Abstract
Chromium (Cr) released from industrial units such as tanneries, textile and electroplating industries is detrimental to the surrounding ecosystems and human health. The focus of the present study was to check the Cr(VI) removal efficiency by marine-derived fungi from liquid broth. Amongst the three Cr(VI) tolerant isolates, #NIOSN-SK56-S19 (Aspergillus sydowii) showed Cr-removal efficiency of 0.01 mg Cr mg−1 biomass resulting in 26% abatement of total Cr with just 2.8 mg of biomass produced during the growth in 300 ppm Cr(VI). Scanning Electron Microscopy revealed aggregation of mycelial biomass with exopolysaccharide, while Electron Dispersive Spectroscopy showed the presence of Cr2O3 inside the biomass indicating presence of active Cr(VI) removal mechanisms. This was further supported when the Cr(VI) removal was monitored using DPC (1,5-diphenylcarbazide) method. The results of this study point to the potential of marine-derived fungal isolates for Cr(VI) removal.
Electronic supplementary material
The online version of this article (10.1007/s12088-018-0719-z) contains supplementary material, which is available to authorized users.
Keywords: Biosorption, Halotolerant, Heavy metal, Hexavalent chromium, Marine-derived fungi
Introduction
Heavy metals mostly belong to the transition element category in the periodic table having densities of more than 5 gm cm−3 [1]. Anthropogenic activities such as effluent release from electroplating, tannery, mining and other industries lead to the release of toxic heavy metals into the environment, thereby polluting groundwater and soil [2, 3]. One such toxic heavy metal is Cr which occurs in + 3 and + 6 oxidation states, the latter being more toxic as it is water soluble and redox active. Various physical and chemical effluent treatment methods employed by industries have high costs and poor heavy metal removal efficiencies [4, 5]. Microbes such as fungi, bacteria possess cellular mechanisms to tolerate toxic metals such as extracellular/intracellular chelation using ligands, metallothioneins, and glutathione; enhanced efflux; subcellular compartmentation; enzymatic reduction; etc. [6–8]. This ability of fungi could be exploited for metal waste remedial purposes such as bio-mining and bioleaching. Fungi, either living or dead, have been reportedly used to bioremediate toxic metals, including biosorption of metals by isolates belonging to genera Aspergillus, Fusarium, Mucor, Penicillium and Saccharomyces [5].
Fungi inhabiting marine habitats such as mangroves and salt pans have been less explored for their metal tolerance potential [9]. These eukaryotes can tolerate high amounts of salts and have excellent efflux mechanisms to exclude cations [10]. Therefore, they can be expected to tolerate high concentrations of heavy metals as well. Various halophilic and halotolerant bacteria have already been reported to be multi-metal resistant to high salt concentrations [11]. Though the biosorption efficiency of heavy metals by terrestrial fungi is well-documented [12–14], marine fungi are sparsely studied for the same. The aim of the present study was to isolate fungi from selected marine niches, followed by screening them for various metal tolerance. Chromium being one the most toxic metals, Cr removal efficiency and its reduction rate have been studied using marine-derived fungi.
Materials and Methods
Sampling Site and Isolation of Halotolerant Fungi
In the present study, fungal cultures were previously isolated from sediments collected from Divar mangroves (15°29′N, 74°12′E at low tides during the year 2012–2014, Supplementary Fig. 1, unpublished work), India and Arabian Sea [cruise track of different sampling stations at 15°30′N, 73°40′E to 12°47′N, 74°01′E (Supplementary Fig. 2). The sediments from the Arabian Sea were collected with the help of box grab from depths ranging up to 3000 m, during February and October 2013 onboard ORV Sindhu Sankalp (SSK046 and SSK056 resp.)]. The sediment salinity of mangroves sediment varied from 32 to 35 PSU, and pH close to 6–6.5 with temperatures between 29 and 40 °C depending on the season. Approximately 0.5 g sediment samples were aseptically suspended in 10 mL of sterile seawater. Soil (sediment) suspension of 0.1 mL was spread-plated on 10, 20 and 30% of sea-salt (data in paper under review) containing different media (HiMedia) like potato dextrose Agar (PDA), Czapek Dox agar (CDA), malt extract agar (MEA), corn meal agar (CMA), Saboraud’s dextrose agar (SDA), Zobell marine agar (ZMA) and 50% sediment agar (SA), so as to isolate maximum numbers of halotolerant fungi. All the media supplemented with antibiotic (Cloxacillin and Ampicillin—750 µg/mL) and fungicide (Cycloheximide—100 µg/mL) to prevent the growth of rapid proliferators. In case of Arabian Sea sediments, the suspension aliquots were spread-plated on 1:5 strength media as above prepared in seawater. The main objective of the study was to isolate halotolerant fungi and check their tolerance to Cr, Cu, Ni, and Cd. All plates were incubated at room temperature (28 °C) for 5 days to a month. Morphologically distinct fungal colonies were sub-cultured to get pure cultures and maintained on CDA at 5 °C.
