Abstract
Incubation with microbial culture supernatants improved essential oil yield from Aquilaria subintegra woodchips. The harvested woodchips were incubated with de man, rogosa and sharpe (MRS) agar, yeast mold (YM) agar medium and six different microbial culture supernatants obtained from Lactobacillus bulgaricus, L. acidophilus, Streptococcus thermophilus, Lactococcus lactis, Saccharomyces carlsbergensis and S. cerevisiae prior to hydrodistillation. Incubation with lactic acid bacteria supernatants provided higher yield of agarwood oil (0.45% w/w) than that obtained from yeast (0.25% w/w), agar media (0.23% w/w) and water (0.22% w/w). The composition of agarwood oil from all media and microbial supernatant incubations was investigated by using gas chromatography-mass spectrometry. Overall, three major volatile profiles were obtained, which corresponded to water soaking (control), as well as, both YM and MRS media, lactic acid bacteria, and yeast supernatant incubations. Sesquiterpenes and their oxygenated derivatives were key components of agarwood oil. Fifty-two volatile components were tentatively identified in all samples. Beta-agarofuran, α-eudesmol, karanone, α-agarofuran and agarospirol were major components present in most of the incubated samples, while S. cerevisiae-incubated A. subintegra provided higher amount of phenyl acetaldehyde. Microbial culture supernatant incubation numerically provided the highest yield of agarwood oil compared to water soaking traditional method, possibly resulting from activity of extracellular enzymes produced by the microbes. Incubation of agarwood with lactic acid bacteria supernatant significantly enhanced oil yields without changing volatile profile/composition of agarwood essential oil, thus this is a promising method for future use.
Keywords: Aquilaria subintegra, Agarwood oil, Culture supernatant, GC–MS, Lactic acid bacteria
Introduction
The genus Aquilaria, comprised of more than 15 species belonging to the Thymelaeaceae family, is indigenous to Southeast Asian countries including Indonesia, Malaysia, Myanmar, The Philippines, Vietnam and Thailand [1]. Aquilaria subintegra, A. malaccensis, A. crassna, A. subintegra, A. agallocha and A. sinensis are major species capable of producing agarwood, which contains economically important essential oil [2]. Agarwood is produced when the Aquilaria tree is infected by various fungi, or in naturally wounded wood [3]. Agarwood oil is one of the most expensive essential oils and is widely used in aromatherapy, medicinal applications, incense, perfume, and religious ceremonies in Southeast Asian countries and the Middle East. A. subintegra, a native tree of Thailand and principle source of agarwood, is listed as endangered in Appendix II of the Convention on International Trade in Endangered Species (CITES) of Wild Fauna and Flora [1]. Because of the endangered status of Aquilaria and the huge medicinal and economic importance of its oil, processes for improving agarwood oil yield and composition are required. Recently, key agarwood volatile compounds were identified in the crude extract of the endophytic fungus Diaporthe sp. MFLUCC16-0051 isolated from A. subintegra heartwood. These compounds greatly resembled those found in agarwood oil produced from the host plant Aquileria [4]. Thus, endophytes could provide an alternative method for producing agarwood oil. Processes for improving agarwood oil yield have also been investigated. Traditionally, harvested agarwood chips have been soaked in water, before subjected to hydrodistillation, which aims to increase oil yield [5]. Treatment of A. crassna with an enzyme mixture comprising cellulase, xylase, alcalase and rohalase, prior to extracting agarwood oil by using supercritical fluid extraction, significantly increased extraction yield [1]. With this precedent, pre-hydrodistillation fermentation with various microbes has been used to investigate impact on yield and composition of the oil.
