of free radicals is more than ability of the system to readily detoxify the body from them, as their accumulation results in cellular damage [30]. Two concurrent pathways of oxidative stress are: (a) the production of reactive oxygen species (ROS) such as hydro peroxides (HOO•), singlet oxygen, and hydrogen peroxide (H2O2) and (b) the exhaustion of naturally occurring antioxidant reserves of the system [31].
Lead has a strong electron sharing property that helps in the covalent bond formation. The covalent bonds are formed between the lead moiety and the sulfhydryl groups in the antioxidant enzymes making them most vulnerable targets for lead, ultimately making them inactive. Lead also binds with sulfhydryl groups of reduced glutathione (GSH) rendering it inactive [32]. Besides, lead inactivates δ-aminolevulinic acid dehydratase (ALAD), glutathione reductase, glutathione peroxidase, and GST (Glutathione-S-transferase) enzymes and further reduces GSH levels [33]. Other antioxidant enzymes which are inactivated by lead are superoxide dismutase (SOD) and catalase (CAT) because of its ability to replace the zinc ions which serve as co-factors for these antioxidant enzymes. Lead targets sulfhydryl groups also of these enzymes. Decreased concentration of SOD decreases the clearance of superoxide radical whereas reduced CAT impairs the superoxide radical (O2 −•) scavenging [34].
The ROS formed due to lead poisoning also causes lipid peroxidation of red blood cell (RBC) membranes as they take electrons from the cell membranes and damages the cell by denaturing lipid that formed the membrane. RBCs have very limited reservoirs of antioxidant enzymes to counter the effect of ROS and they are unable to replenish these enzymes back as they lack rough endoplasmic reticulum [35]. Moreover, ALA which is increased in the blood due to inhibition of ALAD by lead also generates hydrogen peroxide and superoxide radical which goes to interact with oxy-Hb resulting in the generation of hydroxyl radicals making RBCs highly vulnerable to oxidative stress and resulting in hemolysis [16]. Excessive generation of ROS is a reason behind reproductive toxicity caused by lead. A study of the male reproductive system has revealed positive correlation between seminal plasma lead and spermatozoa ROS levels [36]. Instead, in people with extended lead exposure, increased activity of SOD was established which might have resulted from its property to clear the burden of increasing ROS production due to lead exposure [37]. Oxidative stress also seems to be involved in the development of renal toxicity induced by the environmental lead exposure that leads to substantial pathological lesions on the renal systems of both men and animals [38]. ROS also damages nucleic acids and inhibits the repair of DNA [39]. Oxidative stress was studied by low level lead exposure in first grade Uruguayan children, proposing its possible adverse effects on oxidative stress [40].
Hematological Changes
Lead exposure leads to hematological changes [41] that result due to the poisoning of three enzymes of heme biosynthesis pathway: ALAD, ferrochelatase, and δ- aminolevulinic acid synthetase (ALAS) [17]. Also, RBCs become more fragile as their membrane is damaged because lead disrupts the maintenance of the cell membrane [42]. The combined effect of these two processes leads to anemia [43]. Lead poisoning results into two forms of anaemia: (a) Frank anaemia, is hypochromic and normocytic or microcytic with a reticulocytosis. It appears only when there is chronic lead intoxication and (b) haemolytic anaemia that is related to acute high levels of lead exposure [44].
Build-up of ALA in plasma and it’s excretion in urinedue to influence of lead on ALAD is observed even at BLL of <10 μg/dl. ALAD inhibition by lead occurs at BLL of 10–20 μg/dl. Biosynthesis of heme does not reduce until BLL reaches about 55 μg/dl as at such high levels the action of ALAD is restricted by 80–90% [45]. Only as high as BLL of 50 μg/dl interprets into anaemia besides hematological influences at lower concentrations also.
Neurological Effect
Most sensitive organ to lead exposure is brain [46]. Neurons may be directly or indirectly harmed by accumulation of heme precursors such as ALA due to influence of lead on ALAD [47]. Lead also hinders the development of neurotransmitters and organisation of ion channels [48]. Fundamental cellular processes such as intra and intercellular signalling, cell adhesion, protein folding and maturation, apoptosis, ionic transportation, enzyme regulation and release of neurotransmitters have been significantly influenced by the ionic mechanism of lead action [49] in which lead substitutes bivalent cations such as Ca2+, Mg2+, Fe2+ and monovalent cations such as Na+ [50]. Neurological deficits are principally due to this mechanism. As lead replaces calcium ions, it becomes capable to cross the blood–brain barrier (BBB) at a considerable rate and is taken up by the calcium ATPase pump [3]. Once there, it accumulates in astroglial cells as they contain lead-binding proteins. However its toxic effects are more pronounced in immature astroglial cells (developing nervous system) that lack lead-binding proteins. It damages the astroglial cells and blocks the formation of myelin sheath, which are both involved in the development of BBB [41].
Lead has a dual effect on neurotransmitter release: spontaneous neurotransmitter release is boosted while stimulated release is inhibited [51]. It inhibits the release of neurotransmitters such as glutamate which is important for functions like learning. The main target of lead toxicity is assumed to be N-methyl-D-aspartate receptors as lead operates by blocking them and lead exposure also decreases the amount of gene for this receptor [52]. Protein kinase C, a key neurotransmitter can also be affected by replacement of calcium by lead as it regulates long-term neural excitation and memory storage [51]. Sodium ion concentration is also affected by lead as it impairs the normal functioning of the sodium-dependent vital processes such as generation of action potentials in the excitatory tissues for cell to cell communication, uptake of neurotransmitters (choline, dopamine, and GABA) and regulation of uptake and retention of calcium by synaptosomes [51].
Both arms of the nervous system, peripheral and central are affected by lead exposure. Yet, the central nervous system is affected more in children while peripheral nervous system in adults [53]. In central nervous system of developing child, synapse formation in cerebral cortex is greatly affected by lead [3]. Magnetic resonance imaging of the adults which were exposed to increased lead levels during their childhood shows decreased brain volume especially in the prefrontal cortex [46]. Lead acetate can be found stored in the cerebellum disturbing its physiology as well as causing neurotoxicity, cellular deterioration and possibly cellular death [54]. In peripheral nervous system it causes peripheral neuropathy by decreasing motor activity due to loss of myelin sheath which isolates the nerves, therefore completely damages the nerve impulse transduction resulting in deficiency of muscular coordination, fatigue and muscular weakness particularly of the exterior muscles [55]. Lead poisoning also reduces the number of neurons and decreases neuronal growth [56].
Many studies were published reporting that very low BLL may impact children’s cognitive abilities like learning deficits, lowered intelligence quotient (IQ) and behavioural problems [25, 55, 57]. As reduced academic performance was found to be associated with BLL lower than 5 μg/dl [58], therefore no lower threshold can be set for the dose–response relationship below which lead exposure is treated as safe. A higher risk for developmental disabilities is seen in children with BLL greater than 10 μg/dl. It was also reported that in BLL between 5 and 35 μg/dl, there is decrement of IQ of 2–4 points for each μg/dl increase in lead levels [59]. Winneke et al. [60] in their study argued that for every 10 μg/dl increase in blood lead, there is a loss of 4–7 IQ points. At low level of lead exposure children might be hyperactive, easily irritated and non-attentive but at higher levels of lead toxicity they might have decreased intelligence, delayed growth, hearing loss possessing only short-term memory and even results in permanent brain damage and death [46]. Prenatal and early childhood exposure to lead has been shown to be associated with violent crimes in adulthood [57].