Screening for Heavy Metal Tolerance
Based on growth and colony morphology, 234 salt tolerant fungal isolates were selected for primary screening at 500 ppm of metal (Nickel, Copper, Chromium and Cadmium) concentration in CDA. Filter sterilized metal solution [stock solutions of Ni (1 M—58,693 ppm), Cu (1 M—63,546 ppm), Cr (100 mM—10,400 ppm) and Cd (2 M—224,820 ppm)] was added to autoclaved agar media to give a desired concentration of the concerned metal. Fungal isolates were initially screened at 500 ppm of all four metals in batches of 15 isolates at a time. As the number of Cr-tolerant isolates was very less compared to other metals, the concentration for checking Cr-tolerance was reduced to 250 ppm. One week old isolates grown in CDA prepared in seawater were cut in the form of 7 mm discs and placed inverted on metal agar plates for 10 days. The subsequent screening was carried out at 750 ppm of Cu, Cd, Ni and 500 ppm of Cr for selected 75 isolates that grew in previous screening. Sixteen isolates showing growth in the first screening, were further screened at 1000 ppm of Cu, Cd and Ni and 750 ppm of Cr. Further, 15 isolates from these were screened and 1250 ppm of Cu, Cd and Ni and at 1000 ppm of Cr. Metal Tolerance Index (MTI) in percent, was calculated for the selected 15 isolates using the following formula [15]:
Identification of Selected Isolates by ITS Sequencing
DNA extraction for three isolates that showed high tolerance to Cr was done using ZR Fungal/Bacterial DNA MicroPrep Kit (Zymo Research Corp., USA). PCR amplification of the ITS region was performed using ITS primers (ITS1—TCCGTAGGTGAACCTGCGG and ITS4—TCCTCCGCTTATTGATATGC) [16]. Amplification program followed was—initial denaturation step at 95 °C for 5 min; denaturation step at 95 °C for 1 min; annealing temperature of 52 °C for 45 s; elongation step at 72 °C for 1 min and a final elongation step at 72 °C for 10 min. Step 2, 3 and 4 were repeated for 35 cycles. The amplicons were stored at 4 °C and then purified using AxyPrep PCR Cleanup Kit (Axygen Biosciences, USA). The purified PCR products were then uni-directionally sequenced using Applied Biosystems (ABI) 3730 DNA stretch sequencer, with the XL upgrade and the ABI prism big dye terminator version 3.1 Cycle sequencing ready reaction kit. The raw sequences were analyzed using the BLAST tool [17] to get the closest reference sequences available at NCBI. The partial ITS sequences were then submitted to GenBank database. Phylogenetic tree was constructed for the ITS sequences of all selected three isolates along with ITS sequences of type strains and other isolates showing closest hits, using the Neighbor-Joining method at bootstrap value of 1000 using the MEGA software version 5.05 [18].
Determination of Chromium Content Residual Broth Using Atomic Absorption Spectroscopy (AAS)
Three isolates that showed tolerance to chromium (750 ppm screening) were selected to estimate their Cr removal efficiency from liquid broth. Starter inoculum was prepared by incubating one 7 mm disc of isolate cut from the periphery of growing culture, in 100 mL Erlenmeyer flasks with 20 mL medium (CDB in distilled water). Before the onset of sporulation, the mycelia were broken evenly using glass beads and, 1 mL of this inoculum suspension was inoculated into 20 mL of CDB prepared in distilled water containing increasing concentrations of Cr(VI) (0, 50, 100, 200, and 300 ppm) in triplicates. The pH of the broth was adjusted to 5 using 6N HCl. After incubation for one week at 80 rpm at 28 °C (room temperature), the contents of the flasks (duplicates) were filtered through 0.45 µm Millipore filter paper to separate broth from biomass. The filtrate was acidified using 2% of trace metal grade HNO3 and Cr (total) content in broth was estimated using AAS (Thermo Scientific Solar S series, Thermo Electron Corporation, Cambridge). Further, the biomass was dried, weighed and acid digested using 1:1 ratio of HNO3: HClO4 [modified from 19] to check if biomass was involved in the removal of Cr (total). The remnants of digestion were dissolved in 2% of trace metal grade HNO3 prepared in MilliQ water, and Cr (total) content was estimated using AAS.
Estimation of Hexavalent Chromium Reduction Rate
The selected isolates were inoculated in 20 mL medium (CDB in distilled water) in the form of a disc (as mentioned before) as starter inoculum. The mycelial matt was crushed using sterile glass beads before the onset of sporulation. One mL of this crushed mycelial suspension was inoculated in 20 mL of CDB supplemented with 50 ppm of Cr (maximum biomass was obtained at this concentration from the previous experiment, therefore this concentration was chosen). The pH of the medium supplemented with Cr was adjusted to 5 using 6N HCl, and these flasks with mycelial inoculum were incubated for 1 week at 80 rpm at room temperature. Cr(VI) was estimated spectrophotometrically at 540 nm (UV2450, Shimadzu Corporation, Japan) using DPC method [20] every day. Further to detect whether varying chromium oxidation states gave different absorption spectra, the selected isolates were inoculated in 250 mL Erlenmeyer flasks with 100 mL medium (CDB in distilled water) amended with 50 ppm of Cr (pH adjusted to 5). The flasks with mycelial inoculum were incubated for 20 days at 80 rpm at room temperature. Every alternate day 1 mL of medium was filtered using syringe filter (0.22 µm) and spectrophotometrically analyzed using UV–VIS spectrophotometer (UV-2450, Shimadzu Corporation, Japan).
Morphological Analysis of Mycelia Grown in the Presence of Chromium Using Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS) Analysis
The selected isolates were grown in CDB prepared in distilled water and supplemented with Cr(VI) to compare their mycelial morphology in presence and absence of Cr(VI), using scanning electron microscopy following a modified protocol from [21]. The biomass fixed in 2% glutaraldehyde was dehydrated with series of ethanol concentrations. The dehydrated mycelia were then gold-coated (SPI Module Sputter coater, USA) and observed using scanning electron microscope (JSM 5800 LV SEM and EDS, Japan). The same set of samples were used for EDS analysis.
Results and Discussion
Isolation of Marine-Derived Fungi from Mangrove and Sea Sediments
Divar mangroves being located along the Mandovi estuary (Supplementary Fig. 1), face a direct tidal effect along with the constant influence of disturbances caused by barge movement carrying metal ore, across the estuary. The off-shore sediment samples collected from the Arabian Sea (Supplementary Fig. 2) sea-bed are comparatively less affected by anthropogenic activities. A total of 380 fungal isolates were obtained, out of which 145 isolates from mangrove sediments and 175 isolates from Arabian Sea sediments survived on sub-culturing, while 60 isolates did not grow further. The isolates were maintained on CDA plates till further studies. Coastal marine habitats such as mangroves and mudflats are rich in organic matter [22] and harbor diverse forms of fungi [23], which are the initiators for any decomposition processes [22]. Due to the presence of recalcitrant organic material, these sediments act as bio-filters for heavy metals [24] and other toxic substances. Mangroves and salt pans are expected to harbor heavy metal-tolerant bacteria and fungi as these microbes are well adapted to exclude sodium ions out of their system [25] and can also do the same with metals.
