Abstract
Fungi associated with black point were isolated from three highly susceptible wheat genotypes in the North China Plain. The 21 isolates represented 11 fungal genera. The most prevalent genera were Alternaria (isolation frequency of 56.7%), Bipolaris (16.1%), and Fusarium (6.0%). The other eight genera were Curvularia, Aspergillus, Cladosporium, Exserohilum, Epicoccum, Nigrospora, Penicillium, and Ulocladium; their isolation frequencies ranged from 0.8 to 4.8%. The pathogenicity of the isolates was individually assessed in the greenhouse by inoculating wheat plants with spore suspensions. Ten of the 21 isolates caused significantly higher incidences of black point than that the controls. These isolates belonged to eight fungal species (A. alternata, B. sorokiniana, B. crotonis, B. cynodontis, C. spicifera, F. equiseti, E. rostratum, and E. sorghinum) based on morphological traits and phylogenetic analysis. The average incidences of black point in the eight fungal species were 32.4, 54.3, 43.0, 41.9, 37.2, 38.8, 50.1, and 34.1%, respectively. B. sorokiniana and A. alternata were determined to be the most important pathogens in the North China Plain based on fungal prevalence and symptom severity. This study is the first to identify E. rostratum as a major pathogen causing black point in wheat.
Electronic supplementary material
The online version of this article (10.1007/s12088-018-0709-1) contains supplementary material, which is available to authorized users.
Keywords: Triticum aestivum, Black point, Exserohilum, Alternaria, Bipolaris
Introduction
The North China Plain (NCP) is one of the most important agricultural regions in China [1]. Black point is a widespread and severe grain disease in this area, as well as other countries, and leads to economic losses for farmers [2, 3]. Black point also reduces grain quality, decreases seed germination, and inhibits seedling growth [4–6]. The incidence of black point in wheat germplasm in the NCP varies from 0.3 to 66.7%, and 62.5% of 403 germplasm studied between 2010 and 2012 were classified as susceptible under field conditions [6]. This indicates that most cultivars and new lines bred in the NCP are susceptible to black point; however, the resistance of wheat to black point varies significantly by genotype [6].
The most common approaches for controlling this disease are growing resistant varieties of wheat, cultural controls, chemical controls, and biological controls. Among these, planting resistant cultivars is the most economical and environmentally sustainable [7]. There are few genetic studies on wheat black point resistance because the pathogen is complex; which complicates breeding for disease resistance.
More than 100 fungal species have been isolated from wheat grains infected with black point. Numerous fungal genera have been associated with black point, such as Alternaria, Bipolaris, Curvularia, Drechslera, Epicoccum, Fusarium, Nigrospora, Penicillium, and Sclerotium. Of these, fungi belonging to Alternaria, Bipolaris, and Fusarium are the most widely observed [4, 5, 8, 9]. However, except Fusarium proliferatum [5], there has been no conclusive evidence that black point is a direct result of fungal infection. What’s more, the dominant pathogens and their pathogenicities in the NCP remain unknown.
To effectively screen wheat germplasm that are resistant to black point and breed wheat cultivars that are resistant to the predominant pathogens, the first step is to clarify the identities of the predominant pathogens and their pathogenicities. Here, we report on the major fungal pathogens causing black point in wheat in NCP, as well as their pathogenicities in the greenhouse.
Materials and Methods
Wheat Lines
Three highly susceptible wheat lines, one spring wheat line (Pinzhisuo6) and two semi-winter wheat lines (Wanyuanbai and Jicheng2), were used to isolate fungal species and determine their pathogenicities. These lines were selected from 403 wheat germplasm collection grown in field and exposed to black point incidence under natural conditions in the NCP [6].
Fungal Isolation
Wheat lines were planted in the experimental farm of Henan Agricultural University (longitude 113°42′E, latitude 34°44′N, elevation 111.3 m) during the 2012–2013 wheat growing season. Each of the three wheat lines was planted in two 75-plant rows. Black point kernels were collected at the GS 87 stage of the Zadoks scale [10], which is when the symptom was clearly visible. Ten spikes from each of the three replications were collected and brought to laboratory.
The diseased kernels were surface sterilized in 1% sodium hypochlorite (NaOCl) solution for 3 min, rinsed three times with sterile distilled water, and plated aseptically in separate 9 cm Petri dishes. Each Petri dish contained ten kernels from one wheat line. The Petri dishes were arranged in a completely randomized fashion, incubated at 25 °C in the dark for 9 days, and checked every 2–3 days for the growth of microorganisms.
