Abstract
Bacterial persisters (defined as dormant, non-dividing cells with globally reduced metabolism) are the major cause of recurrent infections. As they neither grow nor die in presence of antibiotics, it is difficult to eradicate these cells using antibiotics, even at higher concentrations. Reports of metabolites (which help in waking up of these inactive cells) enabled eradication of bacterial persistence by aminoglycosides, suggest the new potential strategy to improve antibiotic therapy. Here we propose, mannitol enabled elimination of Salmonella persister cells by the nisin–antibiotic combination. For this, persister cells were developed and characterized for their typical properties such as non-replicative state and metabolic dormancy. Different carbon sources viz. glucose, glycerol, and mannitol were used, each as an adjunct to ampicillin for the eradication of persister cells. The maximum (but not complete) killing was observed with mannitol–ampicillin, out of all the combinations used. However, significant elimination (about 78%) could be observed, when nisin (an antimicrobial peptide) was used with ampicillin in presence of mannitol, which might have mediated the transfer of antibiotic–nisin combination at the same time when the cells tried to grab the carbon molecule. Further, the effectiveness of the trio was confirmed by flow cytometry. Overall, our findings highlight the potential of this trio-combination for developing it as an option for tackling Salmonella persister cells.
Keywords: Mannitol, Nisin, Persister cells, Salmonella Typhi, Trio-combination
Introduction
Treatment of bacterial infections is getting complicated by both bacterial resistance and persistence. Bacterial persistence is often responsible for recurrent bouts of the associated diseases. Persisters are not the same as antibiotic-resistant bacteria as the latter survive because of genetic mutations that protect them from antibiotics. On the other hand, persisters survive by essentially pretending dead in presence of stress. They shut down their normal metabolic functions, lie inactive in the body and refuse to gobble up the antibiotics designed to poison them. Salmonella Typhi is one such organism that is posing a major threat to human health due to its ability to cause a repeated relapse of typhoid fever [1]. Salmonella has the ability to persist within the macrophage. After getting phagocytized by the macrophages, a small proportion of Salmonella population gets transformed into persisters due to the action of various factors such as toxin–antitoxin modules, low pH, metabolic limitations and oxidative stress [2, 3]. Due to the dormancy of organisms, antibiotic treatment is unsuccessful in controlling relapse of bacterial infections. In this context, antimicrobial peptides are gaining attention as an alternative strategy for fighting against these dormant cells. The major target of antimicrobial peptides (AMPs) is membrane integrity, which is essential for the survival of bacteria irrespective of the metabolic state of the cell [4–6]. In the present study, the successful use of trio-combination of nisin, antibiotic and sugar as an effective cocktail to eradicate Salmonella persisters is being reported for the first time.
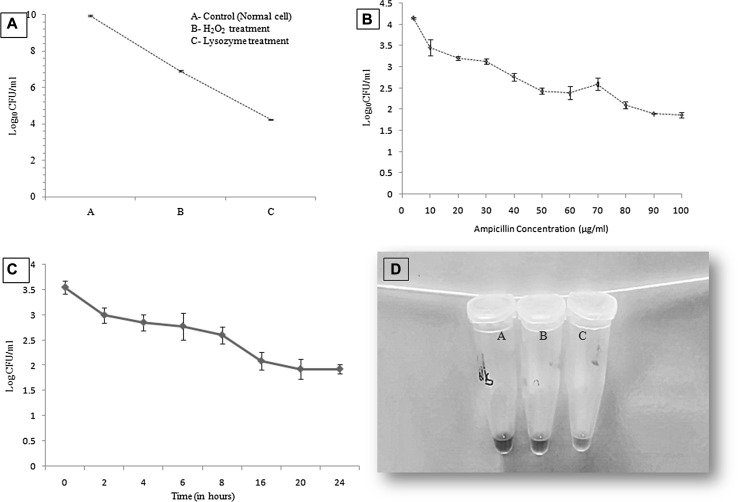