Screening of the Fungal Isolates for Heavy Metal Tolerance
Majority of the isolates showed tolerance to 500 ppm concentration of Ni, Cu, and Cd, but as the concentration increased further, the number of isolates tolerating the heavy metals decreased. Cadmium and Chromium showed the least MTI values, proving to be more toxic than the other two metals. It was observed that as the concentration of metal increased in the agar plate, the diameter of the fungal colony decreased. Incipient inhibition of mycelial growth due to Ni stress was reported to be 50–100 ppm for A. niger and Trichoderma viride, while total inhibition of growth was attained at 1000 ppm [26]. In such cases, only metal tolerant and metal resistant species survive, while the rest cease to grow. Similar results were seen with respect to the toxicity of metals, where the number of lead-tolerant isolates decreased with increase in lead concentration on PDA [4]. Most of the fungal isolates were tolerant to Ni on the agar plate (152 isolates) followed by Cu (61 isolates) and Cd (54 isolates), while least tolerant to Cr (16 isolates) at 500 ppm concentration. Chromium was found to be the most toxic amongst all the metals under consideration, where only four isolates were able to grow at 750 ppm concentration, and one isolate grew at 1000 ppm concentration. Some of the isolates showed a reduction in sporulation at higher metal concentrations, while some completely stopped growing (Supplementary Fig. 3). Reduction in sporulation has been reported when A. terreus and Alternaria alternata were grown in increasing concentrations of Cu on CDA [27]. Some of the fungal colonies showed blue coloration on Cu-containing agar plates. Change in colour of the biomass can be attributed to the accumulation of metal inside the mycelia as reported in case of formation of copper oxalate crystals when subjected to copper stress [28].
On calculating MTI percentage for 15 selected isolates (Table 1), it was observed that MTI% decreased with increasing metal concentration, while some isolates (#NIOSN-M98, #NIOSN-SK56-S19) showed a sudden increase in metal tolerance at higher metal concentration and later showed a reduction in tolerance to metal. Some isolates like #NIOSN-M20, #NIOSN-M29, #NIOSN-M110, and #NIOSN-SK56-S19 also showed 100% MTI, as the growth in metal plate exceeded growth in control plate. Tolerance index more than 1.0 was reported in a study where the fungal culture was subjected to adaptive tolerance to metal [29]. Another study reported up to 7 times higher fungal activity in soil supplemented with Cu when compared to control without metal [30]. In both these studies, it appears that the presence of metal is stimulating the growth, which might be happening in some cases in the present study where there is a sudden increase in metal tolerance. MTI values were used to further short-list the isolates for molecular identification.
Table 1.
Metal tolerance index for 15 fungal isolates subjected to different concentrations of metals
| Culture nos. | Metal tolerance index (MTI%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nickel (ppm) | Copper (ppm) | Cadmium (ppm) | Chromium (ppm) | |||||||||||||
| 500 | 750 | 1000 | 1250 | 500 | 750 | 1000 | 1250 | 500 | 750 | 1000 | 1250 | 250 | 500 | 750 | 1000 | |
| #NIOSN-M20 | 100 | 70 | 58 | 46 | 64 | 76 | 36 | 22 | 50 | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| #NIOSN-M29 | 100 | 70 | 36 | 40 | 64 | 80 | 38 | 22 | 40 | 36 | 38 | 46 | Nil | Nil | Nil | Nil |
| #NIOSN-M38 | 34 | 43 | Nil | Nil | 40 | 43 | Nil | Nil | 34 | 43 | Nil | Nil | Nil | Nil | Nil | Nil |
| #NIOSN-M56 | 36 | 24 | Nil | Nil | Nil | Nil | Nil | Nil | 22 | Nil | Nil | Nil | 36 | 42 | Nil | Nil |
| #NIOSN-M84 | Nil | 84 | 34 | 48 | Nil | 60 | 52 | Nil | 44 | 48 | 34 | 56 | 32 | Nil | Nil | Nil |
| #NIOSN-M86 | 90 | 44 | Nil | Nil | 30 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | 30 | 42 | 34 | Nil |
| #NIOSN-M98 | 43 | 57 | 68 | 45 | 75 | 81 | 57 | 28 | 34 | 32 | 36 | 51 | Nil | Nil | Nil | Nil |
| #NIOSN-M110 | 100 | 82 | 48 | 50 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | 42 | 42 | Nil | Nil |
| #NIOSN-SK56-S19 | 88 | 100 | 58 | 42 | Nil | Nil | Nil | Nil | 30 | Nil | Nil | Nil | 34 | 40 | 22 | 32 |
| #NIOSN-SK56-S43 | 78 | 72 | Nil | Nil | Nil | 68 | 30 | Nil | 24 | Nil | Nil | Nil | 22 | Nil | Nil | Nil |
| #NIOSN-SK56-S52 | 94 | 90 | 60 | 36 | Nil | Nil | Nil | Nil | 32 | Nil | Nil | Nil | 46 | 46 | 32 | Nil |
