Abstract
The health beneficial attributes of bifidobacteria and its safe association with the host gut has increased its significance as a probiotic. However delivering probiotic bifidobacteria with Minimum Biological Value (MBV) through product has always been a challenge. In the present study, an attempt was made to maintain the viability of native isolate of Bifidobacterium longum CFR 815j and deliver through ice-cream. B. longum CFR815j was microencapsulated in alginate starch capsules by emulsification followed by evaluation of bead stability in simulated gastrointestinal conditions. After incorporation in ice-cream, the effect on chemical properties, sensory parameters and meltdown characteristics of the product were also evaluated. Survival studies of B. longum revealed higher counts than 107 in the product which is essential for probiotic bacteria to exhibit beneficial effect. Further, all the properties of this ice-cream were comparable to the regular ice-cream. Our studies conclude that encapsulation was able to maintain the requisite MBV of bifidobacteria in ice-cream without affecting the sensory characteristics.
Electronic supplementary material
The online version of this article (10.1007/s12088-018-0720-6) contains supplementary material, which is available to authorized users.
Keywords: Bifidobacterium, Probiotic, Encapsulation, Ice cream
Introduction
Bifidobacteria supplementation as a probiotic is known to improve health of the host as well as prevent and alleviate various diseases [1]. Probiotics are available as pharmaceutical preparation and food formulations; the latter is generally preferred vis-à-vis former [2]. To have the desired effect, the organisms should be delivered in a viable form at an efficacious dosage (generally considered 107–108 cfu/day). However, the viability of the organisms declines during food processing and storage. Maintaining the viability of the bacteria till the shelf life of the product has been a major challenge for the probiotic industry. The food matrix influences the probiotic survival in the formulation throughout its shelf life, safe passage of probiotic to the targeted site and also the health benefits to the consumer [3].
Ice-cream is a popular product among people of all the age groups [4]. They are mainly composed of milk proteins, fat, and lactose, which can serve as a good matrix for delivery of probiotic bacteria. The pH range of 5.6–6.5 and the frozen storage condition may also facilitate the survival of the probiotic strains during storage [4]. However, the survival of bacteria in the product is influenced by many factors such as stress related to acidity, redox potential, pH, freezing, oxygen, sugar concentration, osmotic effects and mechanical shearing [5]. Encapsulation is a recent technique and can help in improving the viability of the bacteria in the product. It protects the bacteria during detrimental conditions of low pH, bile salts, the presence of oxygen, freezing and freeze-drying processes, etc. There are very few studies which report ice-cream as a medium of delivery for bifidobacteria [5–7]. Further, bifidobacteria a highly health beneficial probiotic, being anaerobic and less acid tolerant, ensuring its viability in such products has been a challenge. Encapsulation using alginate-starch and cryoprotectant (glycerol) has been reported for incorporation in yoghurt [8] however its efficiency in ice cream has not been evaluated.
In the present study, efficiency of encapsulation to improve the survivability of B. longum CFR815j, a native isolate in ice-cream by using combination of alginate starch and cryoprotectant (glucose) for encapsulation was evaluated.
Materials and Methods
Bacteria and Growth Conditions
Bifidobacterium longum CFR815j used in the study is a native laboratory isolate reported by Achi and Halami (data unpublished). B. longum CFR815j was activated from frozen stock culture by two successive subculturing in de Man, Rogosa and Sharpe (MRS) broth containing 0.05% w/v cysteine hydrochloride and incubating under anaerobic conditions at 37 °C for 24 h. Microbiological media and chemicals used in the study were procured by Hi Media Pvt Ltd, Mumbai, India.
Encapsulation Process
B. longum CFR815j was activated and the broth was centrifuged at 8000 rpm for 10 min. The biomass was harvested, washed twice with phosphate buffered saline (PBS) and resuspended in PBS. This fresh suspension was used for encapsulation.
For encapsulation by emulsification process method originally reported by Sheu and Marshall [9] and Sultana et al. [8] for alginate and alginate-starch combination was used respectively. Briefly, fresh cell suspension was mixed with 3.6% sodium alginate containing cryoprotective agent (6% glucose) at 1:4 ratio. This mix was then added dropwise to ghee containing tween 80 (0.2%) at 1:5 ratio. A uniform turbid solution was obtained by magnetic stirring for 10 min. Then 0.05 M calcium chloride solution was added quickly but gently (20 ml/s) till the water oil emulsion is broken. The beads were collected by low speed centrifugation at 350×g for 10 min at 4 °C. Combination of alginate-starch encapsulation material was prepared by adding 2% starch to 3% alginate mix containing 6% glucose and was compared to alginate alone.