The infected tissues were separated and placed on potato dextrose agar (PDA) medium under sterile conditions. After incubation at 25 °C in the dark for 7 days, fungi arising from the grain were sub cultured and characterized based on their colony and conidial morphologies based on the monographs of Lu [11] and the literature of Manamgoda et al. [12]. Single-spore isolations from these cultures according to Chomnunti et al. [13] yielded a total of 21 isolates.
Pathogenicity Evaluation
Conidial suspensions of the 21 isolates were prepared according Mahto et al. [14]. The concentration of the conidia in suspensions was adjusted to 3 × 105 conidia per mL. Three highly susceptible wheat lines (previously mentioned) were used to determine the pathogenicity under greenhouse conditions between 2014 and 2016.
The experiment was conducted with a randomized block design with three replicates. Each replicate consisted of 132 plants from each wheat line planted in 22 plastic basins (15-cm diameter and 20-cm depth). At Zadoks’ stage GS 65 [10], spikes were treated with conidial suspensions using a hand sprayer. Parallel controls were inoculated with sterile distilled water (CKs). Following inoculation, the spikes were covered with a transparent plastic bag for 24 h. After removing the bag, the spikes were sprayed with sterile distilled water until saturation. Water was sprayed at 8:00 a.m., 12:00 a.m., 2:00 p.m., and 4:00 p.m., each day, for 7 days.
The spikes were harvested when they reached Zadoks’ stage GS 92 [10], sun-dried, and separately threshed by hand. The number of the total and diseased kernels (the black or brown discoloration > 1 mm) was recorded and the incidences of black point were calculated and converted into percentages.
Re-isolation of Pathogens
Black point kernels were placed on PDA medium as described previously after sterilization. Each Petri dish contained six kernels inoculated with a single isolate of one wheat line. There were six replications for each isolate from each wheat line, which were arranged in a completely randomized design. A non-inoculated check (CKs) was used as a control. The sporulated fungi from each kernel were examined after incubation at 25 °C for 7 days in the dark.
Statistical Analysis
The incidences of black point were calculated as the mean of the replicates (n = 3). Differences between the 21 isolates within wheat lines were calculated using the statistical software package SPSS 13.0. Analysis of variance (ANOVA) with Duncan’s multiple range test and Dunnett’s tests method at P < 0.01 were performed. A general linear model was used to perform an interaction analysis of wheat lines, isolates, and years.
Phylogenetic Analysis
Ten isolates, causing significantly higher incidences of black point than those of the control, were chosen for species identification using phylogenetic analysis. These isolates were grown on PDA at 25 °C in the dark for 7 days. Fungal DNA was prepared according to Manamgoda et al. [15].
The ITS (internal transcribed spacers and intervening 5.8S nrDNA), GPDH (partial glyceraldehyde-3-phosphate dehydrogenase gene), and TEF (partial translation elongation factor 1-alpha gene) regions were amplified according to Manamgoda et al. [15]. The reaction volume was 25 μL, and contained: 50 ng DNA, 12.5 μL 2× Taq Master Mix, and 1 μL 10 mM of each primer. Purified PCR products were sequenced by BGI-Shenzhen.
The ITS sequences of the ten isolates were identified using BLAST (NCBI) to determine the preliminary genus of the isolates. Then, the six fungal genera out of ten isolates were separately analyzed using the available sequences in the Mycobank (http://www.mycobank.org/quicksearch.aspx) and in the literature [12, 16–19]. To distinguish closely related species, five isolates of the genus Bipolaris were subjected to a combined multi-gene analysis using ITS, GPDH, and TEF. The GenBank accession numbers of the sequences used in this study are available in Supplementary Tables S1 and S2. DNA sequences were edited and aligned using SeqMan (DNA STAR Package) and Clustal X [20].
The substitution model was chosen using the Akaike information criterion (AIC) in MrModeltest V. 2.2 [21]. The dataset was analyzed with the RAxML BlackBox online server [22] using maximum likelihood and MrBayes 3.1 for Bayesian inference [23]. To minimize clutter, highly consistent sequences from same genus were eliminated when constructing phylogenetic trees.
