Abstract
The aim of this study was to evaluate the effect of farnesol on the production of acids and hydrolytic enzymes by biofilms of Streptococcus mutans and Candida albicans. The present study also evaluated the time-kill curve and the effect of farnesol on matrix composition and structure of single-species and dual-species biofilms. Farnesol, at subinhibitory concentrations, showed a significant reduction in S. mutans biofilm acid production, but did not alter C. albicans hydrolytic enzyme production. The number of cultivable cells of both microorganisms was significantly reduced after 8 h of contact with farnesol. Extracellular matrix protein content was reduced for biofilms formed in the presence of farnesol. In addition, confocal laser scanning and scanning electron microscopy displayed structural alterations in all biofilms treated with farnesol, which included reduction in viable cells and extracellular matrix. In conclusion, farnesol showed favorable properties controlling some virulence factors of S. mutans and C. albicans biofilms. These findings should stimulate further studies using this quorum-sensing molecule, combined with other drugs, to prevent or treat biofilm-associated oral diseases.
Keywords: Biofilms, Fungi, Quorum sensing, Streptococci, Virulence
Introduction
The microorganisms that colonize the oral cavity have specific characteristics which make them virulent when interacting with external factors, as in the case of the bacterium Streptococcus mutans, which uses sucrose from the host diet to produce cariogenic biofilms [1]. S. mutans adheres to dental surfaces in the presence of sucrose through the formation of exopolysaccharides (EPS) in a process mediated by glycosyltransferases. In addition, this bacterial species is able to develop in environments with low pH, as well as to produce acids from dietary carbohydrates [2]. Taken together, these virulence factors contribute to the dissolution of tooth enamel and development of carious lesions
Other microorganisms, such as fungi, also inhabit the oral environment. Because of this, Candida albicans fungus deserves attention [2]. It can colonize surfaces such as denture acrylic resins and medical devices, which in the latter case can lead to a high mortality rate of hospitalized patients [3]. According to the literature, an extracellular carbohydrate present in the biofilm matrix of C. albicans, β- 1,3 glucan, appears to be involved in drug sequestration, thereby assisting in antimicrobial resistance [4]. The basic composition of the extracellular matrix of Candida albicans biofilm is 55% protein, 25% carbohydrates, 15% lipids, and 5% deoxyribonucleic acids and sugars, such as arabinose, glucose, and xylose [5]. Moreover, Candida species can produce hydrolytic enzymes, which contribute to their invasion in the host tissue through digestion or destruction of the cell membrane [6]. These enzymes can also attack the cells and molecules of the host immune system [6]. The production of hydrolytic enzymes and the capacity to form biofilms [7] on oral tissues and abiotic surfaces are considered major virulence factors related to Candida species [6].
Although S. mutans is considered the main causative or etiological agent of dental caries [8], studies have shown that the association with C. albicans [9] in dental biofilms causes more aggressive caries in comparison to a biofilm formed only by S. mutans [2], especially in the presence of sucrose [10]. Considering this, in conjunction with biofilm resistance to conventional drugs, alternative treatments for dental caries are stimulated. Accordingly, the use of some quorum-sensing (QS) molecules as antimicrobials has been reported [11–14]. These molecules regulate gene expression, cellular differentiation, and other functions [11–15]. Among the QS molecules, farnesol, an acyclic alcohol secreted by Candida species such as C. albicans and C. dubliniensis [16], has received attention for presenting antibiofilm activity. Farnesol also influences other species of fungi and bacteria [17].
Despite the antibiofilm effect of farnesol having been explored in previous studies, the effect of this QS molecule on the production of acids and hydrolytic enzymes (proteinase, phospholipase and hemolytic) by S. mutans and C. albicans biofilms remains unknown. Thus, the aim of this study was to assess the effect of farnesol on the abovementioned virulence factors of S. mutans and C. albicans biofilms. In addition, the current study evaluated the time-kill curve of S. mutans and C. albicans in the presence of farnesol, as well as the effect of this molecule on the extracellular matrix composition and the structure of single and mixed biofilms of the same species.