Enumeration of Encapsulated Bifidobacteria
For release of the bacteria 1 g of beads was suspended in 9 ml of PBS (pH 7) with shaking at room temperature. Sample was then serially diluted and plated on Bifidobacterium agar. After incubation, under anaerobic conditions at 37 °C for 48 h the viable count was determined.
Scanning Electron Microscopy
The morphology of the beads were examined under SEM (Zeiss EVO LS15, Germany).
Tolerance to Acid and Bile Stress
The beads were studied for their stability at low pH (2.5) and bile salt (2%) as described by Shi et al. [10]. Briefly, 0.5 ml of culture/encapsulated microspheres were added into different tubes containing 4.5 ml of simulated gastric fluid (SGF) pH 2.5 or 2% bile salt solution and incubated at 37 °C for 90 min. After the specified time the viable cells were determined as mentioned above.
Release Study of B. longum CFR815j
In vitro release in simulated gastrointestinal conditions was checked for the beads as per the procedure described by Shi et al. [10]. 0.5 g of beads were added to 50 ml of prewarmed simulated intestinal fluid (50 mM K2HPO4) and incubated at 37 °C. At predetermined intervals, 100 µl of this solution was withdrawn and assayed for amount of released B. longum 815j. The amount of withdrawn sample was replaced with the same amount of fresh medium. The viable count of B. longum CFR815j was carried as mentioned previously.
Preparation of Probiotic Ice-Cream Using Microencapsulated B. longum CFR815j
Alginate starch microspheres formed after emulsification process were further incorporated into ice-creams prepared at a commercial outlet ‘Just Gelato’, Mysuru, Karnataka, India. Preparation of ice-creams was carried out in a 2.5 kg batch. All the liquid ingredients were heated and the solid ingredients were added to it and mixed well. Carrageenan and guar gum were used as stabilizers. The obtained mixture was homogenized and pasteurized at 85 °C for 30 min. Overnight aging of the mix was carried out at 4 °C. Cell suspension containing free cells (45 ml)/Alginate-starch beads (45 g) was then added to the batches respectively. Freezing was carried out in a batch freezer wherein the inlet temperature was 5 °C and the outlet temperature was − 12 °C. Overnight hardening was carried out at − 23 °C. Further storage of the ice-cream was done in − 20 °C freezer.
Viability Studies Under Storage Conditions
Survivability of bacteria was determined after 24 h of freezing and then on 5th, 10th and 15th day of storage. One ml of ice-cream samples were serially diluted in 9 ml of PBS and viable count was determined as described previously.
Analysis of Physiochemical Properties
The ice-cream was further evaluated for meltdown characteristics (as described by Muse et al. [11]), moisture content, protein content (Kjeldahl method) [12], fat content (Rose-Gottlieb method) [13], and sensory characteristics.
Results and Discussions
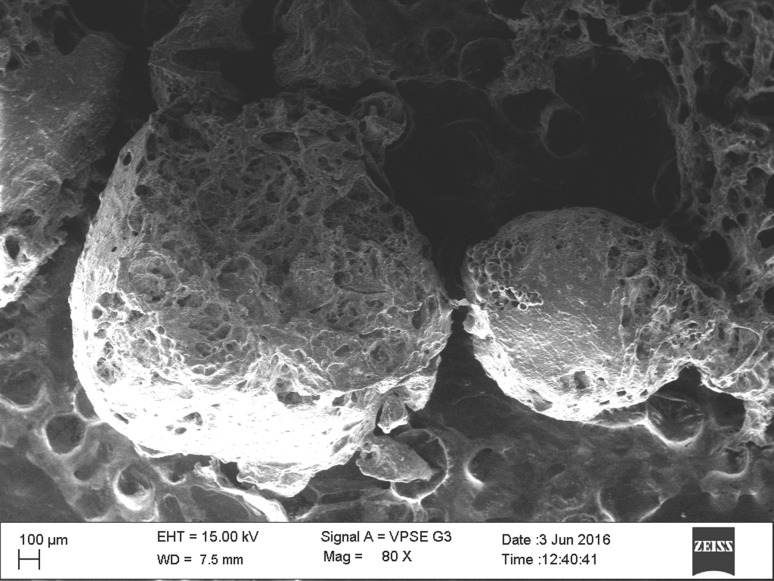
In our studies, emulsification was the method of choice for encapsulation. The addition of starch at a concentration of 2% to the alginate mix led to better recovery of beads per 100 ml of the alginate mix. Recovery of bifidobacteria in a combination of alginate and starch mix (2%) was 2 × 109 cfu/g whereas in alginate alone, it was 14 × 103 cfu/g. Mixing with starch is known to improve the stability of the beads and recovery of bacteria [10, 14]. The beads were found to be spherical in shape and had a size varying from 300 to 800 µm when examined under SEM (Fig. 1, S1). Under low pH conditions the free cell count decreased by 5.96 log cfu/ml whereas the encapsulated bacteria retained the viability of log 7.12 cfu/ml. Previous studies by Sultana et al. [8] and O’riordan et al. [15] have reported no effective protection for encapsulated cells in simulated gastric fluid (SGF) when compared to the free cells. However, an improvement in survival