Microbial strains are important in developing/enhancing flavor and aroma of various foods, such as wine, vinegar, beer, fermented vegetables, milk, preserved soya and fermented meat through production of specific compounds [6]. Microbes produce these compounds during the fermentation process [7]. For example, Leejeerajumnean et al. [8] reported that the amount of some volatile components in soybean was increased, when the bean was fermented with a specific bacterial strain. Ouoba et al. [9] reported significantly high content of pyrazines in African soumbala, when it was fermented by pure Bacillus subtilis. Thus, the amount of volatiles in various samples can be increased by the enzymatic action of specific microbial strains. Enhanced production or improvement of volatile compounds has also been developed based on microbial biosynthesis or biotransformation by using microbial cultures or enzyme preparations [10–12]. Longo and Sanromán [6] reported that chemical compounds are synthesized during fermentation with nutrients, such as, sugars and amino acids. Volatile compounds have been obtained from specific microbial cultures. Precursors or intermediates can be added to the culture medium in order to promote biosynthesis of specific chemical compounds. Thus, fermentation could be used for production of particular constituents. Moreover, chemical compounds could be synthesized by using enzymes such as lipases, proteases and glucosidases to catalyze production of volatile compounds from precursor molecules. For example, glucosidases produced by Vitis, Saccharomyces, Oenococcus, Aspergillus or Candida were used to increase the aroma components of some wines by freeing glycosidically bound volatile terpenes and flavor precursors during or after fermentation [13].
Microbial enzymes are divided into intracellular and extracellular types, which function inside and outside the cell, respectively [14]. Intracellular enzymes are responsible for catalyzing metabolic reactions involved in metabolic pathways, such as glycolysis and photosynthesis, whereas extracellular enzymes are secreted outside the cell in order to function in digestive systems [15]. Extracellular enzymes are major enzymes of microbes and are found in culture supernatants [16]. Recovery of extracellular enzymes is relatively simple and includes methods, such as, centrifugation, filtration, vacuum evaporation and precipitation [17]. Thus, microbial culture supernatants are good sources for obtaining various extracellular enzymes [18, 19]. Therefore, this strategy can be applied for the production of agarwood volatile compounds, as is the case of chemical synthesis by extracellular enzymes in microbial culture supernatants. To date, there is no report describing the application of culture supernatants on agarwood fermentation. In order to develop and improve yield and aroma quality in agarwood oil, the aim of the present study was to incubate microbial culture supernatants from six designated microbes (Lactobacillus bulgaricus, L. acidophilus, Streptococcus thermophilus, Lactococcus lactis, Saccharomyces carlsbergensis, and S. cerevisiae) with A. subintegra woodchips prior to hydrodistillation Subsequently, the yield and chemical composition of agarwood oil produced from the six microbial strains, agar medium incubations and water soaking are compared and analyzed.
Materials and Methods
Agarwood Samples
Stem wood chips of A. subintegra were collected in Chanthaburi province, Thailand. A botanist identified the plant and a voucher herbarium specimen (QBG No. 24155) was deposited at the Queen Sirikit Botanical Garden, Mae Rim, Chiang Mai, Thailand. The wood chips were dried at room temperature for 1 week prior to pulverizing into a fine powder using a blender (AIM 5CF double ribbon blender; CapPlus Technologies, Phoenix, AZ).
Microbial Strains and Culture Conditions
Saccharomyces carlsbergensis TISTR 5195, S. cerevisiae TISTR 5049, Lactobacillus bulgaricus TISTR 451, L. acidophilus TISTR 1338, Lactococcus lactis TISTR 45, and Streptococcus thermophilus TISTR 458 were obtained from the Thailand Institute of Scientific and Technological Research (TISTR). Lactobacillus bulgaricus TISTR451, L. acidophilus TISTR1338, Lactococcus lactis TISTR45 and Streptococcus thermophilus TISTR458 were routinely cultured on de Man, Rogosa and Sharpe (MRS) agar while S. carlsbergensis TISTR 5195 and S. cerevisiae TISTR 5049 were cultured on Yeast Mold (YM) agar at 37 °C for 24 h. The 20% v/v glycerol bacterial culture of all strains was prepared and stored as stock culture at − 20 °C. For inoculum preparation, a single colony of each microbial strain was subcultured in a test tube containing culture media (3 mL) and incubated at 37 °C for 24 h. An aliquot (1 mL) of the cell suspension was then transferred into a flask containing media (400 mL) and incubated by shaking (170 rpm) at 37 °C. After approximately 24 h incubation, the microbial growth was terminated, when the absorbance of each suspension at 600 nm reached 1.0 (approximately cell density of 108 CFU/mL). Microbial cells were precipitated from the culture media by centrifugation (VWR Galaxy 20R, Pennsylvania) at 6000 rpm at 4 °C for 15 min. The culture supernatant was collected and transferred into a sterile bottle and was kept at 4 °C until use.