Results
Fungal Species Isolated from Black Point Kernels on the NCP
A total of 21 isolates were obtained from 600 black point kernels (200 kernels from each of the three highly susceptible wheat lines). Based on the characteristics of colony and conidial morphology, these isolates belonged to eleven fungal genera: Alternaria, Bipolaris, Fusarium, Curvularia, Aspergillus, Exserohilum, Epicoccum, Nigrospora, Penicillium, Cladosporium, and Ulocladium (Supplementary Table S3). Alternaria was the most prevalent genera (isolation frequency ranged from 53.0 to 61.3% for the three wheat lines), followed by Bipolaris (isolation frequency 12.7–21.0%) and Fusarium (isolation frequency 3.0–9.0%). The isolation frequencies of Curvularia, Aspergillus, Exserohilum, Epicoccum, Nigrospora, Penicillium, Cladosporium, and Ulocladium were 4.8, 3.4, 3.9, 2.7, 2.5, 2.3, 0.9, and 0.8%, respectively.
Pathogenicities of the Select Isolates
The incidences of black point in the three wheat lines induced by the 21 select isolates were significantly different, and ranged from 68.9% (Wanyuanbai inoculated with isolate Ta-BP33 in 2015) to 13.2% (Pinzhisuo6 inoculated with Ta-BP44 in 2014) (Supplementary Table S4).
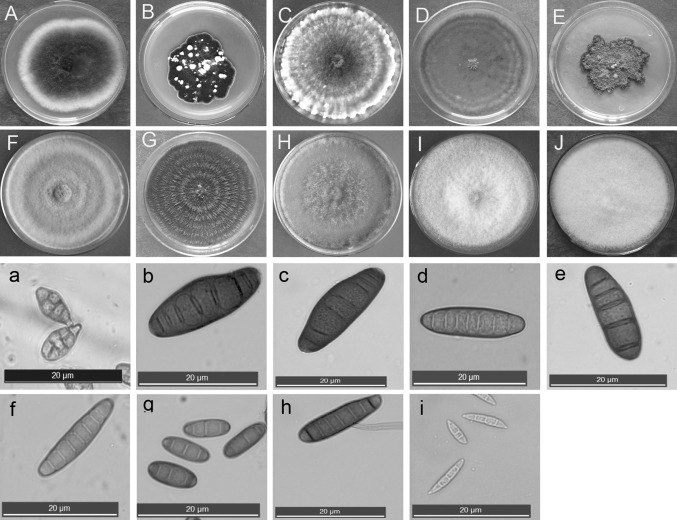
The average incidence of black point for the three wheat lines inoculated with the ten isolates used for phylogenetic analysis (Ta-BP1, Ta-BP2, Ta-BP17, Ta-BP22, Ta-BP33, Ta-BP36, Ta-BP39, Ta-BP48, Ta-BP49, and Ta-BP56) was significantly higher than that of the check over the 3 year study. Among the ten isolates, the incidence of black point was highest across all three wheat lines when inoculated with Ta-BP33 (Bipolaris; 53.7, 60.5, and 55.6% during 2014, 2015, and 2016, respectively), followed by Ta-BP48 (Bipolaris, average incidence of black point ranged from 51.0 to 53.2% over the 3 years) and then Ta-BP17 (Exserohilum; 50.4, 48.1, and 51.7% during 2014, 2015, and 2016, respectively). The incidences of black point caused by three of the isolates, Ta-BP2, Ta-BP36, and Ta-BP56 (Bipolaris), were greater than 40% across all 3 years. The incidences of black point caused by four of the isolates, Ta-BP1 (Alternaria), Ta-BP22 (Fusarium), Ta-BP39, (Curvularia) and Ta-BP49 (Epicoccum), ranged from 32.4 to 38.8%. We examined the colony and conidial morphologies of the ten isolates that caused significantly higher incidences of black point than the check (Fig. 1).
Fig. 1.
The characteristics of colony morphologies (upper) and conidia (lower) of the ten isolates that caused significantly higher incidences of black point in the North China Plain. Isolates were cultured on PDA at 25 °C for 7 days in the dark. The colonies and conidia were 4 recorded with a camera (Canon Powershot A6407) and a microscope (LEICM DM4000B7), respectively. A and a, isolates Ta-BP1; B and b, Ta-BP33; C and c, Ta-BP48; D and d, Ta-BP2; E and e, Ta-BP36; F and f, Ta-BP56; G and g, Ta-BP39; H and h, Ta-BP17; I and i, Ta-BP22, and; J, Ta-BP49 (no conidia of this isolate was seen)
Re-isolation of the Isolates
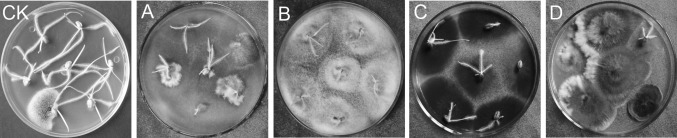
The average re-isolation frequency of the 21 isolates used for the pathogenicity evaluation from all three wheat lines was 78.4%. The re-isolation frequency of the ten isolates that caused severe wheat black point was 94.9%, Isolations from surface-sterilized kernels confirmed that the fungus used for inoculation was responsible for the black point (Table 1, Fig. 2).
