Abstract
Pathogenic variant in COCH are a known cause of DFNA9 autosomal dominant progressive hearing loss and vestibular dysfunction with adult onset. Hitherto, only dominant nonsynonymous variants and in-frame deletions with a presumed dominant negative or gain-of-function effect have been described. Here, we describe two brothers with congenital prelingual deafness and a homozygous nonsense c.292C>T(p.Arg98*) COCH variant, suggesting a loss-of-function effect. Vestibular dysfunction starting in the first decade was observed in the older patient. The heterozygous parents and sibling have normal hearing and vestibular function, except for the mother, who shows vestibular hyporeflexia and abnormal smooth pursuit tests, most likely due to concomitant disease. This is the first report of autosomal recessive inheritance of cochlea-vestibular dysfunction caused by a pathogenic variant in the COCH gene. An earlier onset of hearing impairment and vestibular dysfunction compared to the dominant hearing loss causing COCH variants is observed.
Introduction
The prevalence of permanent bilateral sensorineural hearing loss (HL) of 40 dB or more is estimated at 1–1.5 per 1000 newborns in Western countries [1]. The majority of congenital hearing loss has a monogenic cause [1]. More than 70% of hereditary hearing loss is non-syndromic, mostly with autosomal recessive inheritance pattern [1]. These autosomal recessive forms are predominantly associated with prelingual (often congenital) severe to profound hearing loss, in contrast to the autosomal dominant forms of HL, which are essentially post-lingual [1].
The coagulation factor C homology (COCH) gene (OMIM 603196) is located on chromosome 14 in bands q12–13 [2]. It contains 12 exons and it is structurally organized as a short predicted signal peptide (SP), an N-terminal factor C homology (FCH) domain (later referred to as LCCL for Limulus factor C, Cochlin, Lung gestational protein) and two von-Willebrand factor A-like domains (vWFA-1 and 2) [2]. To date, there are 25 different known COCH mutations: 23 missense and 2 in-frame deletions. Sixteen are located in exons 4 or 5, encoding the LCCL domain and nine are located in the vWFA domains [3–6].
All of the COCH hearing loss causing variants reported today are nonsynonymous variants with a presumed dominant negative or gain-of-function effect, causing progressive late-onset vestibular deterioration and hearing impairment [3–8]. In this paper, we report a novel homozygous c.292 C>T (p.Arg98*) nonsense variant in a consanguineous family with Moroccan roots, causing moderate prelingual sensorineural hearing loss with vestibular dysfunction. The phenotype presents an autosomal recessive mode of inheritance and audiological as well as vestibular characteristics are different from all other known pathogenic variants in COCH.
Case details
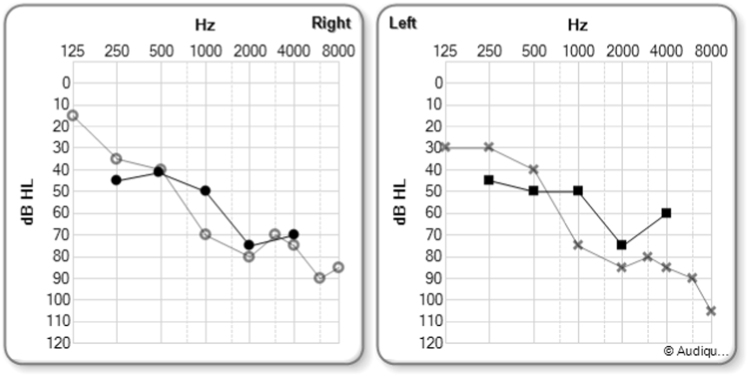
Two brothers were referred to our center (within a 5 years interval) with HL detected by universal newborn hearing screening (NHS) using automated auditory brainstem response audiometry (AABR). They belong to a Belgian family (family AI) with Moroccan roots, consisting of normal hearing consanguineous parents with 4 children, including the two brothers with prelingual (congenital) moderate sensorineural hearing loss (Fig. 1). Both Probands (AI-010 and AI-011) were first seen at age 4 weeks due to bilateral failed hearing screening tests (AI-010 in 2007, AI-011 in 2016). Gestation history and physical examination were unremarkable as well as middle ear otoscopy and high-frequency admittancy tests for both probands. Transient (TEAOE) and distortion product (DPOAE) emissions were absent in all ears. Auditory Brainstem Response audiometry (ABR) during natural sleep showed bilateral moderate hearing loss with thresholds of 50 dB nHL bilaterally in both referred probands. Proband’s AI-010 pure tone audiometry showed a bilateral symmetrical down-sloping sensorineural hearing loss with pure tone average (PTA) (0.5; 1 and 2 kHz) of 47 dB HL (right ear) and 50 dB HL (left ear) at the age of 1 year, slightly declining to 63 dB HL in the right, and 68 dB HL in the left ear at the age of 4 years (Fig. 2) and remained unchanged until present (age of 9 years). The PTA of proband AI-011 were measured 55 dB HL in the right and 57 dB HL in the left ear (aged 7 mounths). Complete physical, serologic, hematologic, urinary, cardiac, renal and ophthalmologic examinations were unremarkable for both brothers and proband’s AI-010 Computed tomography (CT) and magnetic resonance imaging (MR) did not show any typical focal sclerotic lesions in any of the semicircular canals similar described in COCH c.151 C>T (P.Pro51Ser) positive patients [9]. Videonystagmography (VNG) showed bilateral symmetrical, but marked hypo-reflective caloric response with normal, albeit high amplitudes on pendular chair responses in proband AI-010. Proband AI-011 showed normal clinical vestibular tests, including head impulse tests (HIT) and velocity stop tests.