Incubation and Distillation of Agarwood Oil
Powdered A. subintegra woodchips (150 g) were incubated with the supernatant (400 mL) from each microbial culture, medium culture and distilled water at room temperature before being subjected to hydrodistillation using a Clevenger-type apparatus for 48 h. Samples were incubated from 0 to 15 days in order to assess the extraction yield of agarwood oil. Each agarwood oil distillate was dried with anhydrous Na2SO4 and kept at 4 °C until further analysis. All agarwood oil samples were diluted to 1:100 v/v with dichloromethane prior to injection. Each incubation experiment was carried out in five replicates.
Analysis of Agarwood Essential Oil Chemical Composition
The volatile constituents of essential oil obtained from powdered A. subintegra woodchips incubated with various microbial strains were analyzed using a Hewlett Packard model HP6890 gas chromatograph (Agilent Technologies, Palo Alto, CA). The chromatograph was equipped with an HP-5 ms (5% phenylpolymethylsiloxane) capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm; Agilent Technologies) interfaced to an HP model 5973 mass-selective detector. The oven temperature was initially held at 60 °C and then increased by 2 °C/min to 220 °C. The injector and detector temperatures were 250 and 280 °C, respectively. Purified helium was used as the carrier gas at a flow rate of 1 mL/min. EI mass spectra were collected at 70 eV ionization voltages over the range of m/z 29–300. The electron multiplier voltage was 1150 V. The ion source and quadrupole temperatures were set at 230 and 150 °C, respectively. Identification of volatile components was performed by comparison of their retention indices, relative to C8–C19 n-alkanes, and comparison of the mass spectra of individual components with the reference mass spectra in the NIST05 databases with corresponding data of volatile components in agarwood oils. Quantification of all identified components was determined using the MSD ChemStation Data Analysis software (Agilent Technologies, Thailand). Results are presented in terms of percent relative peak areas as no external or internal standards were used in this work. For quantitative analysis, a gas chromatograph model HP 6890 equipped with an FID detector was used. The GC-FID was operated using the same capillary column and chromatographic conditions as described for the GC–MS analysis. The injection temperature was 250 °C with an injection volume of 1 μL in the split mode with a split ratio of 100:1. Helium was used as carrier gas and was maintained in a constant pressure mode.
Statistical Analysis
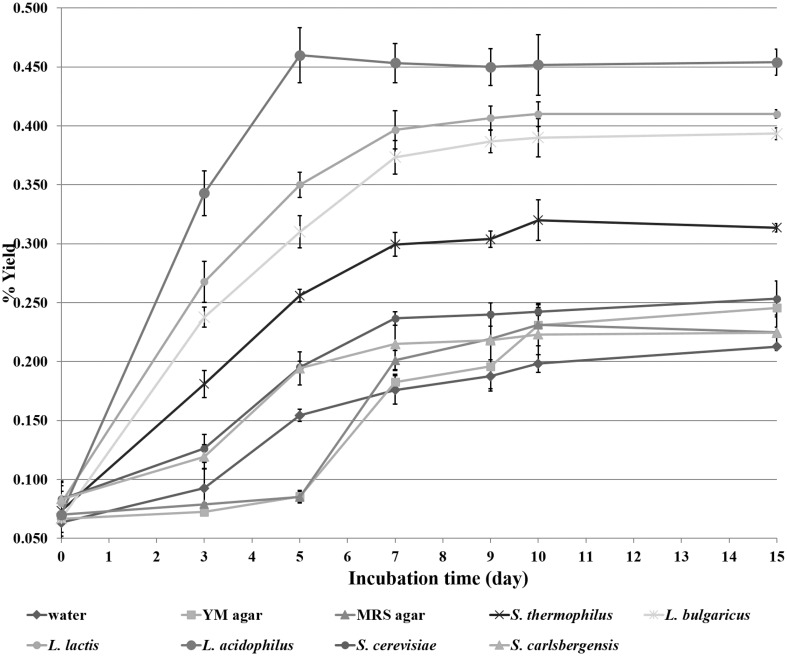
Statistical significance was analyzed by students t test and confidence limits were added at P < 0.05. Results were expressed as mean with error bar from standard deviation showing in Fig. 1.