With a view to restore the activity of previously efficacious drugs; ampicillin was selected for the study as it has been the drug of choice to treat typhoid fever in the past. Minimum inhibitory concentration (MIC) of ampicillin against standard Salmonella enterica serovar Typhi strain Ty2, grown under normal standard condition was recorded as 4.0 µg/ml. To induce the development of Salmonella persister cells, 1% overnight culture was seeded into 20 ml of fresh nutrient broth and incubated for 6 h (which attained O.D600nm 1.0, corresponding to approximately 1010 CFU/ml), washed and suspended in 2 ml of PBS. Thereafter, cells were exposed to environmental stresses (10 mM H2O2, having MIC 0.5 mM and 22.5 µg/ml lysozyme) for 15 min each and enumerated by plating on nutrient agar plates (Fig. 1a). It has been reported that exposure to environment stress before the antibiotic stress helps in turning on stress-responsive cascades, causing a major number of cells to switch on the tolerant state thereby increasing the probability of persisters formation [7, 8]. After exposing the culture to environmental stress, it was treated for 4 h with ampicillin at its MIC and higher than MIC values (4, 10, 20, 30, 40, 50, 60, 70, 80 μg/ml) at standard growth conditions. Earlier work has demonstrated that treatment under these conditions for 4 h eliminates all susceptible non-persister cells [9]. We verified that remaining cells were persisters by increasing the concentration of ampicillin up to 100 μg/ml and noted no further significant decrease in the viability (after 80 μg/ml) by spot plating on normal nutrient agar plates (Fig. 1b) and antibiotic (80 μg/ml) containing nutrient agar plates. On nutrient agar plates, significant growth was observed, whereas no growth was observed on antibiotic containing plates thereby, confirming the formation as well as the property of persister cells that they resume their growth when subjected to normal conditions, unlike when exposed to any stress. Salmonella persisters were isolated under the simulated conditions encountered by the pathogen in the intracellular milieu of macrophages, suggesting the favorable niche of the organisms [10]. Further, biphasic curve, which is considered as a characteristic feature of persisters was studied by time-dependent killing using ampicillin at 80 µg/ml concentration. This resulted in an initial reduction in the colony forming units (CFU/ml) which correspond to sensitive cells followed by a plateau and fraction of cells that survived, indicating that persisters are not vulnerable to even higher concentration of ampicillin (Fig. 1c) [11]. Unlike resistant cells which replicate even under the antibiotic stress, persisters do not divide under such conditions. Isolated persister cells were exposed to normal (nutrient broth) and antibiotic stress (nutrient broth + 80 μg/ml of ampicillin) conditions alternatively, proved the non-dividing state of dormant/persister cells in presence of antibiotic stress. Persister cells also considered as metabolically inactive or slow, because of down-regulation of metabolic genes, in contrast to normal cells [12]. The metabolic state of cells determined using a metabolic marker dye AlamarBlue® (Invitrogen Tech.), indicated the difference in the degree of metabolism of persister cells in presence and absence of stress condition [13] It is based on the ability of metabolically active cells to reduce resazurin (a component of dye) into red color compound resorufin (Fig. 1d).
Fig. 1.