| #NIOSN-SK56-S54 | 75 | 55 | 34 | Nil | 34 | 53 | 30 | Nil | 28 | Nil | Nil | Nil | 36 | 32 | Nil | Nil |
| #NIOSN-SK56-S57 | 76 | 56 | 58 | 24 | 50 | 76 | 32 | Nil | 26 | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| #NIOSN-SK56-S76 | 53 | 58 | 49 | 42 | 27 | 53 | 31 | Nil | 33 | Nil | Nil | Nil | 42 | 36 | 29 | Nil |
| #NIOSN-SK56-C15 | 94 | 72 | 64 | Nil | Nil | Nil | Nil | Nil | 28 | Nil | Nil | Nil | 32 | 40 | Nil | Nil |
The isolates marked in bold were selected for further studies
Identification of Selected Cr-Tolerant Fungal Isolates Using ITS Sequencing
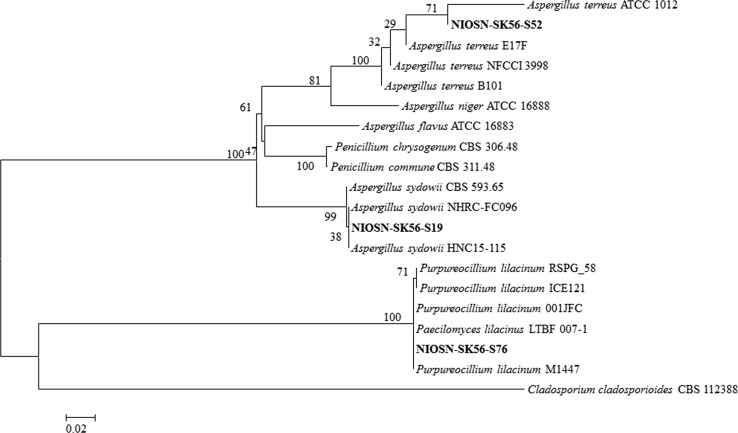
On phylogenetic analysis of ITS rDNA of selected isolates, #NIOSN-SK56-S19, #NIOSN-SK56-S52 and #NIOSN-SK56-S76, they grouped with similar sequences of type strains and were identified as Aspergillus sydowii (KT956258), Aspergillus terreus (KT956259) and Purpureocillium lilacinum respectively (KY788339). The evolutionary tree was constructed using Neighbour-Joining method (Fig. 1). All the isolates belonged to phylum Ascomycota and are reported to be rapid proliferators. The isolates #NIOSN-SK56-S19 (A. sydowii), #NIOSN-SK56-S52 (A. terreus) and #NIOSN-SK56-S76 (P. lilacinum) belonged to orders of Eurotiales and Hypocreales respectively. It has been reported that most of the isolates that could effectively tolerate metal, either as live or dead biomass, belong to these genera [13, 29–31].
Fig. 1.
Phylogenetic analysis of fungal isolates (ITS sequences) inferred from NJ analysis using MEGA software
Determination of Total Chromium Content in Biomass and Residual Broth
It was observed that the isolate P. lilacinum (#NIOSN-SK56-S76) showed substantial growth at all Cr concentrations, whereas isolates A. sydowii (#NIOSN-SK56-S19) and A. terreus (#NIOSN-SK56-S52) showed a decrease in biomass production with an increase in metal concentration (Table 2). Less biomass at higher metal concentrations can be attributed to the ceasing of growth and death due to stress. The concentration of Cr in biomass was also found to decrease with increase in metal concentration in these two isolates, which suggested that the Cr concentration in biomass is directly proportional to the active growth of culture and their biosorption capabilities. Interestingly in the present work, it was found that even minute quantity of biomass of 2.8 mg, as in case of isolate A. sydowii, resulted in up to 26% removal of Cr from the broth at 300 ppm. But the Cr removal efficiency of biomass, in this case, was 0.01 mg Cr mg−1 biomass, when compared to 25.1 mg biomass obtained from 50 ppm flask that resulted in 22% decrease with an efficiency of 0.007 mg Cr mg−1 biomass. Further, isolate P. lilacinum did not show high percent removal although it produced more biomass as compared to other isolates. Despite showing highest total Cr-content in biomass at 300 ppm, the decrease in total Cr content was 14% with an efficiency of 0.008 mg Cr mg−1 biomass. The dead or severely affected biomass, which is also known to be a good biosorbent as reported previously [32, 33] could be responsible for high percent removal of Cr (Table 3). Untreated biomass of the isolates #NIOSN-SK56-S19 and #NIOSN-SK56-S52 had more Cr removal efficiency than previously reported in pre-treated R. arrhizus [34]. Jakubiak et al. [35] reported that live biomass shows more biosorption than dead biomass. Apart from the type of culture affecting metal uptake, pH of the broth also plays an important role in bioavailability of metals. At very low pH, H+ ions compete with metal ions for binding sites, while metal solubility decreases as the pH increases. Therefore an acidic pH is most suitable for these types of studies [36], because of which, a final pH of 5 was chosen for this experiment. At acidic pH, Cr(VI) gives anionic species like Cr2O72−, HCrO4−, and Cr2O42− which get attracted to the protonated surface of fungal mycelia [36, 37]. The acidic pH keeps the Cr ions in soluble form and also facilitates adsorption on the mycelial cell walls. Apart from chitin and melanin components of cell walls, production of extracellular material such as organic acids is also responsible for metal binding [6, 7]. Considering the experimental and instrumental error, it was observed that, on adding the Cr content in broth and biomass, some amount of Cr was left unaccounted, which needs to be investigated further.
Table 2.