Fig. 1.
Yield and incubation time of agarwood oil derived from incubation with different microbial culture supernatants
Results and Discussion
Yield of Agarwood Oil from Different Microbial Incubations
Essential oils of A. subintegra incubated with different microbial supernatants and extracted using a Clevenger-type apparatus appeared as yellow viscous liquids with percentage yields ranging from 0.21 to 0.45 (w/w). Lactic acid bacteria and yeast were employed in this study due to their safety, and common use in food and beverage manufacturing processes. Incubation with various supernatants resulted in different yields and colours of the extracted agarwood oils (Fig. 1). Incubation with lactic acid bacteria supernatants provided significantly higher yield of agarwood oil compared to yeast, the medium incubation, and water soaking, respectively. Among lactic acid bacteria, L. acidophilus TISTR 1338 provided the highest yield (approximately 0.45%), which was two-times that found in water incubation. The color of agarwood oils varied from pale yellow to orange. The colour and physical properties of agarwood oils obtained from incubation with lactic acid bacterial culture supernatants was pale yellow and similar to that obtained by water soaking, and medium incubations. To determine the optimal microbe for incubating A. subintegra woodchips, six safe and culinary microbes were selected. Water, MRS and YM agar medium supernatants showed poor efficiency in extracting essential oil, while lactic acid bacteria and yeast supernatants showed much greater efficiency. Oils obtained from yeast incubations, especially with S. cerevisiae, were orange in color. This may be explained by the presence of higher amounts of yeast alcohol dehydrogenase, which enhanced the rate of conversion of alcohols to aldehydes or ketones during incubation [20]. The volatile profiles of the oil derived from using the three different groups of microbes and media under the same conditions differed. The quantity of individual compounds can differ significantly depending upon the response factor of each microbe. It is clear that lactic acid bacteria gave the highest yield and number of volatile components compared to yeast and media (water, YM and MRS agar) under the same conditions., The efficiency of extraction of A. subintegra volatiles and essential oil decreased slightly, as seen by the lower number of volatiles and % yield, due to the different mechanism of yeast and water. Therefore lactic acid bacteria, especially L. acidophilus, could be appropriate for extracting essential oil from Aquilaria plants. The identified components from this study were similar to those obtained from previous reports [21–24], which reported terpenoids as the major constituents of agarwood essential oil.
Chemical Composition of Agarwood Oil from Different Microbial Incubations
Essential oils were subjected to detailed GC–MS analysis in order to identify their volatile constituents. The GC–MS analysis revealed a total of 70 volatile constituents, 52 of which were common in all three agarwood oil samples (Table 1). The obtained volatile profiles depended on the type of microbe used to incubate the woodchips. Similar volatile profiles were detected in agarwood fermented with different lactic acid bacterial species. S. carlsbergensis and S. cerevisiae also displayed similar volatile profiles. Overall, three major volatile profiles were obtained: one profile corresponded to water soaking (control), as well as, both YM and MRS media, the second profile was derived from lactic acid bacteria, and the third from the yeast supernatant incubation. Sesquiterpenes and their oxygenated derivatives were key components of agarwood oils. Major identified components observed in this work were