of bifidobacteria entrapped in alginate beads containing 0.3% soluble starch in SGF of pH 1.3 compared to alginate alone has also been recorded [16]. In our studies, a decrease of 0.5 log cfu/g microsphere and 6.56 log cfu/g in 2% bile salt solution was observed for encapsulated and free cells respectively at the end of incubation period. This was in accordance to the finding of Sultana et al. [8], wherein a slight decrease in count was observed for encapsulated B. infantis in simulated intestinal fluid (SIF). In-vitro release of microencapsulated B. longum CFR815j was investigated in SIF. It was noted that the capsule does get fractured upon exposure to intestinal fluid, but the fracture stress is constant up to 2 h after which there is an increase in release (Fig S2). Kamalian et al. [17] has reported a gradual increase in release in SIF during the first 150 min of incubation, further the release was noted to be faster in SIF than SGF.
Fig. 1.
Scanning electron microscopic image of the microcapsules
Meltdown characteristic of the ice-cream was analysed after incorporation of encapsulated bacteria. Meltdown rate was initially low i.e. for 10–15 min but after the melting process had started, the melting rate was similar to regular ice-cream (Fig S3). Very slight difference in the melting properties of probiotic and microencapsulated probiotic ice-cream was also reported by El- Sayed et al. [6]. The chemical properties, sensory studies of the ice-cream incorporated with encapsulated B. longum CFR815j were found comparable to the regular ice-cream (Table 1). Similar findings for starch and whey encapsulated Bifidobacterium has been recorded [6, 17]. Survival studies upon storage for a period of 15 days was performed, and it was noticed that there was a count decline of 40% in the ice-cream containing free cells but only a decline of 2.5% in encapsulated probiotic ice-cream. The encapsulated B. longum 815j in ice-cream retained the viable count of 107 cfu/ml (Fig S4). 30% improvement in the survival rate of probiotic bacteria (Lactobacillus casei and Bifidobacterium lactis Bb12) encapsulated using Ca alginate and starch in ice-cream during extended shelf life at − 20 °C has been recorded [7].
Table 1.
Chemical and organoleptic properties of probiotic ice cream compared to regular ice cream
| Samples | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Chemical propertiesa | Organoleptic propertiesa | |||||||
| Moisture (%) | Protein (%) | Fat (%) | pH | Flavour (50)b | Body and texture (40)b | Colour and appearance (10)b | Total (100)b | |
| Control | 68.87 ± 0.038 | 3.09 ± 0.03 | 11.12 ± 0.025 | 6.20 | 46.38 ± 4.12 | 37.75 ± 2.59 | 9.25 ± 0.66 | 93.38 ± 6.52 |
| Non-encapsulated | 67.66 ± 0.0003 | 3.21 ± 0.04 | 11.35 ± 0.307 | 5.90 | 43.00 ± 4.12 | 35.25 ± 2.38 | 8.63 ± 0.69 | 86.88 ± 4.81 |
| Encapsulated | 66.93 ± 0.233 | 3.055 ± 0.005 | 11.28 ± 0.065 | 6.16 | 45.50 ± 3.60 | 33.38 ± 4.47 | 8.75 ± 0.66 | 87.63 ± 6.76 |
aValues expressed as mean ± standard deviation (n = 3)
bMaximum points assigned to an organoleptic property
This study has ensured the viability of native isolate of B. longum in ice-cream. Encapsulation with the combination of starch and alginate can help in improving the viability of bifidobacteria in ice-cream and thus fulfill the criteria of probiotic products by serving the adequate amount of live bacteria. Such ice-creams enriched with bifidobacteria may find high demand among consumers as it ensures health and the consumers can relish the product.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to The Director; CSIR-Central Food Technological Research Institute, Mysuru, India, for providing necessary funds and facilities. SA would like to acknowledge CSIR for granting Senior Research Fellowship. This work was supported by Council of Scientific and Industrial Research, New Delhi under VIIth Five-year plan project (BSC0202).