Acute encephalopathy can occur in children at BLL of 80–100 μg/dl and in adults at BLL of 100–120 μg/dl. The symptoms include irritability, agitation, headaches, confusion, ataxia, drowsiness, convulsions and coma [61, 62]. BLL from 50 μg/dl to about 100 μg/dl in adults was found to be associated with permanent impairment of central nervous system [63]. Chronic high-level lead exposure results in peripheral motor neuropathy. There is convincing evidence of peripheral nerve conduction velocity reduction at lower BLL with a proposed threshold as low as 30 μg/dl while other studies have not seen effects below 70 μg/dl [64, 65]. Neurobehavioural effects including disturbances in reaction time, visual motor performance, hand dexterity, IQ, cognitive performance, anxiety and mood have been observed in lead workers with BLL > 40 μg/100 ml [66, 67]. Baker et al. [68] showed that after reduction in BLL, individual improvement in tension, anger, depression, fatigue and confusion is observed but no substantial improvement was seen in the subtle neurophysiological test results.
Bone
Bones are the primary site for the deposition of lead in our body [69]. Lead is thought to be deposited in two segments in the bones. Cortex, the site for deposition of the non-exchangeable pool is present deep in the bone and the exchangeable pool is found at the bone surface. Lead from exchangeable pool is actively re-absorbed and may get easy access to plasma. After this mobilization, lead from the non-exchangeable pool is shifted to the surface [16]. After sustained exposure of lead for years, a much slower clearance takes place due to prolonged buildup of lead in bones which is re-absorbed into blood over a long period of time [29]. In adults, stable lead isotope procedure presented that nearly 40–70% of the re-absorbed lead in the blood is contributed by bones. Lead mobilization and storage in bones depends upon number of factors such as age, dose, rate of pregnancy, exposure to lead, race, and gestation [41]. Half-life of blood lead is just about 40 days in humans which is increased in the case of pregnant women and children whose bones are in a developing stage. The developing bones in children which go through remodeling allow the lead to be constantly reinstated into the blood stream [15].
Effect on Reproductive System
In women, lead toxicity leads to miscarriage, pregnancy hypertension, infertility, premature membrane rupture, premature delivery and pre-eclampsia [30]. Increased BLL are associated with delayed puberty in girls [70]. BLC > 10 μg/dl is associated with an increased risk of spontaneous abortion, premature delivery and low birth weight [5]. According to another study the risk of spontaneous abortion doubled at maternal BLL of 5–9 μg/dl [71]. In a study, Zhang et al. [72] investigated lead interaction with human chorionic gonadotropin (HCG) and indicated that lead acetate changed the secondary structure of HCG by loosening and destruction of the HCG skeleton and by increasing the hydrophobicity around Tyr residues which resulted in decreased bioactivities of HCG suggesting a direct interactions of lead with sex hormones and suggesting a possible mechanism of lead induced reproductive toxicity at molecular level. Bones store the highest content of lead, metabolic changes during pregnancy mobilises the lead from bones into the blood thus increasing the toxic effects of lead [3]. Pre-natal exposure to maternal BLL of 14 μg/dl may be subjected to increase the possibility of reduced birth weight and premature birth and may be related with an increased hazard of minor developmental abnormalities [57, 73] as higher amount of learning disabilities among school children with biological parents who were lead-poisoned as children 50 years back, is observed [74]. Direct effect of lead on the progressive stages of the foetus was also studied during the gestation period [75].
General effects of lead toxicity on reproductive system in men include: abnormal spermatogenesis (decreased number and motility), reduced libido, abnormal prostatic function, chromosomal damage, changes in serum testosterone and infertility [41]. Lead directly targets testicular spermatogenesis and also the sperms in the epididymis [76]. A number of studies concluded that in men BLL > 40 μg/dl leads to impairment of reproductive functions such as low libido, low semen volume and sperm counts, increased abnormal sperm morphology and decreased sperm motility [17, 77, 78]. Significant effects on reproductive capacity are unlikely below a BLL of around 50 μg/dl but blood lead concentrations > 40 μg/dl may affect sperm morphology and function [5]. In a study on samples of semen and blood from battery manufacturing factory workers, an opposite association between sperm concentration and volume and BLL was observed [77].
Effects on Kidneys
Environmental lead exposure even at low levels is associated with accelerated worsening of chronic renal insufficiency [79]. Renal tubular damage with glycosuria and aminoaciduria can arise as a result of exposure to high lead levels [13]. Energy-dependent processes such as tubular transport is impaired as lead accumulates in the mitochondria [5]. There are two types of nephropathies caused by lead toxicity: Acute and chronic. Morphological changes seen in acute nephropathy are the degenerative changes in the tubular epithelium, presence of nuclear enclosure bodies (which contain lead protein complexes) and functional change is impaired tubular transport. Appearance of protein in the urine is not because of these changes, but it could increase a nonstandard secretion of amino acids, phosphates and glucose, known as Fanconi’s syndrome. However irreversible morphological and functional changes categorized by tubulointerstitial and glomerular variations resulting in hyperuricemia, hypertension and renal breakdown may be seen in chronic nephropathy [43]. Carmignani et al. [80] stated that lead exposure caused pathological changes in the renal system of both men and animals result in renal toxicity due to oxidative stress caused by lead. But some studies suggested that clinically significant damage to kidneys takes place only after long-term exposure and that significant kidney damage does not usually occur in asymptomatic/acute cases. Renal toxicity can be reversible or irreversible, reversible commonly discovered after short term serious exposure of lead acetate in children but irreversible interstitial nephropathy usually noticed during chronic exposure to lead [81]. Lead toxicity leads to histopathological changes in the renal proximal tubular epithelium and results in interstitial nephritis which is usually related to high blood pressure [82].
A study suggests that BLL > 60 μg/dl may cause renal dysfunction but it may be observed even at a low BLL (~10 μg/dl) [29]. A linear correlation between BLL and renal dysfunction was witnessed in the range < 40 to > 70 μg/dl in a study conducted in South Africa but other studies including a large study in smelter workers show no correlation between BLL and sensitive indicators of tubular and glomerular damage [83, 84]. The lowermost level at which lead has an adverse consequence on kidney function is hence not known. Probability of renal damage at lower BLL is also revealed in population studies. A cross-sectional analysis showed that a 10 μg/dl increase in BLL was associated with a 9% fall in creatinine clearance and a longitudinal study projected 0.08 mg/dl increase in serum creatinine levels for tenfold increase in BLL [85, 86].
Cardiovascular System
Lead is projected to alter the permeability of blood vessels and collagen synthesis [87]. Acute and chronic lead poisoning leads to vascular and cardiac damage along with possible serious consequences such as cardiovascular illnesses and hypertension [78]. A study on 220 lead battery workers showed a sturdier association between blood lead and hypertension in 30% of the study population who have a particular type of the ATP1A2 gene [88]. But there is no clear indication of an adverse influence of lead on workers with BLL below 40 μg/dl. The effect of lead exposure on blood pressure remains contentious and there are no relevant new studies to explain this.The studies which have been reported are relatively on significantly smaller populations and the findings of these should also be studied on the larger populations. Many of the researchers have not taken into account confounding variables such as alcohol intake, hemoglobin, obesity and smoking that have a much larger impact on blood pressure [89]. A correlation between BLL and systolic blood pressure was reported in black men and women but not in Caucasians [90].