similar to those previously reported by Pripdeevech et al. [25]. Due to the similarity of volatile profiles (mentioned above), agarwood oils obtained from 5-day incubations with water, L. acidophilus and S. cerevisiae were selected as representative samples to be discussed further, since they provided the highest yields within each group. Forty-seven volatile constituents were tentatively identified in the essential oil of A. subintegra obtained from incubating with water; the majority of the constituents, representing 83.08% of the relative peak area, were comprised of the dominant components β-agarofuran, α-eudesmol, karanone, α-agarofuran, agarospirol and isoamyl dodecanoate. Fifty-one constituents obtained from the A. subintegra essential oil incubated with L. acidophilus and representing 88.35% of the relative peak area, were identified. The principal volatiles were β-agarofuran, α-eudesmol, karanone, α-agarofuran, agarospirol and isoamyl dodecanoate. For the A. subintegra essential oil obtained from incubating with S. cerevisiae, 42 components were identified with the major components being β-agarofuran, phenyl acetaldehyde, α-agarofuran, karanone and α-eudesmol. Three volatile components including indole, E-cinnamyl alcohol and 5-hydroxy-cis-calamenene were detected only in agarwood oil obtained by incubation with lactic acid bacterial culture supernatants. Furfural was found only in the oil obtained by yeast supernatant incubation. Volatile constituents common in water soaking and incubation with all microbial supernatants included β-agarofuran, followed by α-eudesmol, karanone, α-agarofuran, agarospirol and isoamyl dodecanoate, however their concentration differed. For instance, significant differences were found for some other components, such as β-agarofuran (12.63–16.25%), phenyl acetaldehyde (0.91–10.32%), agarospirol (2.38–5.61%) and α-eudesmol (7.17–10.31%). The type of microbe used for incubation was an important factor affecting the generation of metabolites and their relative contents in agarwood oil. Incubation of A. subintegra woodchips induced production of various volatile components dependent on the enzyme production of each microbial strain. Overall, volatile components significantly increased when compared to the original, non-incubated sample. Moreover, the extracellular enzyme showed different substrate specificity to different aroma precursors. This might be due to enzymatic interaction between wood materials and extracellular enzymes within the culture supernatants of lactic acid bacteria and yeast. Naidu et al. [26] reported that compositional and processing properties as well as overall quality of food may be influenced by different extracellular enzymes of lactic acid bacteria secreted in the culture supernatant. To the best of our knowledge, this is the first time to report the use of microbial supernatants for improving the yield and volatile components of agarwood oil. Further investigation should aim at extracting and determining activity of extracellular active enzymes in culture supernatant incubation and their effect on agarwood oil composition. This would greatly aid in better understanding the overall process.
Table 1.
Volatiles in agarwood oil obtained by water soaking (control), L. acidophilus and S. cerevisiae culture supernatant incubations
| No. | Compound | RI | % relative peak area | |||
|---|---|---|---|---|---|---|
| RIcal | RIref | Water | L. acidophilus | S. cerevisiae | ||
| 1 | Furfural | 835 | 828 | – | – | 5.21 |
| 2 | Benzaldehyde | 960 | 952 | 0.09 | 0.10 | 2.90 |
| 3 | Phenyl acetaldehyde | 1057 | 1049 | 0.91 | 1.29 | 10.32 |
| 4 | Cuminaldehyde | 1245 | 1238 | 0.20 | 0.56 | 0.19 |
| 5 | trans-Cinnamaldehyde | 1273 | 1267 | – | 1.47 | 2.21 |
| 6 | Indole | 1296 | 1290 | – | 0.09 | – |