Materials and Methods
Artificial Saliva Medium
Artificial saliva (AS) medium was prepared according to Lamfon et al. [18]. Its composition per 1 L of deionized water was 2 g of yeast extract (Sigma-Aldrich, St Louis, USA), 5 g of peptone (Sigma-Aldrich), 2 g of glucose (Sigma-Aldrich), 1 g of mucin (Sigma-Aldrich), 0.35 g of NaCl (Sigma-Aldrich), 0.2 g of CaCl2 (Sigma-Aldrich) and 0.2 g of KCl (Sigma-Aldrich), as described by Monteiro et al. [19]. The final pH was adjusted to 6.8 using NaOH (Sigma-Aldrich).
Strains and Culture Conditions
The strains of C. albicans (10231) and S. mutans (25175) were purchased from the American Type Culture Collection (ATCC). For C. albicans, colonies subcultured (37 °C, 24 h) on Sabouraud dextrose agar medium (SDA; Difco, Le Pont de Claix, France) were inoculated in 10 ml of Sabouraud dextrose broth (SDB; Difco) and maintained under agitation (120 rpm) at 37 °C for 20 h. Next, yeast cells were centrifuged (8000 rpm, 5 min) and the pellets were washed twice in phosphate buffered saline (PBS; pH 7.0 0.1 M). Afterwards, the cell concentration was adjusted to 1 x 107 cells/mL in AS, using an improved Neubauer chamber. For S. mutans, colonies cultivated (5% CO2, 24 h) on Brain Hearth Infusion (BHI; Difco) agar plates were inoculated in 10 ml of BHI broth medium (Difco) and incubated under static conditions in 5% CO2 at 37 °C for 18 h. Then, bacterial cells were harvested by centrifugation (8000 rpm, 5 min), washed twice in PBS, and the final concentration was spectrophotometrically adjusted to 1 × 108 cells/mL in AS.
Preparation of Farnesol
Farnesol (trans, trans-farnesol; Sigma-Adrich) was first diluted in 7.5% methanol (v/v; Sigma-Aldrich), while a second dilution was performed in AS at the specific concentration for each microbiological assay. Solely methanol at 7.5% was tested as a control and it did not interfere in the cell viability of the microorganisms studied.
Single and Mixed Biofilm Formation in the Presence of Farnesol
Microtiter plates with 96 wells (Costar, Tewksbury, USA) were used for the formation of single and mixed biofilms of C. albicans and S. mutans. A volume of 200 µL of cell suspension (1 × 107 cells/mL in AS for C. albicans and 1 × 108 cells/mL in AS for S. mutans) was added to each well for single biofilms, while 100 µL of each suspension (2 × 107 cells/mL for C. albicans plus 2 × 108 cells/mL for S. mutans) was added in each well for mixed biofilms. The plates were incubated in static conditions in 5% CO2 at 37 °C for 2 h. The 2-h period was based on previous studies which showed that this period was sufficient to promote initial adhesion to abiotic surfaces [19–21].
Subsequently, the AS medium was removed and each well was washed once with PBS to promote the removal of non-adherent cells. Then, farnesol was diluted in AS and added to the wells at the following concentrations: 0.78 and 1.56 mM (corresponding to 1/8 and 1/4 of the minimum inhibitory concentration (MIC) determined for planktonic cells) for assessment of the effect on acid production and enzymatic activity; 3.12 mM, for the time-kill curve assay; 3.12 and 12.5 mM for evaluation of the effect on the extracellular matrix composition and the structure of the biofilms. Afterwards, the plates were stored at 37 °C in 5% CO2 for 48 h, and the media were renewed after 24 h. All farnesol concentrations were based on the Fernandes et al. [22] study. Subinhibitory concentrations (0.78 and 1.56 mM) were used to ensure that the results were not influenced by the death of microorganisms, whereas the inhibitory concentrations (3.12 and 12.5 mM) were applied aiming for biofilm eradication.
Effect of Farnesol on acid Production by S. mutans Biofilms
This assay was performed according to the protocol described by the Hasan et al. [23] study. Briefly, S. mutans biofilms were formed in the presence of subinhibitory concentrations (0.78 and 1.56 mM) of farnesol during 48 h, as detailed above. After this period, the pH of the media was measured and compared with the initial pH values, which were immediately verified after the addition of farnesol-containing saliva in the wells. Chlorhexidine gluconate (CHG) at 0.45 µM (1/4× S. mutans MIC) was used as positive control, while the negative control was AS without farnesol.