Carcinogenicity
The International Agency for Research on Cancer (IARC) has classified inorganic lead compounds as Group 2A carcinogens as they are expected to cause human cancer [91, 92] and tetraethyl lead (organic lead) as a category 3 carcinogen. There is only a single study so far published to support this classification of organic lead as carcinogen in which excess of rectal cancers were observed in production workers [93]. In order to justify the carcinogen status of organic lead compounds, it must be noted that these compounds are metabolized in the body into a number of metabolites including inorganic lead (Group 2A carcinogen) prior to excretion in the urine. The National Toxicity Programme, USA, stated that lead is reasonably expected to be a human carcinogen based on inadequate human studies but ample animal laboratory data and the US Environmental Protection Agency in 2011 stated that lead is a probable human carcinogen [94]. Some analyses on lead-exposed workers have showed variable results with some showing little association with lung cancers while there may be bias because of strong confounders like smoking and arsenic exposure [95]. A study conducted in Canada has been unsuccessful to show any association between lung cancer and exposure to inorganic or organic lead compounds [96]. Some studies have also established an excess of stomach cancers but again confounders such as Helicobacter pylori were not considered [94–96]. There is evidence to suggest an excess of renal cancers but the extremes have been very small and very close to expected levels and studies on central nervous system and brain cancer have failed to show any reliable results. Animal studies have shown good evidence of renal tumors, mainly with exposure to lead acetate [5].
Molecular Basis
ALAD gene polymorphisms are known to influence the BLL and thus directly affect the susceptibility of individuals to lead poisoning. Lead strongly inhibits ALAD enzyme stoichiometrically and at the molecular level, displacing a zinc ion at the metal binding site producing inhibition through a change in the enzyme’s quaternary structure. This results in ALA build-up that can be easily detected in plasma and urine even at BLL < 10 μg/dl [97, 98]. Additionally, this accumulated ALA can stimulate δ-aminobutyric acid receptors in the nervous system owing to its resemblance to δ-aminobutyric acid thus accounting to one of the primary mechanisms of lead-induced neurotoxicity [99]. Another genetic polymorphism study was conducted with respect to vitamin D allele. It was observed that the subjects with BB or Bb allele had significantly raised BLL than those having bb allele [100]. In another study, individuals who were homozygous or heterozygous for ALAD2, had higher BLL than those with ALAD1. These authors concluded that ALAD and vitamin D receptor genes modify lead toxicokinetics [101]. The adverse effects of lead poisoning on the hematopoietic system are due to impairments in the pathway of haemoglobin synthesis disrupting the expression of ALAD, ALAS and ferrochelatase genes [102]. Lead exposure also results in DNA strand breaks induced due to ROS production. This process further leads to replacement of zinc in the DNA binding proteins [103].
Treatment Strategy
Right after the diagnosis of lead poisoning, the first and most critical step prior to the treatment is the removal of patient(s) from the site of exposure followed by elimination of possible sources of lead. The Advisory Committee on Childhood Lead Poisoning Prevention (ACCLPP) - CDC, USA has provided a detailed action plan to fight lead toxicity [19]. Based on the BLL, it has divided the patients into three categories and emphasis on education and treatment strategy has been elaborated (Table 3).
Table 3.
Recommended action plan by ACCLPP for the treatment strategy for the lead poisoning based on BLL for children
| <Reference values | ≥Reference value of 5–≤45 μg/dl | ≥45–≤69 μg/dl | ≥70 μg/dl |
|---|---|---|---|
| Lead education | Lead education | Lead education | Hospitalise and commence chelation therapy (following confirmatory venous blood lead test) in conjugation with consultation from a medical toxicologist or a paediatric environmental health speciality unit. Proceed according to actions of ≥45–≤69 μg/dl |
| Dietary | Dietary | Dietary | |
| Environmental | Environmental | Environmental | |
| Environmental assessment of pre-1978 housing | Follow-up blood lead monitoring | Follow-up blood lead monitoring | |
| Complete history and physical exam | Complete history and physical exam | ||
| Lab work: iron status | Lab work: iron status | ||
| Consider hemoglobin or hemocrit | Free erythrocyte protoporphyrin | ||
| Environmental investigation | Environmental investigation | ||
| Lead hazard reduction | Lead hazard reduction | ||
| Neurodevelopmental monitoring | Neurodevelopmental monitoring | ||
| Abdominal X-ray (if particulate lead ingestion is suspected) with bowel decontamination if indicated | Abdominal X-ray with bowel decontamination if indicated | ||
| Oral chelation therapy | |||
| consider hospitalization if lead safe environment cannot be assured |
Adapted from CDC [19]
Chelation Therapy
The treatment therapy for lead poisoning includes chelation therapy. Chelating agents are organic or inorganic compounds capable of binding metal ions to form complex structures called ‘chelates’ [104]. Thus these agents specifically remove the lead from desired site resulting in reduction of body lead burden. The most significant benefit of the chelation therapy includes the effectiveness against acute poisoning. Other benefits are that they form non-toxic complexes, remove lead from soft tissues and that the oral therapy is available [104]. Some commonly used chelating agents for treating lead poisoning are provided in Table 4 with details on indication and dosage.
Table 4.
Commonly used chelating agents to treat lead poisoning
| Sr. No. | Chelating agent | Indication | Dosage |
|---|---|---|---|
| 1. | Dimercaprol | BLL ≥ 70 μg/dl (Adjunct with CaNa2EDTA if BLL > 100 μg/dl) | 4–5 mg/kg every 4 h for 3–5 days |
| 2. | CaNa2EDTA | BLL ≥ 45 μg/dl or lead encephalopathy | 1000 mg/m2day of 50 mg/kg for 5 days (maximum 1 g/day) IV (in normal saline or D5 W) over 8–12 h IM every 8–12 h Could be repeated after 2-4 day rest period |
| 3. | D-penicillamine | BLL 45–69 μg/dl | Adults: 250 mg every 6 h Children > 6 months: 10–15 mg/kg for 4-12 weeks |
| 4. | DMSA | BLL 45–69 μg/dl | 10 mg/kg or 350 mg/m2 every 8 h for 5 days: then reduce to every 12 h for 14 days. Could be repeated after a 2-week rest period 30 mg/kg/day for at least 5 days with at least 1-week rest period (more efficacious) Not recommended for children <12 months |
Adapted and modified from Kianoush et al. [8]
New Strategies in the Treatment
To overcome the side effects of chelation therapy in lead poisoning, new strategies with alternative or adjunct therapy for the standard pharmacological treatment are being explored [3, 8, 104]. The commonly observed side-effects include: redistribution of toxic metal, essential metal loss, non removal of metal from intracellular sites, hepatotoxicity and nephrotoxicity, poor clinical recovery, pro-oxidant effects, headache, nausea and increased blood pressure. Either two different chelating agents are used or chelating agents are supplemented with anti-oxidants, micronutrients or herbal extracts. In the Table 5, benefits of respective therapeutic strategies are tabulated.
Table 5.