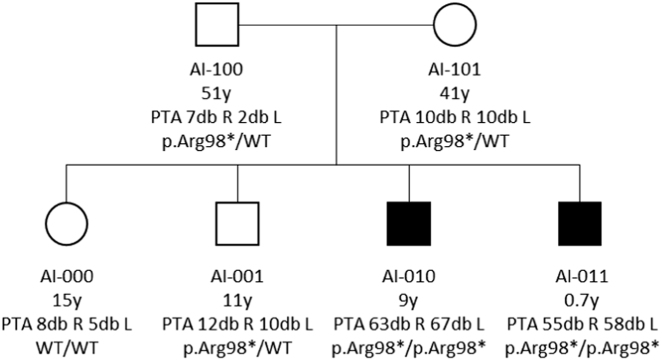
Fig. 1.
The AI-family pedigree. The two hearing impaired brothers (AI-010 and AI-011) are homozygous for the c.292C>T(p.Arg98*) nonsense variant. Pure tone average (PTA) of right (R) and left (L) ears is represented in decibel hearing level (dB HL) for each family member. The older proband shows signs of prolonged vestibular impairment. All other family members, including one older sister (AI-000) and one older brother (AI-001), as well as both parents (AI-100 and AI-101), have normal hearing. All of them, except the older sister (AI-000), are carrying a heterozygous c.292C>T(p.Arg98*) variant
Fig. 2.
Pure Tone Audiometry (PTA) of AI-010 and AI-011, the two hearing impaired brothers of the AI-family. AI-010 is 9 years old and AI-011 is 7 months old. Both are homozygous for c.292C>T(p.Arg98*) variant in COCH. The hearing thresholds of proband AI-010 are depicted with hollow dots for the right and cross signs for the left ear. Those of his younger brother AI-011 are displayed with black dots for the right and squares for the left ear. Both parents (AI-100 and AI-101, resp. 51 and 41 years) have normal PTA, as well as the two older siblings AI-000 (15 y) and AI-001 (11 y) (not shown in this figure). The hearing losses are sensorineural. Note the down-sloping character of the thresholds
All other family members showed normal hearing (Fig. 1). The father (AI-100, aged 51) and older brother (AI-001, aged 11) showed normal clinical vestibular examination as well as unremarkable VNG.
Videonystagmography of the mother (AI-101, aged 41) showed a marked hyporeflexia of the right vestibular organ on caloric tests (76%) with normal caloric response on the left, with balanced pendular function, suggesting long-standing peripheral dysfunction. She also presented signs of central vestibular dysfunction, which are currently under investigation (see Supplementary Material). Table 1 summarizes phenotypic presentation in terms of hearing and vestibular function of all AI-family members.
Table 1.