Effect of Farnesol on Enzymatic Activity of C. albicans Biofilms
Proteinase activity was determined according to the Aoki et al. [24] study. Phospholipase activity was assessed according to the egg yolk agar method [25]. This agar was prepared by adding an egg yolk emulsion (10% v/v) to SDA (13 g), supplemented with NaCl (11.7 g) and CaCl2 (0.11 g) in 184 mL of deionized water [26]. Hemolytic activity was evaluated by using SDA medium supplemented with 7% fresh sheep blood and 3% glucose [27].
For enzymatic activity determination, C. albicans biofilms were developed in the presence of subinhibitory concentrations (0.78 and 1.56 mM) of farnesol, as detailed above. CHG at 1.8 µM (1/4 x C. albicans MIC) was used as positive control, while the negative control was AS without farnesol. After 48 h, the resulting biofilms were scraped from the wells (in PBS) and 10 µL of the biofilm suspension was inoculated on the agar plates. The plates were incubated at 37 °C for 3 (hemolytic activity) and 6 days (proteinase and phospholipase activities). Enzymatic activity was expressed according to the Prz index for proteinase, the Pz index for phospholipase, and the Hz index for hemolytic activity. These indexes represent the ratio between the diameter of the colony and the diameter of the translucent/precipitation zones. Enzymatic activity was graded as high (indexes < 0.4), moderate (indexes from 0.41 to 0.60), low (indexes from 0.61 to 0.99), and absent (indexes = 1) [25, 28].
Time-kill Curve Assay
The method reported by Tong et al. [29] was used to assess the time-kill curve. Falcon tubes were used to dilute farnesol in the inoculum of each strain (1 × 107 cells/mL in AS for C. albicans and 1 × 108 cells/mL in AS for S. mutans) to a concentration of 3.12 mM, and the tubes were incubated at 37 °C for 1, 2, 6, 8, 10, 12, and 24 h. After each period of time, the content of the tubes was diluted in PBS and plated on BHI agar for S. mutans and SDA for C. albicans. The number of colony-forming units (CFUs) was enumerated after 24 to 48 h of incubation at 37 °C.
Effect of Farnesol on Matrix Composition of Biofilms
The extracellular matrix protein content of the biofilms formed in the presence of farnesol was determined by the bicinchoninic acid (BCA kit, Sigma-Aldrich) method, as detailed elsewhere [30]. In turn, the carbohydrate content was measured according to the protocol recommended by Dubois et al. [31]. Absorbance values were correlated with the concentration of proteins and carbohydrates, and the results were expressed as a function of the biofilm dry weight (mg/g dry weight). CHG at 0.37 mM (50× C. albicans MIC) [22] was used as positive control, and AS devoid of farnesol was used as negative control.
Structural Analysis of Biofilms
Confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM) were used for the structural analysis of biofilms exposed to farnesol. Dual-species biofilms of C. albicans ATCC 10231 and S. mutans ATCC 25175 were formed in 24-well plates, with the bottom of the wells previously cut and sterilized by ethylene oxide associated with low temperatures (− 70 °C). After the adhesion phase (2 h), farnesol was added to the wells at 3.12 and 12.5 mM, and the plates were incubated for 48 h to allow biofilm formation. Positive and negative controls were identical to those used in the extracellular matrix composition assay. The resulting biofilms were stained for 15 min using the LIVE/DEAD® BacLight™ (Invitrogen, Carlsbad, CA) dye kit, where 3 μL of SYTO® 9 and 3 μL of propidium iodide were diluted in 3 mL of sterile deionized water. Biofilm confocal imaging was performed using a Leica TCS-SPE confocal scanning laser microscope (Leica Microsystems GmbH, Mannheim, Germany). The images were captured at a magnification of 40 times in immersion oil at 536/617 nm. For SEM analysis, dual-species biofilms were grown as previously described, progressively dehydrated in ethanol, coated with gold, and observed in an S-360 microscope (Leo, Cambridge, USA).