Benefits of new therapeutic strategies for treatment of lead poisoning
| Sr. No. | Therapeutic strategy | Benefits |
|---|---|---|
| 1. | Development of newer chelating agents | Better therapeutic efficacy Access to intracellulary bound metals Lesser adverse drug reactions Better specificity |
| 2. | Combination therapy with two chelating agents | Better chelation efficacy Removal of intra- and extra-cellular metals Prevents metal redistribution Reduction in dose Lesser adverse effects |
| 3. | Chelating agent + Antioxidants | Metal chelation and protection against ROS Reestablish pro/antioxidant status Protects from oxidative stress |
| 4. | Chelating agent + Micronutrients | Modifies toxicokinetics of metals Replenish essential metal loss Cofactors for crucial antioxidant and metabolizing enzymes |
| 5. | Chelating agent + Herbal extract | Plant extracts have been shown to potentiate the efficacy of chelating of agents Herbal drugs are safer according to traditional claims Herbal extracts the benefits of natural chelation properties and antioxidant benefits |
Adapted and modified from Flora and Pachauri [104]. For detailed examples of drugs, please refer to original article
As discussed at length in pathophysiology, the main mechanism by which lead imparts its toxic effects is by enhancing oxidative stress. The main focus of the alternative drugs besides removing lead from the body is to manage oxidative stress caused by lead toxicity. In order to advocate their safety and efficacy as alternate or adjunct therapy for standard pharmacological treatment, more evidences are needed. Following alternative treatments are used:
Thiamine (25–50 mg/kg/day) along with CaNa2EDTA (50 mg/kg/day) for 3 days reduces the oxidative damage [105] in addition to boosting the lead elimination from liver and kidney [106, 107]. Vitamins C and E are also used to diminish lead-induced oxidative stress [105, 108]. N-acetylcysteine (NAC) (800 mg/kg/day) for 5 weeks [109] acts as lead chelator but it also has antioxidant properties as it has reactive groups like amine, thiol and hydroxyl [109, 110]. Infact, according to an in vitro study, N-acetylcysteineamide is preferred alternative than NAC as it has a better cellular permeability, systemic bioavailability, and binding affinity to lead than NAC [110]. Taurine (50-100 mg/kg once daily) with a standard thiol chelator (DMSA 50 mg/kg once daily) for 5 days is effective treatment against sub-chronic lead poisoning to reduce lead burden. However single therapy with Taurine is not able to impart the same effects [111]. Other sulphur containing compounds such as Alpha-lipoic acid (50 mg/kg/day), methionine (100 mg/kg/day) and homocysteine (25 mg/kg/day) administered for 5 weeks are also able to decrease the oxidative stress in lead-exposed tissues [109].
Garlic (Allium sativum) has demonstrated to be significantly effective in reducing BLL in patients with chronic lead poisoning. Dried powdered garlic (400 mg; equivalent to 1200 μg allicin or 2 g fresh garlic) administrated 3 times a day for 4 weeks in a controlled double-blind clinical trial on 117 battery plant workers showed that the neurological effects of lead such as irritability and headache had decreased. Even prevalence of adverse effects was significantly lesser in the garlic group compared to those on treatment with D-penicillamine (250 mg/3 times daily) [112]. Treatment with coriander (Coriandrum sativum) was studied on children having high BLL (163.81 ± 57.19 μg/L) and it was observed that it significantly decreased BLL by supposedly causing renal clearance of lead. But it would be prudent to mention here that more studies are warranted to see the effect of coriander in human lead poisoning [113].
Lead Poisoning: Present Status in India
The National Referral Centre for Lead Poisoning in India (NRCLPI) was established in Bangalore in 2003 to create awareness amongst the general public and to conduct research on this preventable social hazard. Realizing the potential of the outreach of school teachers, NRCLPI with the support of Quality Council of India has initiated mass sensitization programs to educate them regarding lead poisoning. Many national level programmes as mentioned below have been initiated by NRCLPI supported by Quality Council of India [114].
Lead Awareness Programme (LAP) for the general public.
Lead Educator Programme (LEADer) for school teachers.
Short term undergraduate student research projects to kindle the scientific tempo in the student community.
Initially in 1997, a very important study conducted by Dr. T. Venkatesh, now known as ‘The Lead Man of India’, a Prof. at St. Jones National Academy of Health Sciences marked the beginning of involvement of state in fight against lead poisoning. In this study, across the seven metros, BLL of 23,000 children under 12 yrs of age was investigated and 51.3% children had >10 μg/dl, levels which negatively affect the IQ of children [115]. This study was instrumental in introduction of unleaded gasoline in India in 2000 that lead to reduction of lead poisoning in India [116].
PVC pipes that are manufactured by not ensuring the highest quality standards are one of the serious sources of lead poisoning through water as these are used for plumbing and irrigation etc. The Bureau of Indian Standards has laid down quality standards for the same. But as there is no law that prohibits the manufacturing of non-standard products, being cheap, such products find their easy way into the market. Rampant use of these due to ignorance and easy availability has resulted in striking amount of contamination of drinking water with lead in many Indian cities such as Delhi, Kolkata, Kochi, Mumbai, Pune, Nagpur, Nashik and Guwahati [7]. In order to sensitize the health workers about lead poisoning, the National Rural Health Mission initiated an awareness programme for doctors, health workers, and local body representatives across Kerala [117]. According to a 2013 news article published in Times of India, a study conducted by the doctors of Kolkata indicated at least 20% of the city’s children had alarmingly high levels of lead contamination [118].
Accounting to sensitization programs by NRCLPI and arising awareness amongst general public, eco-friendly cultural festivities are now in place. On the advice of NRCLPI, Government bodies have issued notification regarding health hazards of leaded paints; the idols for celebrating Durga Puja or Ganesh Chaturthi are now either being made with unleaded paints or unpainted water soluble eco-friendly material is being used [114]. Realizing the significance of unleaded paints in controlling lead poisoning, Govt. of India, Ministry of Environment, Forest and Climate Change on November 1, 2016 notified that the permissible lead content in the household decorative paints should not exceed 90 ppm under the rule called the Regulation of Lead Contents in Household and Decorative Paints Rules, 2016 [119].
In a high profile case, in 2015, some samples of Maggi noodles from Nestle were found to have almost 17 times high lead than permissible limits [120]. Following this, the Food Safety and Standards Authority of India was ordered by Union Minister to conduct nationwide lab tests on Maggi samples [120]. These tests confirmed presence of alarmingly high lead content which resulted in ban of the Maggi noodles after an intervention from Government and Supreme Court. This forced Nestle to withdraw and destroy Maggi worth 320 crores [121]. It was reintroduced in the markets post a gap of 5 months and only after clearance through lab tests conducted under the supervision of Supreme Court [122].
Prevention
The effective and formal control of exposure of lead workers started only after the pioneering occupational health work of Ronald Lane in 1949 [123]. What he quoted in his paper 68 years ago still stands true. He mentioned that ‘There is only one way to prevent lead poisoning and one way only and that is to make the process safe.’ He also stated that ‘The importance of education of the worker himself must be stressed. He must understand fully the dangers of his work. No attempt must be made to hide it or to minimise it but he must, at the same time be shown his own responsibilities in any safety programme. This needs patience and hard work on the part of the doctor. Complete success will be impossible without the co-operation of the workman.’
Today also the best approach remains to avoid exposure to lead and it is relieving yet important to note that this serious health issue is completely preventable. Preventing lead poisoning before its occurrence is crucial because lead-induced neurocognitive deficits amongst young children are irreversible. The need for prevention is further stressed upon by the concerns about lead remobilization from bones and adverse drug reactions. Children should be educated to frequently wash their hands, discouraged from putting their hands in the mouth. They should constantly be kept under observation if they are residing in old buildings having leaded paint to keep from ingesting the paint chips. Frequent vacuuming and eliminating the use of lead containing objects like jewellery and blinds in the house can also prove helpful. House pipes containing lead or plumbing solder fitted in old houses should be replaced to avoid lead contamination through drinking water. Discontinued usage of leaded gasoline and paints has already been significantly successful in lowering the BLL in America [17]. To regulate and control occupational lead exposure, strict adherence to industry safety regulations is necessary to limit the same. Control of temperature, reduction of aerosol, dust, or fume production, mechanization of the workplace and sufficient local and general ventilation would be helpful in decreasing lead contamination. In addition, worksite safety and personal hygiene i.e. proper use of protective clothes and equipment, is critical to the prevention of occupational lead poisoning. Workers and family members should also be educated to prevent take-home lead poisoning [124].