Determination of total Chromium content in residual broth and biomass of Cr-tolerant isolates
| Isolate number | Concentration of chromium (ppm) | Absolute Cr content (mg) | Dry weight of biomass (mg) | Cr content in digested biomass (mg) | Cr content per mg of dried biomass (mg) | Residual Cr content (mg) in broth | % removal of Cr in broth |
|---|---|---|---|---|---|---|---|
| #NIOSN-SK56-S19 | Control-0 | 0 | 141.8 | 0 | 0 | 0 | Nil |
| 50 | 1 | 25.1 | 0.186 ± 0 | 0.007 | 0.779 ± 0.011 | 22.1 | |
| 100 | 2 | 19.4 | 0.157 ± 0 | 0.008 | 1.947 ± 0 | 2.65 | |
| 200 | 4 | 1.6 | 0.007 ± 0 | 0.004 | 3.417 ± 0.023 | 14.575 | |
| 300 | 6 | 2.8 | 0.029 ± 0 | 0.01 | 4.450 ± 0.023 | 25.833 | |
| #NIOSN-SK56-S52 | Control-0 | 0 | 73.2 | 0 | 0 | 0 | Nil |
| 50 | 1 | 50.4 | 0.39 ± 0 | 0.008 | 0.592 ± 0.016 | 40.8 | |
| 100 | 2 | 34.6 | 0.364 ± 0 | 0.01 | 1.975 ± 0.046 | 1.25 | |
| 200 | 4 | 6.5 | 0.053 ± 0 | 0.008 | 3.565 ± 0.023 | 10.875 | |
| 300 | 6 | 4 | 0.05 ± 0 | 0.013 | 4.598 ± 0.140 | 23.367 | |
| #NIOSN-SK56-S76 | Control-0 | 0 | 169.1 | 0 | 0 | 0 | Nil |
| 50 | 1 | 76.6 | 0.15 ± 0 | 0.002 | 0.751 ± 0.032 | 24.9 | |
| 100 | 2 | 80.7 | 0.28 ± 0 | 0.004 | 2.134 ± 0.040 | Error | |
| 200 | 4 | 50.6 | 0.264 ± 0.1 | 0.005 | 3.286 ± 0.040 | 17.85 | |
| 300 | 6 | 63.6 | 0.523 ± 0 | 0.008 | 5.154 ± 0.161 | 14.1 |
Table 3.
Comparison of Chromium removal using fungal isolates in the present study with that reported in literature
| S. no | Fungal isolate | Isolated from | Sample/effluent | Cr concentration | Cr removal/recovery | References |
|---|---|---|---|---|---|---|
| 1 | Aspergillus niger (pre-treated) | Industrial waste contaminated soil | K2Cr2O7 solution | 4 mM | 18.05 mg Cr g−1 biomass | [2] |
| 2 | Penicillium sp. (pre-treated) | Industrial waste contaminated soil | K2Cr2O7 solution | 4 mM | 19.30 mg Cr g−1 biomass | [2] |
| 3 | Rhizopus arrhizus (pre-treated) | Not mentioned | K2Cr2O7 solution | 100 ppm | 9.02 mg Cr g−1 biomass | [35] |
| 4 | Rhizopus arrhizus (pre-treated) | Not mentioned | K2Cr2O7 solution | 300 ppm | 23.07 mg Cr g−1 biomass | [49] |
| 6 | Rhizopus nigricans (pre-treated) | Not mentioned | K2Cr2O7 solution | 50 mM | 47 mg Cr g−1 biomass | [50] |
| 7 | Trichoderma longibrachiatum | Industrial and sewage waste contaminated water | Potato dextrose broth | 50 ppm | 0.55 mg Cr g−1 biomass | [4] |
| 8 | Trichoderma viridae | Industrial and sewage waste contaminated water | Potato dextrose broth | 50 ppm | 2.55 mg Cr g−1 biomass | [51] |
| 9 | Aspergillus sydowii (#NIOSN-SK56-S19) | Arabian Sea sediment | Czapek Dox broth | 300 ppm | 10 mg Cr g−1 biomass | Present study |
| 10 | Aspergillus terreus (#NIOSN-SK56-S52) | Arabian Sea sediment | Czapek Dox broth | 300 ppm | 13 mg Cr g−1 biomass | Present study |
| 11 | Purpureocillium lilacinum (#NIOSN-SK56-S76) | Arabian Sea sediment | Czapek Dox broth | 300 ppm | 8 mg Cr g−1 biomass | Present study |
Reduction in Cr(VI) Content During the Growth of Fungal Isolates as Determined by DPC Method
On determining the chromium reduction using DPC, which is a method for specific detection of Cr(VI) state, it was observed that isolate A. terreus (#NIOSN-SK56-S52) was the most efficient culture (Fig. 2). It reduced the total Cr(VI) state to Cr(III) in 3 days while isolate A. sydowii reduced the same in 7 days. Surprisingly, isolate P. lilacinum did not participate significantly in the reduction of Cr(VI) to Cr(III) and was able to survive in toxic Cr(VI) state indicating the presence of an alternate mechanism for survival.
Fig. 2.
Determination of Cr(VI) reduction using Diphenylcarbazide (DPC) method
To further investigate, whether chromium under different oxidation states showed different absorption peaks, filtered broth samples were subjected to UV–VIS spectroscopy. Both the isolates belonging to Aspergillus genera, #NIOSN-SK56-S19 (Fig. 3b) and #NIOSN-SK56-S52 (Fig. 3c), showed a shift in peak from 390 to 310 nm where #NIOSN-SK56-S52 showed a gradual increase in intensity while #NIOSN-SK56-S19 exhibited a rapid increase in intensity by the 20th day of incubation. It was observed that the control flask gave a peak at 370 nm at all days (Fig. 3a) indicating there is no change in chromium oxidation state due to any chemical redox reactions. #NIOSN-SK56-S76 (Fig. 3d) did not show any shift in peaks and was comparable to the control. Peak shift from 390 to 310 nm for #NIOSN-SK56-S52 and #NIOSN-SK56-S19 indicated change in Cr form due to biological reduction. Previous studies have also reported absorption peak at 300 nm in UV–VIS spectrum attributing to Cr nanoparticles [38–40]. A separate study reported nano-sized Cr2O3 particles showed absorption peak at 460 nm [41] while another study [42] depicted that the inter-band transitions within the core electrons of chromium to chromium oxide give a peak at 445 nm indicating the presence of Cr2O3 nanoparticles. Cr(VI) being a highly oxidizing agent, easily gets transformed into Cr(III) in the presence of organic matter [43] which might have led to shifts in the peak position as well as its intensity. It was therefore concluded that absorption peaks for chromium changed with respect to oxidation state and not due to nanoparticle formation.
Fig. 3.