Table 1.
The re-isolation frequency of the isolates from diseased kernels of wheat black point in the North China Plain in 2014
| Isolate number | Genus | Re-isolation frequency (%) | |||
|---|---|---|---|---|---|
| Pinzhisuo6 | Wanyuanbai | Jicheng2 | means | ||
| Ta-BP35 | Alternaria | 75.0 | 66.7 | 58.3 | 66.7 |
| Ta-BP1 | 91.7 | 88.9 | 94.4 | 91.7 | |
| Ta-BP3 | 61.1 | 55.6 | 52.8 | 56.5 | |
| Ta-BP6 | 63.9 | 52.8 | 72.2 | 63.0 | |
| Ta-BP33 | Bipolaris | 100.0 | 94.4 | 97.2 | 97.2 |
| Ta-BP48 | 97.2 | 100.0 | 91.7 | 96.3 | |
| Ta-BP36 | 88.9 | 94.4 | 100.0 | 94.4 | |
| Ta-BP2 | 86.1 | 91.7 | 88.9 | 88.9 | |
| Ta-BP56 | 100.0 | 94.4 | 91.7 | 95.4 | |
| Ta-BP22 | Fusarium | 100.0 | 91.7 | 100.0 | 97.2 |
| Ta-BP20 | 52.8 | 58.3 | 63.9 | 58.3 | |
| Ta-BP39 | Curvularia | 100.0 | 100.0 | 94.2 | 98.1 |
| Ta-BP44 | Aspergillus | 80.6 | 91.7 | 83.3 | 85.2 |
| Ta-BP18 | 53.3 | 59.6 | 59.2 | 57.4 | |
| Ta-BP46 | 61.1 | 72.2 | 69.4 | 67.6 | |
| Ta-BP17 | Exserohilum | 97.2 | 100.0 | 100.0 | 99.1 |
| Ta-BP49 | Epicoccum | 91.7 | 88.9 | 92.2 | 90.9 |
| Ta-BP29 | Nigrospora | 66.7 | 72.2 | 75.0 | 71.3 |
| Ta-BP52 | Penicillium | 72.2 | 63.9 | 55.6 | 63.9 |
| Ta-BP7 | Cladosporium | 63.9 | 47.2 | 52.8 | 54.6 |
| Ta-BP19 | Ulocladium | 44.4 | 50.0 | 61.1 | 51.8 |
Fig. 2.
Re-isolation of some isolates from kernels of wheat with black point in the North China Plain in 2014. CK, the wheat kernels inoculated with water; a, Ta-BP17; b, Ta-BP22; c, Ta-BP39, and; d, Ta-BP48. The wheat line was ‘Pinzhisuo6’
Phylogenetic Analysis of the Ten Isolates Causing Severe Black Point in Wheat
Based on colony and conidial morphologies, the ten isolates that caused significantly higher incidences of black point than the check between 2014 and 2016 belong to six fungal genera: Alternaria (Ta-BP1), Bipolaris (Ta-BP2, Ta-BP33, Ta-BP36, Ta-BP48, and Ta-BP56), Fusarium (Ta-BP22), Curvularia (Ta-BP39), Exserohilum (Ta-BP17), and Epicoccum (Ta-BP49) (Supplementary Table S3, Fig. 1). This was confirmed by the BLAST results of their ITS sequences (Supplementary Table S5).
To identify the species of the ten isolates, phylogenetic analysis was conducted. To distinguish closely related species, five isolates from the genus Bipolaris were subjected to a combined multi-gene analysis of the ITS, GPDH, and TEF data. Phylogenetic analysis of the other five isolates was carried out using their ITS sequences. The ten isolates were found to belong to eight fungal species. Three species belonged to the genus Bipolaris (Supplementary Fig. S1): B. sorokiniana (Ta-BP33 and Ta-BP48), B. crotonis (Ta-BP36), and B. coffeana (Ta-BP2 and Ta-BP56). The other five isolates, Ta-BP1, Ta-BP17, Ta-BP22, Ta-BP39, and Ta-BP49, were identified as A. alternata, E. rostratum, F. equiseti, C. spicifera, and E. sorghinum, respectively (Supplementary Figs. S2–S6). The identity of the isolates and their ex-type strains (except Fusarium equiseti) ranged from 98 to 100% (Supplementary Table S5).