Overview of phenotypic presentation of all AI-family members
| AI-100 | AI-101 | AI-000 | AI-001 | AI-010 | AI-011 | |
|---|---|---|---|---|---|---|
| Age (y) | 51 | 41 | 15 | 11 | 9 | 0.7 |
| Gender | Male | Female | Female | Male | Male | Male |
| Phenotype p.Arg98* | +/− | +/− | −/− | +/− | +/+ | +/+ |
| HL (dB) Right ear | 7 | 10 | 8 | 12 | 63 | 55 |
| HL (dB) Left ear | 2 | 10 | 5 | 10 | 67 | 58 |
| HIT test | Normal | Abnormal right side | Normal | Normal | Abnormal right and left side | Normal |
| Smooth pursuit | Normal | Saccadic | Normal | Normal | Normal | Normal |
| Velocity stop tests | N/A | N/A | Normal | Normal | Abnormal right & left VOR | Normal |
| VNG (oculomotor function) | Normal | Saccadic smooth pursuit & hypometric saccades tests | N/A | Normal | Normal | N/A |
| VNG (caloric function) | Normal | Hyporeflexia (76%) right side | N/A | Normal | Hyporeflexia right & left sides (50%) | N/A |
| VNG (pendular function) | Normal | Normal | N/A | Normal | Normal (high amplitude) | N/A |
VNG videonystagmography, N/A not available, HL hearing loss, VOR vestibulo-ocular reflexes. VNG was not carried out on AI-011, the youngest male proband, who is homozygeous for the c.292C>T(p.Arg98*) variant in COCH, due to age limitations. Heterozygous carriers AI-100 and AI-001 show normal vestibular function, both on clinical as well as on functional investigations (VNG). Only heterozygous carrier AI-101, mother of both probands, shows hyporeflexia on the right vestibular organ and abnormal central oculomotor function
Mutation analysis
DNA was prepared from EDTA blood samples by standard procedures. Because first-line Sanger sequencing mutation analysis of the GJB2 coding region after PCR did not reveal a pathogenic variant in GJB2, the DNA of both brothers was screened by Next Generation Sequencing of a Haloplex (Agilent, Santa Clare, CA, USA) enriched gene panel containing 98 genes known to be implicated in non-syndromic hearing loss (Table 2 Supplementary Material).
In both brothers, a homozygous nonsense variant c.292[C>T];[C>T](p.[Arg98*];[Arg98*]) was detected in exon 5 of the COCH gene (reference sequence NM_004086.2/NP_004077.1) (Clinvar ID: SCV000588227). No pathogenic variants that could explain the hearing loss were detected in the other genes analyzed. Sanger sequencing confirmed the presence of this homozygous nonsense variant in the two brothers and showed both parents (AI-100 and AI-101) and one other sib (AI-001) to be heterozygous carriers of this variant. One unaffected sibling (AI-000) did not show c.292C>T(p.Arg98*).
Expression analysis: (Supplementary Material)
RNA of both patients and heterozygous parents was analyzed for COCH expression. Reverse transcriptase–PCR (RT–PCR) amplification and agarose gel electrophoresis of a fragment of the COCH gene showed reduced COCH expression in the heterozygous parents and almost no detectable expression in both patients (Fig. 3 Supplementary Material).
Sequencing analysis (data not shown) and single nucleotide extension analysis showed in both parents a more than 90% reduction of the mutant allele at the RNA level (r.292c>u) compared to the DNA level (Fig 3 Supplementary Material).
Discussion
Nonsynonymous pathogenic variants in the coagulation factor C homology COCH gene cause autosomal dominant adult-onset non-syndromic hearing loss [2, 10–12]. These variants are thought to act in a dominant negative or gain-of-function fashion. Several pathogenic mechanisms have been proposed to explain the effect of dominant COCH mutations. These include misfolding and accumulation of mutant cochlin aggregates, formation of dimers of mutant cochlins and heterodimer formation of mutant-wild type cochlins, disruption of normal cellular trafficking and altered post-translational processing or increase of propensity of covalent protein dimers affecting structural stability [11, 13, 14].
In the current study, we have identified a homozygous c.292C>T(p.Arg98*) variant in a family with autosomal recessive HL. In contrast to the previously reported pathogenic COCH variant (with alleged dominant negative or gain-of-function effect), this variant is expected to be a recessive loss of function variant. It is reported one time in the ExAC variant database (supplementary material). No homozygous loss-of-function variants are reported in the ExAC database (>119,000 alleles) for COCH.
We have performed RNA analysis of the family members, and confirmed that the mutant RNA is the subject of nonsense mediated RNA decay. Also in the coch−/− mouse, absence of cochlin by western blot and RT–PCR analysis is in agreement with this hypothesis [15].