Statistical Analysis
All microbiological assays were carried out in triplicate, on at least three different occasions. Data showed normal distribution (Shapiro–Wilk test) and were submitted to the two-way (for acid production assay) and one-way ANOVA (for all other assays). After, the Holm-Sidak post hoc test was applied using the SigmaPlot 12.0 software (Systat Software Inc., San Jose, USA).
Results
Effect of Farnesol on Acid Production by S. mutans Biofilms
The initial pH value for all groups was approximately 7.0. After 48 h of biofilm formation, farnesol at 0.78 mM and 1.56 mM was able to maintain the same initial pH value, indicating a reduction effect on acid production. For the negative and positive controls, however, final pH values decreased to 5.1 and 5.3, with significant differences between these values and those found for farnesol (at 0.78 and 1.56 mM).
Effect of Farnesol on Enzymatic Activity of C. albicans Biofilms
Farnesol and CHG at subinhibitory concentrations did not reduce the enzymatic activity of C. albicans biofilms, when compared to the negative control. All groups displayed moderate activity for proteinase and phospholipase, and high hemolytic activity.
Time-kill Curve Assay
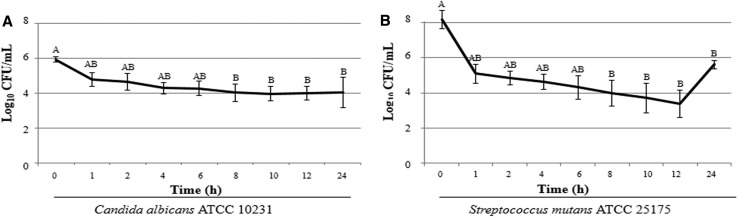
Farnesol became significantly effective after 8 h of contact with C. albicans, reducing the number of CFUs by approximately 2-log10 and maintaining a steady reduction up to 24 h of exposure (Fig. 1a). Similarly, farnesol promoted a significant reduction (~ 4-log10) in the number of CFUs of S. mutans after 8 h (Fig. 1b). In addition, a 2-log10 increase in the S. mutans growth was noted between 12 and 24 h.
Fig. 1.
Time-kill curve for C. albicans ATCC 10231 (a) and S. mutans ATCC 25175 (b) in the presence of farnesol at 3.12 mM
Effect of Farnesol on Matrix Composition of Biofilms
The amount of proteins was significantly reduced for single and mixed biofilms of S. mutans and C. albicans developed in the presence of farnesol and CHG, compared to the negative control group, except for single biofilm of S. mutans exposed to 12.5 mM farnesol (Table 1). No significant differences were noted among the groups for carbohydrate content, regardless of the biofilm.
Table 1.
Extracellular matrix composition of mono- and dual-species biofilms of Candida albicans and Streptococcus mutans grown for 48 h in the presence of different concentrations of farnesol (3.12 and 12.5 mM)
| Matrix composition (mg/g of biofilm dry weight) | C. albicans ATCC 10231 | S. mutans ATCC 25175 | C.albicans ATCC 10231 + S. mutans ATCC 25175 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farnesol (mM) | Chlorhexidine (mM) | Farnesol (mM) | Chlorhexidine (mM) | Farnesol (mM) | Chlorhexidine (mM) | |||||||
| 0 | 3.12 | 12.5 | 0.37 | 0 | 3.12 | 12.5 | 0.37 | 0 | 3.12 | 12.5 | 0.37 | |
| Carbohydrate | 40.27 | 42.07 | 26.22 | 63.23 | 30.55 | 58.15 | 62.81 | 41.73 | 32.84 | 26.54 | 52.49 | 60.27 |
| Protein | 7.81 | 0.00* | 0.84* | 0.00* | 4.76 | 0.05* | 5.80 | 0.00* | 8.75 | 0.00* | 0.17* | 0.14* |
SD values were around 10%