Discussion
The available literature describing the relationship between fungi and black point in wheat are unclear and/or contradictory. Many studies have reported that fungi are associated with black point [4, 5, 9]; however, Williamson showed that there was not a strong association between incidences of black point and infection with A. alternata [24]. We isolated 21 morphologically distinct isolates from diseased wheat kernels of black point and investigated their pathogenicities towards wheat. Of the 21 isolates, ten isolates caused significantly higher incidences of black point than the check. Based on Koch’s Rule, we re-isolated the isolates from the diseased kernels. This proved that these ten isolates were the pathogens responsible for the black point in the wheat; thus, we have proven that fungal pathogens are one of the factors that causes black point in wheat in the NCP.
The ten pathogenic isolates identified in this study were confirmed to be eight fungal species: A. alternata, B. sorokiniana, B. crotonis, B. coffeana, E. rostratum, F. equiseti, C. spicifera, and E. sorghinum. The isolation of Alternaria and Bipolaris from wheat kernels with black point symptoms has been reported [9]; this is consistent with our results. The isolation frequencies of three of the fungal species, F. equiseti, C. spicifera, and E. sorghinum, were not high, but they have also been previously associated with black point symptoms in wheat [5, 8, 9]. However, there have been no previous reports showing Exserohilum to be a cause of black point in wheat. This is the first report showing that E. rostratum is a pathogen causing black point in wheat. Based on the isolation frequencies and symptom severities, B. sorokiniana and A. alternata were determined to be the most important pathogens causing black point in wheat in the NCP.
In addition to pathogens, incidences of black point are directly influenced by environmental conditions [25, 26]. Thus, wheat genotypes with fewer diseased kernels under natural field conditions are not necessarily resistant genotypes, but may rather be the result of inadequate inoculum or unfavorable field conditions. It is, therefore, necessary to screen resistant wheat genotypes by inoculating different black point pathogens under favorable conditions across different years and locations. The eight fungal species that we found lead to significantly higher incidences of black point extend the scope of fungi causing wheat black point and provide pathogenic species for resistance screening in wheat genotypes that can be used to breed disease (black point) resistant varieties.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was funded by the National Science and Technology Pillar Program during the 12th five-year plan period (Grant Number 2015BAD26B01) and the scientific and technological program of Henan province in China (Grant Number 172102110041).
Compliance with Ethical Standards
Conflict of interest
The authors have declared that no competing interests exist.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12088-018-0709-1) contains supplementary material, which is available to authorized users.
References
- 1.Yang H, Zehnder A. China’s regional water scarcity and implications for grain supply and trade. Environ Plan A. 2001;33:79–95. doi: 10.1068/a3352. [DOI] [Google Scholar]
- 2.Conner RL, Thomas JB. Genetic variation and screening techniques for resistance to black point in soft white spring wheat. Can J Plant Pathol. 1985;74:402–407. doi: 10.1080/07060668509501669. [DOI] [Google Scholar]
- 3.Sissons M, Sissons S, Egan N. The black point status of selected tetraploid species and Australian durum wheat and breeding lines. Crop Sci. 2010;50:1279–1286. doi: 10.2135/cropsci2009.08.0439. [DOI] [Google Scholar]
- 4.Rees RG, Martin DJ, Law DP. Black point in bread wheat: effects on quality and germination and fungal associations. Aust J Exp Agric. 1984;127:601–605. doi: 10.1071/EA9840601. [DOI] [Google Scholar]
- 5.Conner RL, Hwang SF, Stevens RR. Fusarium proliferatum: a new causal agent of black point in wheat. Can J Plant Pathol. 1996;18:419–423. doi: 10.1080/07060669609500598. [DOI] [Google Scholar]