| 7 | E-Cinnamyl alcohol | 1309 | 1303 | – | 0.29 | – |
| 8 | p-Vinylguaiacol | 1315 | 1309 | 4.20 | 4.02 | 4.06 |
| 9 | E-Methyl cinnamate | 1382 | 1376 | 0.18 | 0.18 | 1.29 |
| 10 | E-Ethyl cinnamate | 1385 | 1376 | 0.41 | 0.71 | 0.26 |
| 11 | p-Vinylguaiacol | 1406 | 1398 | 0.75 | 1.11 | 1.70 |
| 12 | α-Elemene | 1460 | 1456 | 0.21 | 0.18 | 0.14 |
| 13 | α-Humulene | 1460 | 1456 | 0.08 | 0.36 | 0.22 |
| 14 | β-Agarofuran | 1511 | 1516 | 15.13 | 16.25 | 12.63 |
| 15 | β-Dihydroagarofuran | 1528 | 1520 | 0.63 | 1.08 | 0.35 |
| 16 | α-Bulnesene | 1526 | 1521 | 0.19 | 0.21 | 0.19 |
| 17 | β-Phenyl heptan-3-one | 1832 | 1524 | 0.10 | 0.27 | 0.11 |
| 18 | cis-Calamenene | 1534 | 1529 | 0.11 | 0.12 | 0.00 |
| 19 | α-Agarofuran | 1545 | 1540 | 8.05 | 8.17 | 8.64 |
| 20 | Nor-ketoagarofuran | 1565 | 1555* | 0.23 | 0.25 | 0.34 |
| 21 | γ-Undecalactone | 1575 | 1569 | 0.18 | 0.15 | 0.59 |
| 22 | β-Caryophyllene alcohol | 1578 | 1570 | 2.35 | 2.67 | 1.93 |
| 23 | Caryophyllene oxide | 1589 | 1582 | 1.41 | 0.06 | – |
| 24 | Geranyl isovalerate | 1612 | 1606 | 0.30 | 0.31 | 1.51 |
| 25 | 10-Epi-γ-eudesmol | 1628 | 1622 | 2.16 | 2.24 | 0.29 |
| 26 | Agarospirol | 1638 | 1630 | 5.49 | 5.61 | 2.38 |
| 27 | Epi-α-cadinol | 1645 | 1638 | 0.26 | 0.44 | 0.00 |
| 28 | β-Eudesmol | 1655 | 1649 | 0.73 | 0.79 | 0.24 |
| 29 | α-Cadinol | 1658 | 1652 | 0.19 | 0.47 | 0.30 |
| 30 | α-Eudesmol | 1659 | 1652 | 10.28 | 10.31 | 7.17 |
| 31 | Selin-11-en-4α-ol | 1667 | 1658 | 1.04 | 1.10 | 0.52 |
| 32 | Kusunol | 1668 | 1650* | 0.37 | 0.37 | 0.35 |
| 33 | β-Bisabolol | 1680 | 1674 | 0.27 | 0.31 | 0.34 |
| 34 | 5β,7βH,10α-eudesm-11-en-1α-ol | 1693 | 1687 | 0.21 | 0.34 | 0.18 |
| 35 | 2Z,6Z-Farnesol | 1704 | 1698 | 0.37 | 0.13 | – |
| 36 | Cyperotundone | 1705 | 1699 | 0.57 | 0.74 | 0.61 |
| 37 | β-Sinensal | 1706 | 1699 | 1.61 | 1.65 | 0.73 |
| 38 | Acorenone B | 1706 | 1700 | 1.94 | 1.98 | 1.65 |
| 39 | 5-Hydroxy-cis-calamenene | 1718 | 1713 | – | 0.33 | – |
| 40 | 2E,6Z-Farnesol | 1720 | 1714 | 0.36 | 0.39 | – |
| 41 | E-Nerolidol acetate | 1740 | 1735 | 0.18 | 0.49 | 0.20 |
| 42 | Selina-3,11-dien-14-ol | 1760 | 1753 | 0.31 | 0.25 | – |
| 43 | Selina-4,11-dien-14-al | 1764 | 1758 | 0.81 | 0.87 | – |
| 44 | Cyclocolorenone | 1768 | 1761 | 1.36 | 1.02 | – |
| 45 | Guaia-1(10),11-dien-15-ol | 1781 | 1770 | 0.44 | 0.19 | 1.03 |
| 46 | β-Eudesmol acetate | 1790 | 1784 | 0.24 | 0.17 | 0.25 |
| 47 | α-Bisabolol acetate | 1804 | 1798 | 0.25 | 0.16 | 0.17 |
| 48 | Nootkatone | 1812 | 1806 | 0.24 | 0.25 | – |
| 49 | Karanone | 1818 | 1812* | 9.47 | 9.63 | 7.64 |
| 50 | Oxo-agarospirol | 1828 | 1822* | 2.91 | 2.02 | 2.16 |
| 51 | Isoamyl dodecanoate | 1873 | 1869 | 5.25 | 5.84 | 4.64 |
| 52 | Spathulenol | 1896 | 1890 | 0.05 | 0.36 | 0.22 |
RI Retention indices using a HP-5 ms column, RIcal retention index calculated from retention time, all compounds have matching score ≥ 80% when compared to the NIST mass spectrum database. RIref retention index on HP-5 ms column from references [25, 27, 28]
*Retention indices using a DB-1 column; –, not detected
Conclusion
Lactic acid bacteria are appropriate microbes for use in agarwood incubation, as it can be readily cultured and mass-produced. Incubation of Aquilaria woodchips with lactic acid bacteria also exhibited enhanced yield and increased contents of most volatile compounds of agarwood essential oil. The number of components and yield of volatiles were probably affected by extracellular active enzymes released during incubation. Utilisation of lactic acid bacteria is expected to be valuable for further production of higher yields of agarwood oil. Incubation with lactic acid bacteria provides a safe and relatively inexpensive method to recover more agarwood essential oil in a short period of time.