*Indicates P < 0.05, as compared to the control group, using one-way ANOVA and Holm-Sidak’s post hoc test
Structural Analysis of Biofilms
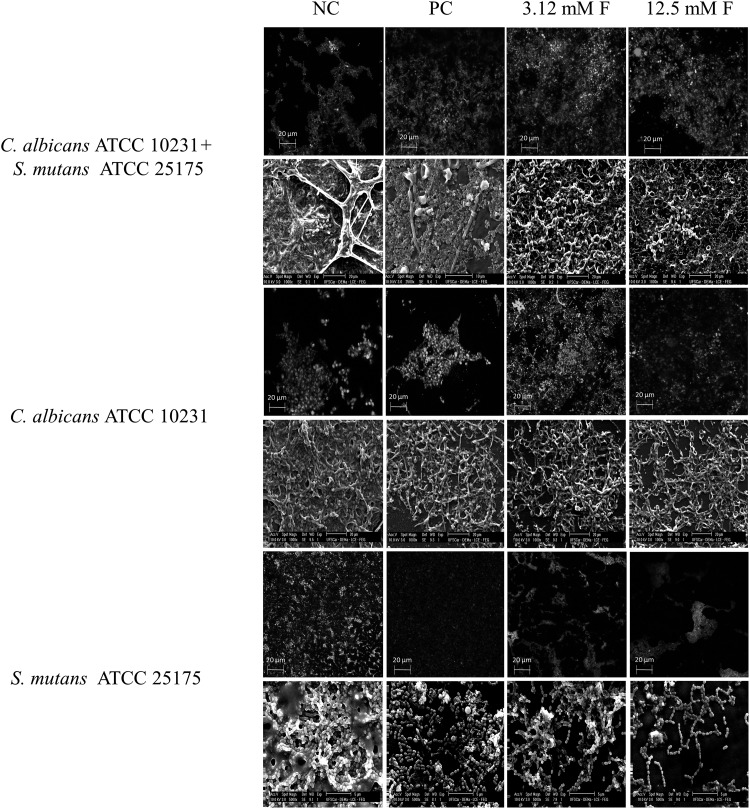
SEM images of the untreated mixed biofilm revealed a dense structure with multilayers of yeasts, hyphae, and bacterial cells immersed in extracellular material covering the entire surface (Fig. 2). The same trend was observed for untreated single biofilms in both S. mutans and C. albicans, which were composed of multilayers of cocci, yeasts, and hyphae. On the other hand, SEM images of all biofilms formed in the presence of farnesol and CHG displayed less compact structures, with cells partially covering the surfaces. CLSM images confirmed the aforementioned findings, revealing lower numbers of viable cells for biofilms formed in the presence of drugs, when compared to the negative controls. Farnesol at 12.5 mM and CHG promoted the highest reductions in cell viability (Fig. 2).
Fig. 2.
Images of confocal laser scanning microscopy (CLSM) and scanning electron microscopy of single-species and dual-species biofilms of C. albicans ATCC 10231 and S. mutans ATCC 25175, developed during 48 h in presence of farnesol at 3.12 mM (3.12 mM F) and 12.5 mM (12.5 mM F). NC negative control (biofilms without farnesol), PC positive control (chlorhexidine gluconate at 0.37 mM). For CLSM, green colors represent viable cells and red colors represent dead cells; magnification: ×40
Discussion
Microorganisms are able to produce biofilms with mechanisms that make them virulent, leading to an imbalance in the health of individuals [32]. The virulence factors are variable and closely related to the ability of microorganisms to produce extracellular matrix. The Gram-positive bacterium, S. mutans, is reported as one of the biggest producers of EPS [33], in addition to producing and tolerating acids, and causing dental caries. Another opportunistic microorganism present in the oral cavity is C. albicans, which can produce enzymes able to attack human tissue [6]. With the increase of resistance to conventional antimicrobial agents, QS molecules have received attention due to their potential as antibiofilm drugs, with great focus on farnesol. Consequently, the effect of this QS molecule was tested on some virulence factors of C. albicans and S. mutans biofilms in this present study.
Interesting results were found for the acid production assay, given that the pH of S. mutans biofilm remained stable at subinhibitory concentrations of farnesol (0.78 and 1.56 mM), which is in accordance with the Jeon et al. [34] study. A plausible explanation for this finding was reported by the same authors who mentioned that farnesol acts on the permeability of the S. mutans proton membrane, which might affect the pH gradient across the membrane, inhibiting the cellular metabolism and, thereby, the production of acids. In contrast, the positive control used in the current study (CHG) significantly reduced the pH from 7.0 to 5.3 after 48 h. The stress caused on the cells by CHG probably stimulated the acid production, resulting in a pH reduction in the environment.