Characteristic features of Persister cells. a Exponential phase S. Typhi cells were subjected to environmental stress (10 mM of H2O2 and 22.5 μg/ml of lysozyme, 15 min each) and enumerated by plating on nutrient agar plates. b After environmental stress cells were further treated for 4 h with different concentrations (at MIC and higher than the MIC) of ampicillin. After 80 µg/ml no significant reduction in viability was recorded and these survived cells were taken as persister cells. c Time-dependent antibiotic (80 µg/ml) killing kinetics of S. Typhi cells showing biphasic curve; a characteristic property of persister cells. The initial reduction in the log10cfu/ml corresponds to sensitive cells followed by a plateau and fraction of cells that survived, indicating S. Typhi persister cells. d Metabolic state difference of persister cells under stress and normal condition: A—Persisters in PBS (Nutrient stress), B—Persisters in antibiotic stress (80ug/ml) and C—Persisters in nutrient media (no stress). Tube A and B do not show the change in color in comparison to tube C, where color is turned pink due to the reduction of resazurin (a component of dye) into red/pink color compound resorufin by metabolically active cells (color figure online)
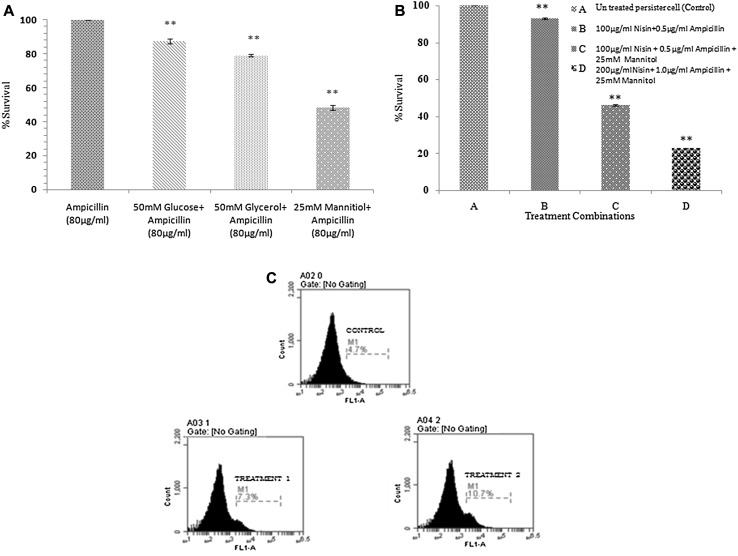
After confirming the characteristic properties of isolated persisters, we further looked into the possible way of eradicating these cells. Recently, it has been found that addition of carbon sources such as mannitol, glucose, fructose, and pyruvate could restore the susceptibility of E. coli persister cells to antibiotics by stimulating their metabolic activity [14–16]. Different carbon sources such as glucose, glycerol, and mannitol were used as an adjunct to ampicillin (80 µg/ml) in two different concentrations (50 and 25 mM). Maximum reduction was observed in mannitol (25 mM) + ampicillin combination followed by glycerol (50 mM) and glucose (50 mM) combination with ampicillin (Fig. 2a). Mannitol in addition to, increasing the metabolic activity of cells by generating proton motive force (PMF) has also been reported to increase the osmolarity of the cells as well as to inhibit starvation and stress-induced defenses. This could be the reasons for the higher effectiveness of mannitol and ampicillin combination out of the rest of the combinations [14, 17, 18]. Moreover, it has been reported that glycerol mediated antibiotic killing of persisters is less efficient than that with mannitol mediated [14]. However, the mannitol–ampicillin combination did not lead to the complete killing of persister cells. The reason could be that the intake of antibiotic by the cell is not carbon source specific but antibiotic specific [14]. Therefore, to further potentiate the influx and killing by the antibiotic, nisin (AMPs) was used along with mannitol. Nisin was selected for the study because it is being used for decades in the food industry as preservative, therefore, is considered safe to use for the in vivo system [5]. Earlier reports from our laboratory have shown that beta-lactam antibiotics could confer higher inhibitory activity when used in combination with nisin [5]. These antibiotics lead to changes in cell morphology and might help in permeabilization of nisin across the bacterial outer membrane, enabling nisin to form pores in the inner membrane. Additionally, this might allow more influx of antibiotics resulting in more inhibition [19].Therefore, in addition to, increasing metabolic activity using mannitol, synergistic action of ampicillin and nisin was exploited for the eradication of persister cells.
Fig. 2.