Acknowledgements
The authors gratefully acknowledge support provided by the Mae Fah Luang University for funding. We are grateful to personnel of the Queen Sirikit Botanical Garden for their help in collection and identification of A. subintegra plants and the Scientific and Technological Instrument Centre of Mae Fah Luang University for their instrumental support concerning the GC–MS.
References
- 1.Yoswathana N. Extraction of agarwood (Aquilaria crassna) oil by using supercritical carbon dioxide extraction and enzyme pretreatment on hydrodistillation. Food Agric Environ. 2013;11:1055–1059. [Google Scholar]
- 2.Hashim YZHY, Ismail NI, Abbas P. Analysis of chemical compounds of agarwood oil from different species by gas chromatography mass spectrometry (GCMS) IIUM Eng J. 2014;15:55–60. [Google Scholar]
- 3.Okudera Y, Ito M. Production of agarwood fragrant constituents in Aquilaria calli and cell suspension cultures. Plant Biotechnol. 2009;26:307–315. doi: 10.5511/plantbiotechnology.26.307. [DOI] [Google Scholar]
- 4.Monggoot S, Popluechai S, Gentekaki E, Pripdeevech P. Fungal endophytes: an alternative source for production of volatile compounds from agarwood oil of Aquilaria subintegra. Microb Ecol. 2017;74:54–61. doi: 10.1007/s00248-016-0908-4. [DOI] [PubMed] [Google Scholar]
- 5.Jok VA, Radzi NC, Hamid KHK. Effect of soaking on the temperature and pH profiles in agarwood extraction. IJLRST. 2014;3:111–113. [Google Scholar]
- 6.Longo MA, Sanromán MA. Production of food aroma compounds: microbial and enzymatic methodologies. Food Technol Biotechnol. 2006;44:335–353. [Google Scholar]
- 7.Welsh FW, Murray WD, Williams RE, Katz I. Microbiological and enzymatic production of flavor and fragrance chemicals. Crit Rev Biotechnol. 1989;9:105–169. doi: 10.3109/07388558909040617. [DOI] [Google Scholar]
- 8.Leejeerajumnean A, Duckham SC, Owens JD, Ames JM. Volatile compounds in bacillus-fermented soybeans. J Sci Food Agric. 2001;81:525–529. doi: 10.1002/jsfa.843. [DOI] [Google Scholar]
- 9.Ouoba LII, Diawara B, Annan NT, Poll L, Jakobsen M. Volatile compounds of Soumbala, a fermented African locust bean (Parkia biglobosa) food condiment. J Appl Microbiol. 2005;99:1413–1421. doi: 10.1111/j.1365-2672.2005.02722.x. [DOI] [PubMed] [Google Scholar]
- 10.Krings U, Berger RG. Biotechnological production of flavors and fragrances. Appl Microbiol Biotechnol. 1998;49:1–8. doi: 10.1007/s002530051129. [DOI] [PubMed] [Google Scholar]
- 11.Vandamme EJ, Soetaert W. Bioflavours and fragrances via fermentation and biocatalysis. J Chem Technol Biotechnol. 2002;77:1323–1332. doi: 10.1002/jctb.722. [DOI] [Google Scholar]
- 12.Aguedo M, Ly MH, Belo I, Teixeira JA, Belin JM, Waché Y. The use of enzymes and microorganisms for the production of aroma compounds from lipids. Food Technol Biotechnol. 2004;42:327–336. [Google Scholar]
- 13.Palmeri R, Spagna G. β-Glucosidase in cellular and acellular form for winemaking application. Enzym Microb Technol. 2007;40:382–389. doi: 10.1016/j.enzmictec.2006.07.007. [DOI] [Google Scholar]