Some dietary deficiencies increase the risk of lead poisoning in children. For example, iron deficiency is associated with elevated BLL. Therefore CDC recommends that children with lead poisoning be placed on iron rich diet [125]. In addition, some studies suggest that iron is able to decrease BLL in children with or without iron deficiency [125]. Calcium (1200 mg/day of elemental calcium as calcium carbonate) inhibits the lead absorption and decreases its bioavailability [6, 126]. It also decreases the lead content in mother’s breast milk [127]. Zinc deficiency worsens the toxicity caused by lead poisoning. Zinc, vitamin C, protein and phosphorus deficiency increase the absorption of ingested lead [125, 126]. Thus it is mandated that these deficiencies be addressed so as to fight the probable lead toxicity as well. Identification of susceptibility through population-based screening methods based on susceptible gene polymorphisms can be of prognostic significance to contain the lead toxicity [128].
Conclusion
Through the plethora of literature discussed in the manuscript so far, it is clear that lead poisoning is a serious yet preventable social environmental hazard. Its treatments though are available but prevention remains most important as the negative neurotoxicological effects manifested in the growing children, pregnant women and foetus cannot be reversed. Because most of the times it is asymptomatic or mimics the characters of some other poisonings/deficiencies, thorough investigations are needed to be done. The most common treatment method i.e. chelation therapy also has its own drawbacks and in most cases rebound or requirement of repeated courses become mandatory. Significant involvement of the State in educating the citizens, constant screening for any probable toxicity and implementation of preventive measures by imparting strict laws seems to be the most effective method to tackle this hazard. Commendable significant initiatives have been taken by the Government of India by enacting the laws against usage of unleaded petrol restricting the amount of lead in paints and initiating other programmes. Still we need to cover a long distance in order to make the environment safe for our health and for the coming generations.
Abbreviations
- ACCLPP
Advisory Committee on Childhood Lead Poisoning Prevention
- ALA
δ-Aminolevulinic acid
- ALAD
δ-Aminolevulinic acid dehydratrase
- ALAS
δ-Aminolevulinic acid synthetase
- BBB
Blood brain barrier
- BLL
Blood lead level
- CAT
Catalase
- CDC
Centres for Disease Control and Prevention
- EP
Erythrocyte protoporphyrin
- GSH
Reduced glutathione
- GST
Glutathione-S-transferase
- HCG
Human chorionic gonadotropin
- IARC
International Agency for Research in Cancer
- IQ
Intelligence quotient
- NAC
N-acetyl cysteine
- NRCLPI
National Referral Centre for Lead Poisoning in India
- RBC
Red blood cell
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
Author Contributions
KKS conceptualized the idea of the review, guided through it, critically read and organized the final manuscript. CS, KT and AS researched the data and drafted the manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors have no conflict of interest with this manuscript.
References
- 1.D’souza HS, Dsouza SA, Menezes G, Venkatesh T. Diagnosis, evaluation, and treatment of lead poisoning in general population. Indian J Clin Biochem. 2011;26:197–200. doi: 10.1007/s12291-011-0122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ChemID plus . Lead and lead compounds. Bethesda, MD: U.S. National Library of Medicine; 2005. [Google Scholar]
- 3.Wani AL, Ara A, Usmani JA. Lead toxicity: a review. Interdiscip Toxicol. 2015;8:55–64. doi: 10.1515/intox-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress Part. I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 5.Gidlow DA. In-depth review: lead toxicity. Occup Med. 2015;65:348–356. doi: 10.1093/occmed/kqv018. [DOI] [PubMed] [Google Scholar]
- 6.Chandran L, Cataldo R. Lead poisoning: basics and new developments. Pediatr Rev. 2010;31:399–405. doi: 10.1542/pir.31-10-399. [DOI] [PubMed] [Google Scholar]
- 7.Multani A. Lead contamination of drinking water in India due to PVC pipes. LEAD Action News. 2001;10(1):12–15. [Google Scholar]
- 8.Kianoush S, Sadeghi M, Balali-Mood M. Recent advances in the clinical management of lead poisoning. Acta Med Iran. 2015;53:327–336. [PubMed] [Google Scholar]
- 9.Sanborn MD, Abelsohn A, Campbell M, Weir E. Identifying and managing adverse environmental health effects: 3. Lead exposure. CMAJ. 2002;166:1287–1292. [PMC free article] [PubMed] [Google Scholar]
- 10.Agency for Toxic Substances and Disease Registry Case Studies in Environmental Medicine (CSEM) Lead Toxicity. Atlanta: US Department of Health and Human Services, Public Health Service; 2010. [Google Scholar]
- 11.Why barns are red: the health risks from lead and their prevention. A resource manual to promote public awareness. Metropolitan Toronto Teaching Health Units and the South Riverdale Community Health Centre, Toronto; 1995.
- 12.Bellinger D, Leviton A, Rubinovitz M, Needleman H, Waternaux C. Longitudinal analysis of prenatal and postnatal lead exposure and early cognitive development. N Engl J Med. 1987;316:1037–1043. doi: 10.1056/NEJM198704233161701. [DOI] [PubMed] [Google Scholar]
- 13.Loghman-Adham M. Renal effects of environmental and occupational lead exposure. Environ Health Perspect. 1997;105:928–938. doi: 10.1289/ehp.97105928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karri SK, Saper RB, Kales SN. Lead encephalopathy due to traditional medicines. Curr Drug Saf. 2008;3:54–59. doi: 10.2174/157488608783333907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbosa F, Jr, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect. 2005;113:1669–1674. doi: 10.1289/ehp.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrick L. Lead toxicity Part. II: The role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev. 2006;11:114. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention/National Center for Environmental Health. Publications List; Nov 25, 2011.
- 18.Update: blood lead levels-United States, 1991–1994. Morbidity and Mortality Weekly Report. 1997;46:141–6. [PubMed]
- 19.Advisory committee on childhood lead poisoning prevention (ACCLPP). CDC; 2012.
- 20.Occupational safety and health guideline for inorganic lead. CDC: U.S. Department of Health and Human Services; 1988.