Treatment of persister cells with different combinations. a The difference in survivability of persister cells after 18 h of treatment with a combination of ampicillin with different carbon sources (50 mM glucose, 50 mM glycerol, and 25 mM mannitol). The maximum reduction in the viability was recorded in mannitol plus ampicillin combination. Asterisks indicate the statistically significant difference (p < 0.01) in different treatment combinations as compare to control of persister cells treated with ampicillin alone (80 μg/ml). b %survival rate after 18 h of treatment with different combinations of nisin, ampicillin with mannitol. The maximum reduction in the viability was recorded in combination C. Asterisks indicate the statistically significant difference (p < 0.01) in different treatment combinations as compare to control of untreated persister cells. c Cells were stained with fluorescein isothiocyanate (FITC, final concentrations 420 nM) after 2 h of treatment; a shift in fluorescence peak was recorded that shows dead population. c(i) control of persister cells, c(ii) Shift was recorded showing dead population after treatment with combination containing 100 µg/ml Nisin + 0.5 µg/ml Ampicillin + 25 mM Mannitol, c(iii) Comparatively more shift was recorded after treatment with combination containing 200 µg/mlNisin + 1.0 µg/ml Ampicillin + 25 mM Mannitol than c(ii) combination
The 100 μg/ml and 0.5 μg/ml concentration of nisin and ampicillin, respectively (in synergism, below their MICs) has been found effective against normal Salmonella [5]. The respective concentrations of both the agents were used in present study to monitor their effect on the persister cells. However, this combination was found to be ineffective to kill these cells that could be due to the limited influx of agents, as the cell is in a dormant state (Fig. 2b). Therefore, the trio of sugar, ampicillin, and nisin was tried to revert the persister phenotype. Nisin and ampicillin at two different concentrations (100 μg/ml nisin + 0.5 μg/ml ampicillin and 200 μg/ml nisin +1 μg/ml ampicillin) were used with a specific concentration of mannitol (25 mM concentration). The trio combination at concentrations, 200 μg/ml nisin +1 μg/ml ampicillin + 25 mM mannitol, was found to be the most effective showing 78% reduction rate of the persister cells (Fig. 2b).
To differentiate between live and dead cells before and after the treatment, flow cytometry analysis was performed. Data revealed that after 2 h of treatment, a shift in fluorescence peak was recorded that shows the dead population in comparison to control, represented in Fig. 2c.
This study highlights three main points: (1) the normal S. Typhi cells were found sensitive to the antibiotic (as per the CSLI guidelines); however, the developed persister cells did not respond to even the much higher concentration of the ampicillin. (2) Persisters partially responded to ampicillin at 80 µg/ml concentrations in the presence of carbon source mannitol (25 mM). Further, (3) nisin- ampicillin (200 and 1 μg/ml, respectively) combination tried to eradicate the persister cells in presence of mannitol (25 mM) was found effective. Considering all these observations, the study revealed that the mentioned trio combination might prove to be helpful in developing the strategic option for combating persister cells.
Statistical Analysis
Experiments were conducted at least three times and data are expressed as mean ± SD. Statistical significance was assessed by one-way analysis of variance (ANOVA). Values less than 0.05 (p < 0.05) were considered as statistically significant.
Acknowledgements
Authors are thankful to the Department of Microbiology, Panjab University Chandigarh for providing the facilities to carry out this work. The authors also acknowledge PURSE Grant from Department of Science and Technology, New Delhi, India, to carry out this work.
Contributor Information
Praveen Rishi, Email: rishiparveen@pu.ac.in, Email: rishipraveen@yahoo.com.
Neha Rani Bhagat, Email: nehabhagat03@gmail.com.
Reena Thakur, Email: reenathakur700@gmail.com.
Preeti Pathania, Email: preetispathania@yahoo.com.