As for production of hydrolytic enzymes by C. albicans biofilms, the proteinase, phospholipase, and hemolytic activity was not affected by farnesol and CHG, both at subinhibitory concentrations. Contrarily, Singh et al. [35] found that farnesol obtained from a plant extract (Usnea longissima) was able to reduce all these virulence factors expressed by a fluconazole-resistant strain of C. albicans. The divergence between the studies might be associated to the protocols used in the evaluation of the enzymatic activity (biofilm x planktonic cells), as well as related to the physiological features of the different Candida strains.
In the current study, a reduction of approximately 1-log10 in the number of CFUs for C. albicans was also identified after 1 h of contact with 3.12 mM farnesol, whereas this decrease was almost 3-log10 for S. mutans (Fig. 1). The initial reduction of S. mutans was higher than that found by Koo et al. [36] (1-log10), which could be explained by the different origins of the evaluated compounds. Koo and colleagues [36] tested a natural farnesol from propolis, while a synthetic farnesol was used in the present study. In agreement with the findings of the present study, de Melo et al. [37] found the highest cell reduction for S. mutans after 8 h of contact with farnesol.
In general, the matrix composition analysis showed that the protein content was reduced after exposure to farnesol at 3.12 and 12.5 mM (Table 1). Furthermore, single-species and dual-species biofilms formed in the presence of this compound, at the same concentrations, showed lower numbers of viable cells and less dense structures, when compared to the untreated biofilms (Fig. 2). Similar results were observed for CHG, which is a broad-spectrum antimicrobial widely used in dentistry. Taken together, these results highlight the action of farnesol on the reduction of biofilms, including an effect on the disintegration of the extracellular matrix. These findings are clinically relevant, since the extracellular matrix represents a barrier to drug penetration. Cell death mediated by farnesol has several mechanisms, such as ROS production and the disruption of mitochondrial function. Moreover, glutathione depletion from microbial cells may occur as a consequence of the formation of complexes between it and farnesol, causing disturbances in intracellular homeostasis and making the cells more susceptible to oxidative stress [38].
In conclusion, farnesol had a significant effect on the virulence factors evaluated, except on the production of hydrolytic enzymes by C. albicans biofilms. Combined with conventional antimicrobials, the effect of this QS molecule on virulence factors of several pathogens should be stimulated in order to fight oral biofilm-associated diseases.
Acknowledgements
The authors thank George Duchow for the English review of the manuscript. This study was supported by the São Paulo Research Foundation (FAPESP, Grant Number 2013/23592-0), Brazil.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Bowen WH, Madison KM, Pearson SK. Influence of desalivation in rats on incidence of caries in intact cagemates. J Dent Res. 1988;67:1316–1318. doi: 10.1177/00220345880670101401. [DOI] [PubMed] [Google Scholar]
- 2.Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, Gonzalez-Begne M, Watson G, Krysan DJ, Bowen WH, Koo H. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/S0966-842X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 4.Nobile CJ, Nett JE, Hernday AD, Homann OR, Deneault JS, Nantel A, Andes DR, Johnson AD, Mitchell AP. Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol. 2009;7:e1000133. doi: 10.1371/journal.pbio.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, Bernhardt J, Lounes-Hadj Sahraoui A, Fontaine J, Sanchez H, Hatfield RD, Ntambi JM, Nett JE, Mitchell AP, Andes DR. Novel entries in a fungal biofilm matrix encyclopedia. Mbio. 2014;5:01333. doi: 10.1128/mBio.01333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaller M, Borelli C, Korting HC, Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48:365–377. doi: 10.1111/j.1439-0507.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 7.Rapala-Kozik M, Bochenska O, Zajac D, Karkowska-Kuleta J, Gogol M, Zawrotniak M, Kozik A. Extracellular proteinases of Candida species pathogenic yeasts. Mol Oral Microbiol. 2017 doi: 10.1111/omi.12206. [DOI] [PubMed] [Google Scholar]