- 21.Patocka J. Organic lead toxicology. Acta Med. 2008;51:209–213. [PubMed] [Google Scholar]
- 22.Nevin R. Understanding international crime trends: the legacy of preschool lead exposure. Environ Res. 2007;104:315–336. doi: 10.1016/j.envres.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 23.American Academics of Pediatrics Lead exposure in children: prevention, detection and management. Pediatrics. 2005;116:1036–1046. doi: 10.1542/peds.2005-1947. [DOI] [PubMed] [Google Scholar]
- 24.Wetmur JG. Influence of the common human δ-aminolevulinate dehydratase polymorphism on lead body burden. Environ Health Perspect. 1994;102:215–219. doi: 10.1289/ehp.94102s3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strayer DS, Rubin E. Environmental and nutritional pathology. In: Rubin R, Strayer DS, editors. Rubins pathology; Clinicopathologic foundations of medicine. 5. Philadelphia: Lippincot; 2008. pp. 253–284. [Google Scholar]
- 26.Ibrahim NM, Eweis EA, El-Beltagi HS, Abdel- Mobdy YE. Effect of lead acetate toxicity on experimental male albino rat. Asian Pac J Trop Biomed. 2012;2:41–46. doi: 10.1016/S2221-1691(11)60187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosnett MJ. Lead. In: Olson KR, editor. Poisoning and drug overdose. 5. New York: McGraw Hill Professional; 2006. [Google Scholar]
- 28.Kosnett MJ, Wedeen RP, Rothenberg SJ, Hipkins KL, Materna BL, Schwartz BS, et al. Recommendations for medical management of adult lead exposure. Environ Health Perspect. 2007;115:463–471. doi: 10.1289/ehp.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant LD. Lead and compounds. In: Lippmann M, editor. Environmental toxicants: human exposures and their health effects. 3. Hoboken, NJ: Wiley; 2008. pp. 757–809. [Google Scholar]
- 30.Flora SJ. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Flora SJS. Nutritional components modify metal absorption, toxic response and chelation therapy. J Nutr Environ Med. 2002;12:53–67. [Google Scholar]
- 32.Hultberg B, Andersson A, Isaksson A. Interaction of metals and thiols in cell damage and glutathione distribution: potentiation of mercury toxicity by dithiothreitol. Toxicology. 2001;156:93–100. doi: 10.1016/s0300-483x(00)00331-0. [DOI] [PubMed] [Google Scholar]
- 33.Ahamed M, Siddiqui MKJ. Low level lead exposure and oxidative stress: current opinions. Clin Chim Acta. 2007;383:57–64. doi: 10.1016/j.cca.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Flora SJ, Saxena G, Mehta A. Reversal of lead-induced neuronal apoptosis by chelation treatment in rats: role of reactive oxygen species and intracellular Ca2+ J Pharmacol Exp Ther. 2007;322:108–116. doi: 10.1124/jpet.107.121996. [DOI] [PubMed] [Google Scholar]
- 35.Corradi M, Goldoni M, Sabbadini M. Acute lead poisoning: a singular case of haemolytic anemia and lead colic. Med Lav. 2011;102:243–249. [PubMed] [Google Scholar]
- 36.Kiziler AR, Aydemir B, Onaran I, Alici B, Ozkara H, Gulyasar T, et al. High levels of cadmium and lead in seminal fluid and blood of smoking men are associated with high oxidative stress and damage in infertile subjects. Biol Trace Elem Res. 2007;120:82–91. doi: 10.1007/s12011-007-8020-8. [DOI] [PubMed] [Google Scholar]
- 37.Kasperczyk S, Birkner E, Kasperczyk A, Zalejska-Fiolka J. Activity of superoxide dismutase and catalase in people protractedly exposed to lead compounds. Ann Agric Environ Med. 2004;11:291–296. [PubMed] [Google Scholar]
- 38.Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O. Lack of reversal of oxidative damage in renal tissues of lead acetate treated rats. Environ Toxicol. 2015;30:1235–1243. doi: 10.1002/tox.21994. [DOI] [PubMed] [Google Scholar]
- 39.Vaziri ND, Khan M. Interplay of reactive oxygen species and nitric oxide in the pathogenesis of experimental lead-induced hypertension. Clin Exp Pharmacol Physiol. 2007;34:920–925. doi: 10.1111/j.1440-1681.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- 40.Roy A, Queirolo E, Peregalli F, Manay N, Martinez G, Kordas K. Association of blood lead levels with urinary F2-8α isoprostane and 8-hydroxy-2-deoxy-guanosine concentrations in first-grade Uruguayan children. Environ Res. 2015;140:127–135. doi: 10.1016/j.envres.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Assi MA, Hezmee MNM, Haron AW, Sabri MYM, Rajion MA. The detrimental effects of lead on human and animal health. Vet World. 2016;9:660–671. doi: 10.14202/vetworld.2016.660-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, et al. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Baranowska-Bosiacka I, Gutowska I, Rybicka M, Nowacki P, Chlubek D. Neurotoxicity of lead. Hypothetical molecular mechanisms of synaptic function disorders. Neurol Neurochir Pol J. 2012;46:569–578. doi: 10.5114/ninp.2012.31607. [DOI] [PubMed] [Google Scholar]
- 44.Vij AG, Dhundasi SA. Hemopoietic, hemostatic and mutagenic effects of lead and possible prevention by zinc and vitamin C. Al Ameen J Med Sci. 2009;2:27–36. [Google Scholar]
- 45.Ahamed M, Verma S, Kumar A, Siddiqui MKJ. Environmental exposure to lead and its correlation with biochemical indices in children. Sci Total Environ. 2005;46:48–55. doi: 10.1016/j.scitotenv.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 46.Cleveland LM, Minter ML, Cobb KA, Scott AA, German VF. Lead hazards for pregnant women and children. Am J Nurs. 2008;108:40–49. doi: 10.1097/01.NAJ.0000339156.09233.de. [DOI] [PubMed] [Google Scholar]
- 47.Fujita H, Nishitani C, Ogawa K. Lead, chemical porphyria, and heme as a biological mediator. Tohoku J Exp Med. 2002;196:53–64. doi: 10.1620/tjem.196.53. [DOI] [PubMed] [Google Scholar]
- 48.Casaret LJ, Klaassen CD, Doull J, editors. Toxic effects of metals. Casarett and Doull’s toxicology: the basic science of poisons. 7. New York: McGraw Hill Professional; 2007. [Google Scholar]
- 49.Garza A, Vega R, Soto E. Cellular mechanisms of lead neurotoxicity. Med Sci Monit. 2006;12:RA57–RA65. [PubMed] [Google Scholar]
- 50.Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126:5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- 51.Bressler J, Kim KA, Chakraborti T, Goldstein G. Molecular mechanisms of lead neurotoxicity. Neurochem Res. 1999;24:595–600. doi: 10.1023/a:1022596115897. [DOI] [PubMed] [Google Scholar]
- 52.Kosnett MJ. Lead. In: Brent J, editor. Critical care toxicology: diagnosis and management of the critically poisoned patient. Houston: Gulf Professional Publishing; 2005. p. 822. [Google Scholar]
- 53.Brent JA. Review of medical toxicology. J Clin Toxicol. 2006;44:355. [Google Scholar]
- 54.Mtui E, Gruener G, FitzGerald MJT. Clinical neuroanatomy and neuroscience. Philadelphia, PA: Elsevier Health Sciences; 2011. [Google Scholar]
- 55.Sanders T, Liu Y, Buchner V, Tchounwou PB. Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health. 2009;24:15–46. doi: 10.1515/reveh.2009.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearson HA, Schonfeld DJ. Lead. In: Rudolph CD, editor. Rudolph’s pediatrics. 21. New York: McGraw-Hill Professional; 2003. pp. 1016–1056. [Google Scholar]
- 57.Park SK, O’Neill MS, Vokonas PS, Sparrow D, Wright RO, Coull B, et al. Air pollution and heart rate variability: effect modification by chronic lead exposure. Epidemiology. 2008;19:111–120. doi: 10.1097/EDE.0b013e31815c408a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellinger DC. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008;20:172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- 59.Brunton LL, Goodman LS, Blumenthal D, Buxton I, Parker KL, editors. Goodman and Gillmans manual of pharmocology and theraupetics. New York: McGraw Hill Professional; 2007. [Google Scholar]
- 60.Winneke G, Brockhaus A, Ewers U, Kramer U, Neuf M. Results from the European multicenter study on lead neurotoxicity in children: implications for risk assessment. Neurotoxicol Teratol. 1990;12:553–559. doi: 10.1016/0892-0362(90)90022-5. [DOI] [PubMed] [Google Scholar]
- 61.International Programme on Chemical Safety (IPCS) Evaluation—monograph on lead, inorganic. Geneva: World Health Organization; 2007. [Google Scholar]
- 62.National Poisons Information Service (NPIS) US. Lead. TOXBASE, Health Protection Agency; 2010.