Absorption spectra of cell-free culture broth obtained growth of fungal isolates in chromium supplemented culture broth using UV–VIS spectrophotometry. a Control. b Isolate A. sydowii. c Isolate A. terreus. d Isolate P. lilacinum. The arrows indicate the Cr peaks in all the four spectra
SEM and EDS Analysis
The effect of metal stress on fungal surface morphology was studied by using SEM. Isolate A. sydowii was seen to produce an extracellular thread-like substance (Fig. 4b) at 50 ppm Cr stress, while such thread-like substance was not observed in control (absence of metal) (Fig. 4a). Extracellular aggregation in between the mycelial network was seen in A. terreus (Fig. 4d) when subjected to Cr stress and this focuses on an important point that aggregates could be the possible sites of biosorption in these cultures. SEM image of P. lilacinum (Fig. 4f—arrow) showed irregularly shaped mycelia, while the mycelia in control were found to be healthy and intact (Fig. 4e—arrow). Interesting observation for this particular isolate was that it efficiently produced more biomass (Table 2) under metal stress but did not produce any extracellular material that could possibly adsorb the metal onto its surface as evident from SEM image (Fig. 4f) to combat the stress. This particular isolate also did not aid in the reduction of Cr(VI) to Cr(III) as mentioned earlier. Primary sites for biosorption to occur are the cell walls that contain huge proportions of chitin, melanin and phenolic compounds which provide oxygen-rich metal-binding sites [31, 44, 45]. This extracellular material could be most probably responsible for biosorption, as exopolysaccharide (EPS) is known to biosorb heavy metals [46]. Nanoparticle aggregation was reported on the mycelial cell wall of Pleurotus eryngii exposed to Al2O3 compared to the non-exposed mycelia [35]. Subbaiah and Yun [14] reported the presence of pores and deformed mycelia when used as biosorbent in an aqueous solution of Ni.
Fig. 4.

SEM images fungal isolates grown at 50 ppm Cr and without Cr. a Isolate A. sydowii grown in 0 ppm Cr. b Isolate A. sydowii grown in 50 ppm Cr showing production of extracellular material. c Isolate A. terreus grown in 0 ppm Cr. d Isolate A. terreus grown in 50 ppm Cr showing extracellular aggregation within mycelial growth. e Isolate P. lilacinum grown in 0 ppm Cr. f Isolate P. lilacinum grown in 50 ppm Cr showing deflation of mycelia
Gold-sputtered mycelia used for SEM analysis were also studied for EDS analysis to understand the form of Cr present within the biomass. The salt used in the broth was K2Cr2O7 which gives Chromium anion with an oxidation state of + 6. On EDS analysis, the compound detected in the samples was Cr2O3, with an oxidation state of + 3 (Fig. 5). Therefore the presence of Cr2O3 in the mycelia of the isolates highlights the ability of the organisms to convert Cr+6 to Cr+3 state through a reduction process. Conversion of Cr+6 to Cr+3 state as seen from EDS analysis indicates that these organisms might have multiple metal tolerance mechanisms. This was also supported by the DPC method as discussed above, which is specific to the detection of Cr(VI). The decrease in concentration of Cr(VI) over an incubation period of a week till no more Cr(VI) was measured, indicated a process of conversion of detectable state of Cr(VI) to undetectable form (Cr(III)). This is the first report of fungi causing direct Cr reduction as determined by DPC method as opposed to the extensive literature available for bacteria. Zahoor and Rehman [20] reported up to 85% reduction of Cr(VI) by a Bacillus sp. within 96 h of incubation. In another study, O. intermedium and Brevibacterium sp. were able to reduce Cr(VI) entirely by 72 and 96 h respectively [47]. Reduction of Cr+6 to Cr+4 can occur with two electrons participating in the initial reduction stage and then further getting reduced to Cr+3 in the second step [8]. Reduction of Cr+6 to Cr+3 with Cr+5 as an intermediate in a two-step reaction has also been reported in Pseudomonas ambigua [48]. The results indicated towards not only single but multiple metal tolerance mechanisms existing in these isolates, which needs to be explored further to understand the conversion of toxic Cr(VI) to lesser-toxic form Cr(III).
Fig. 5.
EDS spectra of fungal isolates grown at 300 ppm Cr showing the presence of Cr2O3. a Isolate A. sydowii. b Isolate A. terreus. c Isolate P. lilacinum. The arrows indicate the Cr peaks in all the three spectra
Conclusion
The diversity of microorganisms from marine environments is of growing interest and have already found applications in various fields of science. The salt-tolerant species isolated from marine environments, able to grow at high concentrations of salt, possessing a high resistance to heavy metals have excellent potential as agents for reduction of pollution in saline conditions and also in non-saline environments. The analysis carried out in this study serves as an indication of the potential of various marine-derived fungal isolates for metal bioremediation. The fungi isolated from marine environments such as mangroves and open ocean sediments tolerated high concentrations of heavy metals and even converted toxic Cr+6 to lesser toxic Cr+3 form as seen in this study. Further studies are required before proposing the ability of the cultures to be used for designing bioremediation strategy to remove harmful metals from the effluent discharges of tanneries or dye industries which are rich in chromium.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The first author is thankful to Council of Scientific and Industrial Research (CSIR) for the fellowship (Ref No.: 18-12/2011(ii)EU-V). All the authors are thankful to Head, BOD and Director, CSIR-NIO for the facilities. The studies were funded through the Project PSC0206. The authors are thankful to Mr. Areef Sardar for carrying out SEM and EDS analysis. This work is part of the doctoral thesis to be submitted to Goa University in Department of Microbiology. The manuscript is NIO contribution No. 6186.
Compliance with Ethical Standards
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12088-018-0719-z) contains supplementary material, which is available to authorized users.