- 8.Marcenes W, Kassebaum NJ, Bernabe E, Flaxman A, Naghavi M, Lopez A, Murray CJ. Global burden of oral conditions in 1990–2010: a systematic analysis. J Dent Res. 2013;92:592–597. doi: 10.1177/0022034513490168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raja M, Hannan A, Ali K. Association of oral candidal carriage with dental caries in children. Caries Res. 2010;44:272–276. doi: 10.1159/000314675. [DOI] [PubMed] [Google Scholar]
- 10.Peleg AY, Hogan DA, Mylonakis E. Medically important bacterial-fungal interactions. Nat Rev Microbiol. 2010;8:340–349. doi: 10.1038/nrmicro2313. [DOI] [PubMed] [Google Scholar]
- 11.Zhang LH, Dong YH. Quorum sensing and signal interference: diverse implications. Mol Microbiol. 2004;53:1563–1571. doi: 10.1111/j.1365-2958.2004.04234.x. [DOI] [PubMed] [Google Scholar]
- 12.Pacheco AR, Sperandio V. Inter-kingdom signaling: chemical language between bacteria and host. Curr Opin Microbiol. 2009;12:192–198. doi: 10.1016/j.mib.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koul S, Kalia VC. Multiplicity of quorum quenching enzymes: a potential mechanism to limit quorum sensing bacterial population. Indian J Microbiol. 2017;57:100–108. doi: 10.1007/s12088-016-0633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Tu F, Gui Z, Lu X, Chu W. Antibiotic resistance profiles and quorum sensing-dependent virulence factors in clinical isolates of pseudomonas aeruginosa. Indian J Microbiol. 2013;53:163–167. doi: 10.1007/s12088-013-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu M, Zhang C, Mu Y, Shen Q, Feng Y. Indole affects biofilm formation in bacteria. Indian J Microbiol. 2010;50:362–368. doi: 10.1007/s12088-011-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martins M, Henriques M, Azeredo J, Rocha SM, Coimbra MA, Oliveira R. Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryot Cell. 2007;6:2429–2436. doi: 10.1128/EC.00252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semighini CP, Hornby JM, Dumitru R, Nickerson KW, Harris SD. Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol Microbiol. 2006;59:753–764. doi: 10.1111/j.1365-2958.2005.04976.x. [DOI] [PubMed] [Google Scholar]
- 18.Lamfon H, Porter SR, McCullough M, Pratten J. Formation of Candida albicans biofilms on non-shedding oral surfaces. Eur J Oral Sci. 2003;111:465–471. doi: 10.1111/j.0909-8836.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 19.Monteiro DR, Gorup LF, Silva S, Negri M, de Camargo ER, Oliveira R, Barbosa DB, Henriques M. Silver colloidal nanoparticles: antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling. 2011;27:711–719. doi: 10.1080/08927014.2011.599101. [DOI] [PubMed] [Google Scholar]
- 20.Arias LS, Delbem AC, Fernandes RA, Barbosa DB, Monteiro DR. Activity of tyrosol against single and mixed-species oral biofilms. J Appl Microbiol. 2016;120:1240–1249. doi: 10.1111/jam.13070. [DOI] [PubMed] [Google Scholar]
- 21.Alves F, de Oliveira Mima EG, Passador RCP, Bagnato VS, Jorge JH, Pavarina AC. Virulence factors of fluconazole-susceptible and fluconazole-resistant Candida albicans after antimicrobial photodynamic therapy. Lasers Med Sci. 2017;32:815–826. doi: 10.1007/s10103-017-2177-y. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes RA, Monteiro DR, Arias LS, Fernandes GL, Delbem AC, Barbosa DB. Biofilm formation by Candida albicans and Streptococcus mutans in the presence of farnesol: a quantitative evaluation. Biofouling. 2016;32:329–338. doi: 10.1080/08927014.2016.1144053. [DOI] [PubMed] [Google Scholar]