- 6.Li QY, Qin Z, Jiang YM, Shen CC, Duan ZB, Niu JS. Screening wheat genotypes for resistance to black point and the effects of diseased kernels on seed germination. J Plant Dis Prot. 2014;121:79–88. doi: 10.1007/BF03356495. [DOI] [Google Scholar]
- 7.El-Gremi SM, Draz IS, Youssef WAE. Biological control of pathogens associated with kernel black point disease of wheat. Crop Prot. 2017;91:13–19. doi: 10.1016/j.cropro.2016.08.034. [DOI] [Google Scholar]
- 8.Sisterna MN, Sarandon SJ. Preliminary studies on the natural incidence of wheat black point under different fertilization levels and tillage systems in Argentina. Plant Pathol J. 2005;4:26–28. doi: 10.3923/ppj.2005.26.28. [DOI] [Google Scholar]
- 9.Malaker PK, Mian IH. Population dynamics of mycoflora and incidence of black point disease in wheat grains. Bangladesh J Agric Res. 2010;35:1–10. doi: 10.3329/bjar.v35i1.5861. [DOI] [Google Scholar]
- 10.Zadoks J, Chang T, Konzak C. A decimal code for the growth stages of cereals. Weed Res. 1974;14:415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x. [DOI] [Google Scholar]
- 11.Lu JY. Plant pathogenic mycology. Beijing: China Agriculture Press; 2001. [Google Scholar]
- 12.Manamgoda DS, Rossman AY, Castlebury LA, Crous PW, Madrid H, Chukeatirote E, Hyde KD. The genus Bipolaris. Stud Mycol. 2014;79:221–288. doi: 10.1016/j.simyco.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chomnunti P, Schoch CL, Aguirre-Hudson B, Ko-Ko TW, Hongsanan S, Jones EB, Kodsueb R, Phookamsak R, Chukeatirote E, Bahkali AH, Hyde KD. Capnodiaceae. Fungal Divers. 2011;51:103–134. doi: 10.1007/s13225-011-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahto BN, Gurung S, Adhikari TB. Assessing genetic resistance to spot blotch, stagonospora nodorum blotch and tan spot in wheat from Nepal. Eur J Plant Pathol. 2011;131:249–260. doi: 10.1007/s10658-011-9803-5. [DOI] [Google Scholar]
- 15.Manamgoda DS, Cai L, McKenzie EHC, Crous PW, Madrid H, Chukeatirote E, Shivas RG, Tan YP, Hyde KD. A phylogenetic and taxonomic re-evaluation of the Bipolaris–Cochliobolus–Curvularia complex. Fungal Divers. 2012;56:131–144. doi: 10.1007/s13225-012-0189-2. [DOI] [Google Scholar]
- 16.Woudenberg JHC, Groenewald JZ, Binder M, Crous PW. Alternaria redefined. Stud Mycol. 2013;75:171–212. doi: 10.3114/sim0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YM, Hong JH, Lee H, Ahn BJ, Kim GH, Kim JJ. Phylogenetic analysis of the genus Fusarium and their antifungal activity against wood-decay and sapstain fungi. Holzforschung. 2013;67:473–478. [Google Scholar]
- 18.Chowdhary A, Hagen F, Curfsbreuker I, Madrid H, Hoog GSD, Meis JF. In vitro activities of eight antifungal drugs against a global collection of genotyped Exserohilum isolates. Antimicrob Agents Chemother. 2015;59:6642–6645. doi: 10.1128/AAC.01218-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q, Jiang JR, Zhang GZ, Cai L, Crous PW. Resolving the Phoma enigma. Stud Mycol. 2015;82:137–217. doi: 10.1016/j.simyco.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nylander JAA. Mr Model Test v2. Uppsala: Evolutionary Biology Center, University of Uppsala; 2004. [Google Scholar]
- 22.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 23.Huelsenbeck JP, Ronquist F. Bayesian analysis of molecular evolution using MrBayes. In: Nielsen R, editor. Statistical methods in molecular evolution. New York: Springer; 2005. pp. 183–232. [Google Scholar]
- 24.Williamson PM. Black point of wheat. In vitro production of symptoms, enzymes involved, and association with Alternaria alternata. Aust J Agric Res. 1997;48:13–19. doi: 10.1071/A96068. [DOI] [Google Scholar]
- 25.Clarke MP, Gooding MJ, Jones SA. The effects of irrigation, nitrogen fertilizer and grain size on Hagberg falling number, specific weight and black point of winter wheat. J Sci Food Agric. 2004;84:227–236. doi: 10.1002/jsfa.1657. [DOI] [Google Scholar]
- 26.Fernandez MR, Sissons M, Conner RL, Wang H, Clarke JM. Influence of biotic and abiotic factors on dark discoloration of durum wheat kernels. Crop Sci. 2011;51:1205–1214. doi: 10.2135/cropsci2010.07.0433. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.