Funding
This work was funded by Council of Scientific and Industrial Research (CSIR), New Delhi under VIIth Five-year plan project (BSC0202).
Compliance with Ethical Standards
Conflict of interest
The Authors declared that they have no conflict of interests.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12088-018-0720-6) contains supplementary material, which is available to authorized users.
References
- 1.Achi SC, Halami PM. Bifidobacterial probiotics through fermented foods. In: Kalia VC, Shouche Y, Purohit HJ, Rahi P, editors. Mining of microbial wealth and metagenomics. Singapore: Springer; 2017. pp. 267–285. [Google Scholar]
- 2.Saxelin M. Probiotic formulations and applications, the current probiotics market, and changes in the marketplace: a European perspective. Clin Infect Dis. 2008;46:S76–S79. doi: 10.1086/523337. [DOI] [PubMed] [Google Scholar]
- 3.Sanders ME, Marco ML. Food formats for effective delivery of probiotics. Annu Rev Food Sci Technol. 2010;1:65–85. doi: 10.1146/annurev.food.080708.100743. [DOI] [PubMed] [Google Scholar]
- 4.Cruz AG, Antunes AE, Sousa ALO, Faria JA, Saad SM. Ice-cream as a probiotic food carrier. Food Res Int. 2009;42:1233–1239. doi: 10.1016/j.foodres.2009.03.020. [DOI] [Google Scholar]
- 5.Mohammadi R, Mortazavian AM, Khosrokhavar R, da Cruz AG. Probiotic ice cream: viability of probiotic bacteria and sensory properties. Ann Microbiol. 2011;61:411–424. doi: 10.1007/s13213-010-0188-z. [DOI] [Google Scholar]
- 6.EL-Sayed HS, Salama HH, EL-Sayed SM. Production of synbiotic ice cream. Int J ChemTech Res. 2014;7:138–147. [Google Scholar]
- 7.Homayouni A, Azizi A, Ehsani MR, Yarmand MS, Razavi SH. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem. 2008;111:50–55. doi: 10.1016/j.foodchem.2008.03.036. [DOI] [Google Scholar]
- 8.Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K. Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yogurt. Int J Food Microbiol. 2000;62:47–55. doi: 10.1016/S0168-1605(00)00380-9. [DOI] [PubMed] [Google Scholar]
- 9.Sheu TY, Marshall RT. Microentrapment of lactobacilli in calcium alginate gels and solubilisation there from. J Food Sci. 1993;58:557–561. doi: 10.1111/j.1365-2621.1993.tb04323.x. [DOI] [Google Scholar]
- 10.Shi L, Li Z, Li D, Xu M, Chen H, Zhang Z, Tand Z. Encapsulation of probiotic Lactobacillus bulgaricus in alginate–milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J Food Eng. 2013;117:99–104. doi: 10.1016/j.jfoodeng.2013.02.012. [DOI] [Google Scholar]
- 11.Muse MR, Hartel RW. Ice cream structural elements that affect melting rate and hardness. J Dairy Sci. 2004;87:1–10. doi: 10.3168/jds.S0022-0302(04)73135-5. [DOI] [PubMed] [Google Scholar]
- 12.Gibson RB. The determination of nitrogen by the Kjeldahl method. J Am Chem Soc. 1904;26:105–110. doi: 10.1021/ja01991a014. [DOI] [Google Scholar]
- 13.Standard IDF (1987) 116A, milk based ices and ice-mixes. Determination of fat content—Rose Gottlieb Gravimetric method. International Dairy Federation, Brussels, Belgium
- 14.Krasaekoopt W, Bhandari B, Deeth H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int Dairy J. 2003;13:3–13. doi: 10.1016/S0958-6946(02)00155-3. [DOI] [Google Scholar]
- 15.O’riordan K, Andrews D, Buckle K, Conway P. Evaluation of microencapsulation of a Bifidobacterium strain with starch as an approach to prolonging viability during storage. J Appl Microbiol. 2001;91:1059–1066. doi: 10.1046/j.1365-2672.2001.01472.x. [DOI] [PubMed] [Google Scholar]
- 16.Woo CJ, Lee KY, Heo TR. Improvement of Bifidobacterium longum stability using cell-entrapment technique. J Microbiol Biotechnol. 1999;9:132–139. [Google Scholar]
- 17.Kamalian N, Mirhosseini H, Mustafa S, Manap MYA. Effect of alginate and chitosan on viability and release behavior of Bifidobacterium pseudocatenulatum G4 in simulated gastrointestinal fluid. Carbohydr Polym. 2014;111:700–706. doi: 10.1016/j.carbpol.2014.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.