- 63.Billings RJ, Berkowitz RJ, Watson G. Teeth. Pediatrics. 2004;113:1120–1127. [PubMed] [Google Scholar]
- 64.Triebig G, Weltle D, Valentin H. Investigations on neurotoxicity of chemical substances at the workplace. V. Determination of the motor and sensory nerve conduction velocity in persons occupationally exposed to lead. Int Arch Occup Environ Health. 1984;53:189–203. doi: 10.1007/BF00398813. [DOI] [PubMed] [Google Scholar]
- 65.Davis JM, Svendsgaard DJ. Nerve conduction velocity and lead: a critical review and meta-analysis. In: Johnson BL, Anger WK, Durao A, Xinteras C, editors. Advances in neurobehavioural toxicology: applications in environmental and occupational health. Chelsea, MI: Michigan Lewis Publishers; 1990. pp. 353–376. [Google Scholar]
- 66.Stollery BT, Banks HA, Broadbent DE, Lee WR. Cognitive functioning in lead workers. Br J Ind Med. 1989;46:698–707. doi: 10.1136/oem.46.10.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanninen H, Aitio A, Kovala T, Luukkonen R, Matikainen E, Mannelin T, et al. Occupational exposure to lead and neuropsychological dysfunction. Occup Environ Med. 1998;55:202–209. doi: 10.1136/oem.55.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baker EL, White RF, Pothier LJ, Berkey CS, Dinse GE, Travers PH, et al. Occupational lead neurotoxicity: improvement in behavioural effects after reduction of exposure. Br J Ind Med. 1985;42:507–516. doi: 10.1136/oem.42.8.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Renner R. Exposure on tap: drinking water as an overlooked source of lead. Environ Health Perspect. 2010;118:A68–A72. doi: 10.1289/ehp.118-a68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schoeters G, Hond ED, Dhooge W, Larebeke NV, Leijs M. Endocrine disruptors and abnormalities of pubertal development. Basic Clin Pharmacol & Toxicol. 2008;102:168–175. doi: 10.1111/j.1742-7843.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- 71.Borja-Aburto VH, Hertz-Picciotto I, Rojas Lopez M, Farias P, Rios C, Blanco J. Blood lead levels measured prospectively and risk of spontaneous abortion. Am J Epidemiol. 1999;150:590–597. doi: 10.1093/oxfordjournals.aje.a010057. [DOI] [PubMed] [Google Scholar]
- 72.Zhang H, Liu Y, Zhang R, Liu R, Chen Y. Binding mode investigations on the interaction of lead (II) Acetate with human chorionic gonadotropin. J Phys Chem B. 2014;118:9644–9650. doi: 10.1021/jp505565s. [DOI] [PubMed] [Google Scholar]
- 73.Health Protection Agency . Lead toxicological overview. Version 3. London: H.P.A; 2012. [Google Scholar]
- 74.Hu H. Knowledge of diagnosis and reproductive history among survivors of childhood plumbism. Am J Public Health. 1991;81:1070–1072. doi: 10.2105/ajph.81.8.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saleh HA, El-Aziz GA, El-Fark MM, El-Gohary M. Effect of maternal lead exposure on craniofacial ossification in rat fetuses and the role of antioxidant therapy. Anat Histol Embryol. 2009;38:392–399. doi: 10.1111/j.1439-0264.2009.00960.x. [DOI] [PubMed] [Google Scholar]
- 76.Wadi S, Ahmad G. Effects of lead on the male reproductive system in mice. J Toxicol Environ Health A. 1999;56:513–521. doi: 10.1080/009841099157953. [DOI] [PubMed] [Google Scholar]
- 77.Telisman S, Cvitkovic P, Jurasovic J, Pizent A, Gavella M, Rocic B. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect. 2000;108:45. doi: 10.1289/ehp.0010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease-a systematic review. Environ Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu CC, Lin JL, Lin-Tan DT. Environmental exposure to lead and progression of chronic renal diseases: a four-year prospective longitudinal study. J Am Soc Nephrol. 2004;15:1016–1022. doi: 10.1097/01.asn.0000118529.01681.4f. [DOI] [PubMed] [Google Scholar]
- 80.Carmignani M, Volpe AR, Boscolo P, Qiao N, DiGioacchino M, Grilli A, et al. Catcholamine and nitric oxide systems as targets of chronic lead exposure in inducing selective functional impairment. Life Sci J. 2000;68:401–415. doi: 10.1016/s0024-3205(00)00954-1. [DOI] [PubMed] [Google Scholar]
- 81.Finley J. Compositions and methods for the prevention and treatment of diseases or conditions associated with oxidative stress, inflammation, and metabolic dysregulation. U. S. Patent No.8, 652, 518.U. S. Patent and Trademark Office, Washington, DC; 2014.
- 82.Nisar MF, Nasir I, Shaheen S, Khalid A, Tazeen N. Chronic lead acetate nephrotoxicity: a histological study on albino rats. Ann King Edw Med Univ. 2014;17:239. [Google Scholar]
- 83.Gerhardsson L, Chettle DR, Englyst V, Nordberg GF, Nyhlin H, Scott MC, et al. Kidney effects in long term exposed lead smelter workers. Br J Ind Med. 1992;49:186–192. doi: 10.1136/oem.49.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ehrlich R, Robins T, Jordaan E, Miller S, Mbuli S, Selby P, et al. Lead absorption and renal dysfunction in a South African battery factory. Occup Environ Med. 1998;55:453–460. doi: 10.1136/oem.55.7.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Staessen JA, Lauwerys RR, Buchet JP, Bulpitt CJ, Rondia D, Vanrenterghem Y, et al. Impairment of renal function with increasing blood lead concentrations in the general population. N Engl J Med. 1992;327:151–156. doi: 10.1056/NEJM199207163270303. [DOI] [PubMed] [Google Scholar]
- 86.Tsaih SW, Korrick S, Schwartz J, Amarasiriwardena C, Aro A, Sparrow D, et al. Lead, diabetes, hypertension, and renal function: the normative aging study. Environ Health Perspect. 2004;112:1178–1182. doi: 10.1289/ehp.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Needleman H. Lead poisoning. Annu Rev Med. 2004;55:209–222. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- 88.Glenn BS, Stewart WF, Schwartz BS, Bressler J. Relation of alleles of the sodium potassium adenosine triphosphatase alpha 2 gene with blood pressure and lead exposure. Am J Epidemiol. 2001;153:537–545. doi: 10.1093/aje/153.6.537. [DOI] [PubMed] [Google Scholar]
- 89.Cheng Y, Schwartz J, Sparrow D, Aro A, Weiss ST, Hu H. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension: the normative aging study. Am J Epidemiol. 2001;153:164–171. doi: 10.1093/aje/153.2.164. [DOI] [PubMed] [Google Scholar]
- 90.Vupputuri S, He J, Muntner P, Bazzano LA, Whelton PK, Batuman V. Blood lead level is associated with elevated blood pressure in blacks. Hypertension. 2003;41:463–468. doi: 10.1161/01.HYP.0000055015.39788.29. [DOI] [PubMed] [Google Scholar]
- 91.Statement on the 2006 UK Total Diet Study of Metals and Other Elements. Committee on Toxicity of Chemicals in Food Consumer Products and the Environment (COT). London: Food Standards Agency; 2008.
- 92.International Agency for the Research on Cancer (IARC). Inorganic and organic lead compounds. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 87. Lyon, France; 2006. [PMC free article] [PubMed]
- 93.Fayerweather WE, Karns ME, Nuwayhid IA, Nelson TJ. Case-control study of cancer risk in tetraethyl lead manufacturing. Am J Ind Med. 1997;3:28–35. doi: 10.1002/(sici)1097-0274(199701)31:1<28::aid-ajim5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 94.National Toxicity Program. Department of Health and Human Carcinogens. 12th ed. Department of Health and Human Services US; 2011.