- 14.Fr B, Griebe T, Nielsen PH. Enzymatic activity in the activated-sludge floc matrix. Appl Microbiol Biotechnol. 1995;43:755–761. doi: 10.1007/BF00164784. [DOI] [PubMed] [Google Scholar]
- 15.Rathnam CKM, Edwards GE. Intracellular localization of certain photosynthetic enzymes in bundle sheath cells of plants possessing the C4 pathway of photosynthesis. Arch Biochem Biophys. 1975;171:14–225. doi: 10.1016/0003-9861(75)90026-0. [DOI] [PubMed] [Google Scholar]
- 16.Saifuddin N, Wong CW, Yasumira AA. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. J Chem. 2009;6:61–70. [Google Scholar]
- 17.Volesky B, Luong JH, Aunstrup K. Microbial enzymes: production, purification, and isolation. Crit Rev Biotechnol. 1984;2:119–146. doi: 10.3109/07388558409082583. [DOI] [Google Scholar]
- 18.Cui Y, Chatterjee A, Liu Y, Dumenyo CK, Chatterjee AK. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-l-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J Bacteriol. 1995;177:5108–5115. doi: 10.1128/jb.177.17.5108-5115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez-Porro C, Martin S, Mellado E, Ventosa A. Diversity of moderately halophilic bacteria producing extracellular hydrolytic enzymes. J Appl Microbiol. 2003;94:295–300. doi: 10.1046/j.1365-2672.2003.01834.x. [DOI] [PubMed] [Google Scholar]
- 20.Men L, Wang Y. The oxidation of yeast alcohol dehydrogenase-1 by hydrogen peroxide in vitro. J Proteome Res. 2007;6:216–225. doi: 10.1021/pr0603809. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara M, Tsuneya T. Components of the agarwood smoke on heating. J Essent Oil Res. 1993;6:120–123. [Google Scholar]
- 22.Näf R, Velluz A, Brauchli R, Thommen W. Agarwood oil (Aquilaria agallocha Roxb.). Its composition and eight new valencane-, eremophilane- and vetispirane-derivatives. Flavour Fragr J. 1995;10:147–152. doi: 10.1002/ffj.2730100306. [DOI] [Google Scholar]
- 23.Meier M, Kohlenberg B, Braun NA. Isolation of anisyl acetone from agarwood oil. J Essent Oil Res. 2003;8:340–345. [Google Scholar]
- 24.Ito M, Okimoto K, Yagura T, Hinda G, Kiuchi F, Shimada Y. Induction of sesquiterpenoid production by methyl jasmonate in Aquilaria sinensis cell suspension culture. J Essent Oil Res. 2005;17:175–180. doi: 10.1080/10412905.2005.9698867. [DOI] [Google Scholar]
- 25.Pripdeevech P, Khummueng W, Park SK. Identification of odor-active components of agarwood essential oils from Thailand by solid phase microextraction-GC/MS and GC-O. J Essent Oil Res. 2011;23:46–53. doi: 10.1080/10412905.2011.9700468. [DOI] [Google Scholar]
- 26.Naidu AS, Bidlack WR, Clemens RA. Probiotic spectra of lactic acid bacteria (LAB) Crit Rev Food Sci Nutr. 1999;38:13–126. doi: 10.1080/10408699991279187. [DOI] [PubMed] [Google Scholar]
- 27.Adams RP. Identification of essential oil components by gas chromatograph/mass spectrometry. 4. Carol Stream: Allured Publishing Corporation; 2007. [Google Scholar]
- 28.Babushok VI, Linstrom PJ, Zenkevich IG. Retention indices for frequently reported compounds of plant essential oils. J Phys Chem Ref Data. 2011;40:1–47. doi: 10.1063/1.3653552. [DOI] [Google Scholar]