References
- 1.Parry SM, Palmer SR, Slader J, Humphrey T, South East Wales Infectious Disease Liaison Group Risk factors for Salmonella food poisoning in the domestic kitchen—a case control study. Epidemiol Infect. 2002;129:277–285. doi: 10.1017/S0950268802007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helaine S, Kugelberg E. Bacterial persisters: formation, eradication, and experimental systems. Trends Microbiol. 2014;22:417–424. doi: 10.1016/j.tim.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Cheverton AM, Gollan B, Przydacz M, Wong CT, Mylona A, Hare SA, Helaine S. A Salmonella toxin promotes persister formation through acetylation of tRNA. Mol Cell. 2016;63:86–96. doi: 10.1016/j.molcel.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals. 2013;6:1543–1575. doi: 10.3390/ph6121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh AP, Prabha V, Rishi P. Value addition in the efficacy of conventional antibiotics by nisin against Salmonella. PLoS ONE. 2013;8:e76844. doi: 10.1371/journal.pone.0076844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh AP, Prabha V, Rishi P. Efficacy of cryptdin-2 as an adjunct to antibiotics from various generations against Salmonella. Indian J Microbiol. 2014;54:323–328. doi: 10.1007/s12088-014-0463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong SH, Wang X, O’Connor HF, Benedik MJ, Wood TK. Bacterial persistence increases as environmental fitness decreases. Microb Biotechnol. 2012;5:509–522. doi: 10.1111/j.1751-7915.2011.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhargava N, Sharma P, Capalash N. Pyocyanin stimulates quorum sensing mediated tolerance to oxidative stress and increases persister cells population in Acinetobacter baumannii. Infect Immun. 2014;82:3417–3425. doi: 10.1128/IAI.01600-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett. 2004;230:13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 10.Silva-Herzog E, McDonald EM, Crooks AL, Detweiler CS. Physiologic stresses reveal a Salmonella persister state and TA family toxins modulate tolerance to these stresses. PLoS ONE. 2015;10:e0141343. doi: 10.1371/journal.pone.0141343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R, Ray P, Das A, Sharma M. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J Med Microbiol. 2009;58:1067–1073. doi: 10.1099/jmm.0.009720-0. [DOI] [PubMed] [Google Scholar]
- 12.Shah D, Zhang Z, Khodursky AB, Kaldalu N, Kurg K, Lewis K. Persisters: a distinct physiological state of E. coli. BMC Microbiol. 2006;6:53. doi: 10.1186/1471-2180-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaldalu N, Hauryliuk V, Tenson T. Persisters as elusive as ever. Appl Microbiol Biotechnol. 2016;100:6545–6553. doi: 10.1007/s00253-016-7648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473(7346):216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JS, Heo P, Yang TJ, Lee KS, Cho DH, Kim BT, Suh JH, Lim HJ, Shin D, Kim SK, Kweon DH. Selective killing of bacterial persisters by a single chemical compound without affecting normal antibiotic-sensitive cells. Antimicrob Agents Chemother. 2011;55:5380–5383. doi: 10.1128/AAC.00708-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orman MA, Brynildsen MP. Establishment of a method to rapidly assay bacterial persister metabolism. Antimicrob Agents Chemother. 2013;57:4398–4409. doi: 10.1128/AAC.00372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman LR, Richardson AR, Houston LS, Kulasekara HD, Martens-Habbena W, Klausen M, Burns JL, Stahl DA, Hassett DJ, Fang FC, Miller SI. Nutrient availability as a mechanism for selection of antibiotic tolerant Pseudomonas aeruginosa within the CF airway. PLoS Pathog. 2010;6:e1000712. doi: 10.1371/journal.ppat.1000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barraud N, Buson A, Jarolimek W, Rice SA. Mannitol enhances antibiotic sensitivity of persister bacteria in Pseudomonas aeruginosa biofilms. PLoS ONE. 2013;8:e84220. doi: 10.1371/journal.pone.0084220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh AP, Preet S, Rishi P. Nisin/β-lactam adjunct therapy against Salmonella enterica serovar Typhimurium: a mechanistic approach. J Antimicrob Chemother. 2014;69:1877–1887. doi: 10.1093/jac/dku049. [DOI] [PubMed] [Google Scholar]