- 95.Lundstrom NG, Nordberg G, Englyst V, Gerhardsson L, Hagmar L, Jin T, et al. Cumulative lead exposure in relation to mortality and lung cancer morbidity in a cohort of primary smelter workers. Scand J Work Environ Health. 1997;23:24–30. doi: 10.5271/sjweh.174. [DOI] [PubMed] [Google Scholar]
- 96.Wynant W, Siemiatycki J, Parent ME, Rousseau MC. Occupational exposure to lead and lung cancer: results from two case-control studies in Montreal, Canada. Occup Environ Med. 2013;70:164–170. doi: 10.1136/oemed-2012-100931. [DOI] [PubMed] [Google Scholar]
- 97.Wetmur JG, Kaya AH, Plewinska M, Desnick RJ. Molecular characterization of the human -aminolevulinatedehydratase 2 (ALAD2) allele: implications for molecular screening of individuals for genetic susceptibility to lead poisoning. Am J Hum Genet. 1991;49:757–763. [PMC free article] [PubMed] [Google Scholar]
- 98.Schwartz BS, Lee BK, Stewart W, Ahn KD, Springer K, Kelsey K. Associations of δ-aminolevulinic acid dehydratase genotype with plant, exposure duration, and blood lead and zinc protoporphyrin levels in Korean lead workers. Am J Epidemiol. 1995;142:738–745. doi: 10.1093/oxfordjournals.aje.a117705. [DOI] [PubMed] [Google Scholar]
- 99.Agency for Toxic Substances and Disease Registry . Interaction profile for chlorpyrifos, lead, mercury, and methyl mercury. Atlanta, GA: United State Department of Health and Human Services; 2006. [PubMed] [Google Scholar]
- 100.Jain NB, Laden F, Guller U, Shankar A, Kazani S, Garshick E. Relation between blood lead levels and childhood anemia in India. Am J Epidemiol. 2005;161:968–973. doi: 10.1093/aje/kwi126. [DOI] [PubMed] [Google Scholar]
- 101.Schwartz BS, Lee BK, Lee GS, Stewart WF, Simon D, Kelsey K, et al. Associations of blood lead, dimercaptosuccinic acid chelatable lead, and tibia lead with polymorphisms in the vitamin D receptor and [delta] aminolevulinic acid dehydratase genes. Environ Health Perspect. 2000;108:949–954. doi: 10.1289/ehp.00108949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Piomelli S. Childhood lead poisoning. Pediatr Clin North Am J. 2002;49:1285–1304. doi: 10.1016/s0031-3955(02)00097-4. [DOI] [PubMed] [Google Scholar]
- 103.Kumar A, Prasad MNV, Achary VMM, Panda BB. Elucidation of lead-induced oxidative stress in Talinumtriangulare roots by analysis of antioxidant responses and DNA damage at cellular level. Environ Sci Pollut Res. 2013;20:4551–4561. doi: 10.1007/s11356-012-1354-6. [DOI] [PubMed] [Google Scholar]
- 104.Flora SJS, Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010;7:2745–2788. doi: 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang C, Liang J, Zhang C, Bi Y, Shi X, Shi Q. Effect of ascorbic acid and thiamine supplementation at different concentrations on lead toxicity in liver. Ann Occup Hyg. 2007;51:563–569. doi: 10.1093/annhyg/mem036. [DOI] [PubMed] [Google Scholar]
- 106.Kim JS, Hamilton DL, Blakley BR, Roussraux CG. The effects of thiamine on lead metabolism: organ distribution of lead. Can J Vet Res. 1992;56:256–259. [PMC free article] [PubMed] [Google Scholar]
- 107.Najarnezhad V, Aslani MR, Balali-Mood M. The therapeutic potential of thiamine for treatment of experimentally induced sub-acute lead poisoning in sheep. Comp Clin Patol. 2010;19:69–73. [Google Scholar]
- 108.Sajitha GR, Jose R, Andrews A, Ajantha KG, Augustine P, Augusti KT, et al. Garlic oil and vitamin E prevent the adverse effects of lead acetate and ethanol separately as well as in combination in the drinking water of rats. Indian J Clin Biochem. 2010;25:280–288. doi: 10.1007/s12291-010-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caylak E, Aytekin M, Halifeoglu I. Antioxidant effects of methionine, alpha-lipoic acid, N-acetylcysteine and homocysteine on lead-induced oxidative stress to erythrocytes in rats. Exp Toxicol Pathol. 2008;60:289–294. doi: 10.1016/j.etp.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 110.Chen W, Ercal N, Huynh T, Volkov A, Chusuei CC. Characterizing N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA) binding for lead poisoning treatment. J Colloid Interface Sci. 2012;371:144–149. doi: 10.1016/j.jcis.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Flora SJ, Pande M, Bhadauria S, Kannan GM. Combined administration of taurine and meso 2, 3 dimercaptosuccinic acid in the treatment of chronic lead intoxication in rats. Hum Exp Toxicol. 2004;23:157–166. doi: 10.1191/0960327104ht432oa. [DOI] [PubMed] [Google Scholar]
- 112.Kianoush S, Balali-Mood M, Mousavi SR, Moradi V, Sadeghi M, Dadpour B, et al. Comparison of therapeutic effects of garlic and d-Penicillamine in patients with chronic occupational lead poisoning. Basic Clin Pharmacol Toxicol. 2012;110:476–481. doi: 10.1111/j.1742-7843.2011.00841.x. [DOI] [PubMed] [Google Scholar]
- 113.Deldar K, Nazemi E, Balali MM, Emami SA, MohammadPour AH, Tafaghodi M, et al. Effect of Coriandrum sativum L. extract on lead excretion in 3-7 year old children. J Birjand Univ Med Sci. 2008;15:11–19. [Google Scholar]
- 114.Venkatesh T. Lead is hampering the Quality of our lives. In: Gyani GJ, editor. Quality India. 2008. pp. 22–23. [Google Scholar]
- 115.van Alphen M. Lead poisoning in India. LEAD Action News 1999;7(1):2–3.
- 116.Singh AK, Singh M. Lead decline in the Indian environment resulting from the petrol-lead phase-out programme. Sci Total Environ. 2006;368:686–694. doi: 10.1016/j.scitotenv.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 117.Maya C. Lead poisoning: warning bells toll. The Hindu. Updated on Aug 22, 2011.
- 118.Mitra P. Lead poisoning affects 20% Kolkata kids. Times of India; Jul 14, 2013.
- 119.Regulation on Lead contents in Household and Decorative Paints Rules, 2016. The Gazzette of India, Ministry of Environment, Forest and Climate Change notification, New Delhi; Nov 1, 2016.
- 120.Chatterjee P. Explained: the controversy surrounding Maggi Noodles. Indian Express Updated on Jun 5, 2015.
- 121.After Maggi and Knorr, Top Ramen noodles withdrawn from market. Hindustan times Updated on Jun 30, 2015.
- 122.New stocks of Maggi noodles cleared by labs, likely to hit stores this month: Nestle. Indian Express. Updated on Nov 4, 2015.
- 123.Lane RE. The care of the lead worker. Br J Ind Med. 1949;6:125–143. doi: 10.1136/oem.6.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chey H, Buchanan S. Toxins in everyday life. Prim Care. 2008;35:707–727. doi: 10.1016/j.pop.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 125.Warniment C, Tsang K, Galazka SS. Lead poisoning in children. Am Fam Physician. 2010;81:751–757. [PubMed] [Google Scholar]
- 126.Woolf AD, Goldman R, Bellinger DC. Update on the clinical management of childhood lead poisoning. Pediatr Clin North Am. 2007;54:271–294. doi: 10.1016/j.pcl.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 127.Levin R, Brown MJ, Kashtock ME, Jacobs E, Whelan EA, Rodman J, et al. Lead exposures in U.S. Children, 2008: implications for prevention. Environ Health Perspect. 2008;2008(116):1285–1293. doi: 10.1289/ehp.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaik AP, Sultana SA, Alsaeed AH. Lead exposure: a summary of global studies and the need for new studies from Saudi Arabia. Dis Markers. 2014;2014:415160–415166. doi: 10.1155/2014/415160. [DOI] [PMC free article] [PubMed] [Google Scholar]