References
- 1.Beveridge TJ, Hughes MN, Leung KT, Poole RK, Savvaidis I, Silver S, Trevors JT. Metal-microbe interactions: contemporary approaches. Adv Microb Physiol. 1997;38:177–243. doi: 10.1016/S0065-2911(08)60158-7. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad I, Ansari MI, Aqil F. Biosorption of Ni, Cr and Cd by metal tolerant Aspergillus niger and Penicillium sp. using single and multi-metal solution. Indian J Exp Biol. 2006;44:73–76. [PubMed] [Google Scholar]
- 3.Hasan HAH. Role of rock phosphate in alleviation of heavy metals stress on Fusarium oxysporum. Plant Soil Environ. 2007;53:1–6. doi: 10.17221/2288-PSE. [DOI] [Google Scholar]
- 4.Joshi PK, Swarup A, Maheshwari S, Kumar R, Singh N. Bioremediation of heavy metals in liquid media through fungi isolated from contaminated sources. Indian J Microbiol. 2011;51:482–487. doi: 10.1007/s12088-011-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishnoi NR, Garima Fungus- an alternative for bioremediation of heavy metal containing wastewater: a review. J Sci Ind Res India. 2005;64:93–100. [Google Scholar]
- 6.Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Biol. 2002;53:1–11. [PubMed] [Google Scholar]
- 7.Bellion M, Courbot M, Jacob C, Blaudez Chalot M. Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi—a minireview. FEMS Microbiol Lett. 2006;254:173–181. doi: 10.1111/j.1574-6968.2005.00044.x. [DOI] [PubMed] [Google Scholar]
- 8.Borut P, Istvan P, Peter R, Pesti M. Interference of chromium with biological systems in yeasts and fungi: a review. J Basic Microbiol. 2010;50:21–36. doi: 10.1002/jobm.200900170. [DOI] [PubMed] [Google Scholar]
- 9.Nazareth S, Gaitonde S, Marbaniang T. Metal resistance of halotolerant fungi from mangroves and salterns of Goa, India. Kavaka. 2012;40:15–21. [Google Scholar]
- 10.Gunde-Cimerman N, Ramos J, Plemenitas A. Halotolerant and halophilic fungi: a review. Mycol Res. 2009;113:1231–1241. doi: 10.1016/j.mycres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Hamedi J, Fatemeh M, Hamed KSP. Biotechnological exploitation of actinobacterial members. In: Maheshwari DK, Saraf M, editors. Halophiles—biodiversity and sustainable exploitation. Switzerland: Springer; 2015. [Google Scholar]
- 12.Rulcker CK, Allard B, Schnurer J. Interactions between a soil fungus, Trichoderma harzianum, and IIb metals-adsorption of mycelium and production of complexing metabolites. Biometals. 1993;6:223–230. [Google Scholar]
- 13.Parvathi K, Nareshkumar R, Nagendran R. Biosorption of manganese by Aspergillus niger and Saccharomyces cerevisiae. World J Microbiol Biot. 2007;23:671–676. doi: 10.1007/s11274-006-9281-7. [DOI] [Google Scholar]
- 14.Subbaiah MV, Yun YS. Biosorption of nickel (II) from aqueous solution by the fungal mat of Trametes versicolor (Rainbow) biomass: equilibrium, kinetics, and thermodynamic studies. Biotechnol Bioprocess Eng. 2013;18:280–288. doi: 10.1007/s12257-012-0401-y. [DOI] [Google Scholar]
- 15.Akhtar S, Hassan MM, Ahmad R, Suthor V, Yasin M. Metal tolerance potential of filamentous fungi isolated from soils irrigated with untreated municipal effluent. Soil Environ. 2013;32:55–62. [Google Scholar]
- 16.White TJ, Bruns T, Lee S, Taylor J, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, et al., editors. PCR protocols: a guide to methods and applications. New York: Academic Press; 1990. [Google Scholar]
- 17.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 18.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hseu ZY. Evaluating heavy metal contents in nine composts using four digestion methods. Biores Technol. 2004;95:53–59. doi: 10.1016/j.biortech.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Pfalaum R, Howick L. The chromium-diphenylcarbazide reaction. J Am Chem Soc . 1956;78:4862–4866. doi: 10.1021/ja01600a014. [DOI] [Google Scholar]
- 21.Damare VS. Diversity of thraustochytrid protists isolated from brown alga, Sargassum cinereum using 18S rDNA sequencing and their morphological response to heavy metals. J Mar Biol Assoc UK. 2015;95:265–276. doi: 10.1017/S0025315414001696. [DOI] [Google Scholar]
- 22.Holguin G, Vazquez P, Bashan Y. The role of sediment microorganisms in the productivity, conservation and rehabilitation of mangrove ecosystems: an overview. Biol Fertil Soils. 2001;33:265–278. doi: 10.1007/s003740000319. [DOI] [Google Scholar]
- 23.Raghukumar S, Raghukumar C, Manohar CS. Fungi living in diverse extreme habitats of the marine environment. Kavaka. 2014;42:145–153. [Google Scholar]
- 24.Chakraborty P, Ramteke D, Chakraborty S. Geochemical partitioning of Cu and Ni in mangrove sediments: relationships with their bioavailability. Mar Pollut Bull. 2015;93:194–201. doi: 10.1016/j.marpolbul.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Nayak SS, Gonsalves V, Nazareth SW. Isolation and salt tolerance of halophilic fungi from mangroves and solar salterns in Goa-India. Indian J Geomar Sci. 2012;41:164–172. [Google Scholar]
- 26.Babich H, Gamba-Vitalo C, Stotsky G. Comparative toxicity of nickel to mycelia proliferation and spore formation of selected fungi. Arch Environ Contam Toxicol. 1982;11:465–468. doi: 10.1007/BF01056073. [DOI] [Google Scholar]
- 27.Al-Abboud MA, Alawlaqi MM. Biouptake of copper and their impact on fungal fatty acids. Aust J Basic Appl Sci. 2011;5:283–290. [Google Scholar]