- 23.Hasan S, Danishuddin M, Adil M, Singh K, Verma PK, Khan AU. Efficacy of E. officinalis on the cariogenic properties of Streptococcus mutans: a novel and alternative approach to suppress quorum-sensing mechanism. PLoS ONE. 2012;7:e40319. doi: 10.1371/journal.pone.0040319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoki S, Ito-Kuwa S, Nakamura Y, Masuhara T. Comparative pathogenicity of a wild-type strain and respiratory mutants of Candida albicans in mice. Zentralblatt fur Bakteriologie: Int J Med Microbiol. 1990;273:332–343. doi: 10.1016/S0934-8840(11)80437-8. [DOI] [PubMed] [Google Scholar]
- 25.Price MF, Wilkinson ID, Gentry LO. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia. 1982;20:7–14. doi: 10.1080/00362178285380031. [DOI] [PubMed] [Google Scholar]
- 26.Tsang CS, Chu FC, Leung WK, Jin LJ, Samaranayake LP, Siu SC. Phospholipase, proteinase and haemolytic activities of Candida albicans isolated from oral cavities of patients with type 2 diabetes mellitus. J Med Microbiol. 2007;56:1393–1398. doi: 10.1099/jmm.0.47303-0. [DOI] [PubMed] [Google Scholar]
- 27.Sacristan B, Blanco MT, Galan-Ladero MA, Blanco J, Perez-Giraldo C, Gomez-Garcia AC. Aspartyl proteinase, phospholipase, hemolytic activities and biofilm production of Candida albicans isolated from bronchial aspirates of ICU patients. Med Mycol. 2011;49:94–97. doi: 10.3109/13693786.2010.482947. [DOI] [PubMed] [Google Scholar]
- 28.Williamson MI, Samaranayake LP, MacFarlane TW. Phospholipase activity as a criterion for biotyping Candida albicans. J Med Vet Mycol. 1986;24:415–417. doi: 10.1080/02681218680000631. [DOI] [PubMed] [Google Scholar]
- 29.Tong Z, Zhang L, Ling J, Jian Y, Huang L, Deng D. An in vitro study on the effect of free amino acids alone or in combination with nisin on biofilms as well as on planktonic bacteria of Streptococcus mutans. PLoS ONE. 2014;9:e99513. doi: 10.1371/journal.pone.0099513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou LN, Yao WF, Liu J, Shang J, Shan MQ, Zhang L, Ding AW. Protective effect of different solvent extracts from platycladi cacumen carbonisatum on LPS-induced human umbilical vein endothelial cells damage. Zhongguo Zhong Yao Za Zhi. 2013;38:3933–3938. [PubMed] [Google Scholar]
- 31.Dubois MGK, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;38:3933–3938. [Google Scholar]
- 32.Klein MI, Hwang G, Santos PH, Campanella OH, Koo H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front Cell Infect Microbiol. 2015;13:5–10. doi: 10.3389/fcimb.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury JA. The role of sucrose in cariogenic dental biofilm formation–new insight. J Dent Res. 2006;85:878–887. doi: 10.1177/154405910608501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeon JG, Pandit S, Xiao J, Gregoire S, Falsetta ML, Klein MI, Koo H. Influences of trans-trans farnesol, a membrane-targeting sesquiterpenoid, on Streptococcus mutans physiology and survival within mixed-species oral biofilms. Int J Oral Sci. 2011;3:98–106. doi: 10.4248/IJOS11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh BN, Upreti DK, Singh BR, Pandey G, Verma S, Roy S, Naqvi AH, Rawat AK. Quercetin sensitizes fluconazole-resistant candida albicans to induce apoptotic cell death by modulating quorum sensing. Antimicrob Agents Chemother. 2015;59:2153–2168. doi: 10.1128/AAC.03599-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother. 2002;46:1302–1309. doi: 10.1128/AAC.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Melo NI, de Carvalho CE, Fracarolli L, Cunha WR, Veneziani RC, Martins CH, Crotti AE. Antimicrobial activity of the essential oil of Tetradenia riparia (Hochst.) Codd. (Lamiaceae) against cariogenic bacteria. Braz J Microbiol. 2015;46:519–525. doi: 10.1590/S1517-838246246220140649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polke M, Leonhardt I, Kurzai O, Jacobsen ID. Farnesol signalling in Candida albicans–more than just communication. Crit Rev Microbiol. 2017;44:230–243. doi: 10.1080/1040841X.2017.1337711. [DOI] [PubMed] [Google Scholar]