- 28.Zapotoczny S, Jurkiewicz A, Tylko G, Anielska T, Turnau K. Accumulation of copper by Acremonium pinkertoniae, a fungus isolated from industrial wastes. Microbiol Res. 2007;162:219–228. doi: 10.1016/j.micres.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Valix M, Loon LO. Adaptive tolerance behaviour of fungi in heavy metals. Miner Eng. 2003;16:193–198. doi: 10.1016/S0892-6875(03)00004-9. [DOI] [Google Scholar]
- 30.Rajapaksha RMCP, Tobor-Kaplon MA, Baath E. Metal toxicity affects fungal and bacterial activities in soil differently. Appl Environ Microbiol. 2004;70:2966–2973. doi: 10.1128/AEM.70.5.2966-2973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akthar MN, Sastry KS, Mohan PM. Mechanism of metal ion biosorption by fungal biomass. Biometals. 1996;9:21–28. doi: 10.1007/BF00188086. [DOI] [Google Scholar]
- 32.Faryal R, Lodhi A, Hameed A. Isolation, Characterization and biosorption of Zinc by indigenous fungal strains Aspergillus fumigatus RH05 and Aspergillus flavus RH07. Pak J Bot. 2006;38:817–832. [Google Scholar]
- 33.Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH. Biosorption of heavy metals : a review. J Chem Sci Technol. 2014;3:74–102. [Google Scholar]
- 34.Merrie JS, Sheela R, Saswathi N, Prakasham RS, Ramakrishna SV. Biosorption of chromium VI using Rhizopus arrhizus. Indian J Exp Biol. 1998;36:1052–1055. [Google Scholar]
- 35.Jakubiak M, Giska I, Asztemborska M, Bystrzejewska-Piotrowska G. Bioaccumulation and biosorption of inorganic nanoparticles: factors affecting the efficiency of nanoparticle mycoextraction by liquid- grown mycelia of Pleurotus eryngii and Trametes versicolor. Mycol Prog. 2014;13:525–532. doi: 10.1007/s11557-013-0933-3. [DOI] [Google Scholar]
- 36.Coogeevaram S, Dhanarani S, Park J, Dexilin M, Thamaraiselvi K. Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J Hazard Mater. 2007;146:270–277. doi: 10.1016/j.jhazmat.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Sen M. A comparative study on biosorption of Cr(VI) by Fusarium solani under different growth conditions. Open J Appl Sci. 2012;2:146–152. doi: 10.4236/ojapps.2012.23021. [DOI] [Google Scholar]
- 38.Annamalai K, Nair AM, Chinnaraju S, Kuppusamy S. Chromium (III) nanoparticles synthesis using the biosorption and bioreduction with Bacillus subtilis: effect of pH and temperature. Int J ChemTech Res. 2014;6:1910–1912. [Google Scholar]
- 39.Annamalai K, Nair AM, Chinnaraju S, Kuppusamy S. Removal of chromium from contaminated effluent and simultaneously green nanoparticle synthesis using Bacillus subtilis. Malaya J Biosci. 2014;1:13–18. [Google Scholar]
- 40.Chandra S, Kumar A. Spectral, thermal and morphological studies of chromium nanoparticles. Spectrochim Acta A. 2013;102:250–255. doi: 10.1016/j.saa.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Jaswal VS, Arora AK, Kinger M, Gupta VD, Singh J. Synthesis and characterization of chromium oxide nanoparticles. Orient J Chem. 2014;30:559–566. doi: 10.13005/ojc/300220. [DOI] [Google Scholar]
- 42.Mohite PT, Kumar AR, Zinjarde SS. Biotransformation of hexavalent chromium into extracellular chromium (III) oxide nanoparticles using Schwanniomyces occidentalis. Biotechnol Lett. 2016;38:441–446. doi: 10.1007/s10529-015-2009-8. [DOI] [PubMed] [Google Scholar]
- 43.Focardi S, Pepi M, Focardi SE. Microbial reduction of hexavalent chromium as a mechanism of detoxification and possible bioremediation applications. In: Chamy R, Rosenkranz F, editors. Biodegradation-life of science. Rijeka: InTech; 2013. pp. 321–347. [Google Scholar]
- 44.Baldrian P, Gabriel J, et al. Adsorption of heavy metal on microbial biomass: Use of biosorption for removal metals from metal solutions. In: Sasek V, et al., editors. The utilization of bioremediation to reduce soil contamination: problems and solutions. Dordrecht: Springer; 2003. [Google Scholar]
- 45.White C, Wilkinson SC, Gadd GM. The role of microorganisms in biosorption of toxic metals and radio nuclides. Int Biodeterior Biodegrad. 1995 [Google Scholar]
- 46.Liu Y, Lam MC, Fang HHP. Adsorption of heavy metals by EPS of activated sludge. Water Sci Technol. 2001;43:59–66. [PubMed] [Google Scholar]
- 47.Faisal M, Hasnain S. Comparative study of Cr(VI) uptake and reduction in industrial effluent by Ochrobactrum intermedium and Brevibacterium sp. Biotechnol Lett. 2004;26:1623–1628. doi: 10.1007/s10529-004-3184-1. [DOI] [PubMed] [Google Scholar]
- 48.Wang YT, Shen H. Bacterial reduction of hexavalent chromium. J Ind Microbiol. 1995;14:159–163. doi: 10.1007/BF01569898. [DOI] [PubMed] [Google Scholar]
- 49.Prakasham RS, Merrie JS, Sheela R, Saswathi N, Ramakrishna SV. Biosorption of chromium VI by free and immobilized Rhizopus arrhizus. Environ Pollut. 1999;104:421–427. doi: 10.1016/S0269-7491(98)00174-2. [DOI] [Google Scholar]
- 50.Sudha BR, Abraham TE. Biosorption of Cr(VI) from aqueous solution by Rhizopus nigricans. Bioresour Technol. 2001;79:73–81. doi: 10.1016/S0960-8524(00)00107-3. [DOI] [PubMed] [Google Scholar]
- 51.Kumar R, Singh P, Dhir B, Sharma AK, Mehta D. Potential of some fungal and bacterial species in bioremediation of heavy metals. J Nucl Phys. 2014;1:213–223. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.