The majority of the currently known pathogenic variants in COCH cause hearing impairment that typically starts with a high-frequency loss in the 2nd to 4th decade and show a progressive decline to very poor auditory performance in the 6th or 7th decade [2, 10–12]. A missense variant located in the vWFA1 domain, namely p.Cys162Tyr, segregates in a Chinese family with autosomal dominant post-lingual hearing loss, albeit appearing at a much younger mean age of onset (17 years) [16]. The phenotype observed with the homozygous COCH c.292C>T(p.Arg98*) nonsense variant is that of bilateral down-sloping sensorineural hearing loss, which is already present at birth, and therefore readily detectable by NHS. Human bi-allelic loss-of-function COCH variants therefore seem to cause an earlier age of onset compared to the dominant gain-of-function variants. This is different compared to the coch mouse models, where coch−/− mice show no signs of hearing loss in the first year of life and show elevated thresholds or absent ABRs at the high frequencies at the age of 21 months. Heterozygous cochG88E/+ and homozygous cochG88E/G88E mice show hearing loss at all frequencies at this age [17]. Similar to the coch+/− heterozygous mice, and a previously reported woman harboring a heterozygous COCH c.146dupT(p.Cys50Leufs*8) frameshift variant [18], also heterozygous carriers of the c.292C>T(p.Arg98*) variant show normal hearing. It is noteworthy that in the mouse models, coch G88E/G88E and coch−/−, the vestibular dysfunction for both the dominant and recessive models precedes loss of hearing function. Moreover, coch−/− mice also show vestibular malfunction, although only at later ages [17]. No vestibular dysfunction or hearing loss has been reported in the heterozygous carriers in our family, except for patient AI-101, the proband’s mother. However there are several arguments suggesting that this may be unrelated to her COCH carrier status: the vestibular dysfunction is restricted to only one vestibular organ, the long-standing hyporeflexia could have appeared congenitally or at very young age and finally concomitant central degenerative disease cannot be ruled out in this patient. Further clinical and functional vestibular follow-up to determine the vestibular phenotype at older age has to be performed in patients and heterozygous carriers.
Unfortunately, no additional family members of older generations are available for testing at the moment.
Electronic supplementary material
case details, mutation analyze, rna analysis
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1038/s41431-017-0066-2) contains supplementary material, which is available to authorized users.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shearer AE, Hildebrand MS, Smith RJH. Hereditary Hearing Loss and Deafness Overview.In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mefford HC, Stephens K, Amemiya A, Ledbetter N, editors.GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017.1999 Feb 14 [updated 2017 Jul 27]. NCBI Bookshelf:https://www.ncbi.nlm.nih.gov/books/NBK1434.
- 2.Robertson NG, Khetarpal U, Gutierrez-Espelata GA, Bieber FR, Morton CC. Isolation of novel and known genes from a human fetal cochlear cDNA library using subtractive hybridization and differential screening. Genomics. 1994;23:42–50. doi: 10.1006/geno.1994.1457. [DOI] [PubMed] [Google Scholar]
- 3.Bae SH, Robertson NG, Cho HJ, et al. Identification of pathogenic mechanisms of COCH mutations, abolished cochlin secretion, and intracellular aggregate formation: genotype-phenotype correlations in DFNA9 deafness and vestibular disorder. Hum Mutat. 2014;35:1506–13. doi: 10.1002/humu.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung J, Choi JY, Kim HS, et al. Novel COCH p.V123E mutation, causative of DFNA9 sensorineural hearing loss and vestibular disorder, shows impaired cochlin post-translational cleavage and secretion. Human Mut. 2015;36:1168–75. doi: 10.1002/humu.22855. [DOI] [PubMed] [Google Scholar]
- 5.Tsukada K, Ichinose A, Miyagawa M, et al. Detailed hearing and vestibular profiles in the patients with COCH mutations. Ann Otol Rhinol Laryngol. 2015;124:100S. doi: 10.1177/0003489415573074. [DOI] [PubMed] [Google Scholar]
- 6.Hildebrand MS, Luke Gandolfo AES, et al. A novel mutation in COCH-implications for genotype-phenotype correlations in DFNA9 hearing loss. Laryngoscope. 2010;120:2489–93. doi: 10.1002/lary.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picciani R, Desai K, Guduric-Fuchs J, Cogliati T, Morton CC, Bhattacharya SK. Cochlin in the eye: functional implications. Prog Retin Eye Res. 2007;26:453–69. doi: 10.1016/j.preteyeres.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Usami SI, Takahashi K, Yuge I, et al. Mutations in the COCH gene are a frequent cause of autosomal dominant progressive cochleo-vestibular dysfunction, but not of Meniere’s disease. Eur J Hum Genet. 2003;11:744–8. doi: 10.1038/sj.ejhg.5201043. [DOI] [PubMed] [Google Scholar]
- 9.de Varebeke SP, Termote B, Van Camp G, et al. Focal sclerosis of semicircular canals with severe DFNA9 hearing impairment caused by a P51S COCH-mutation: is there a link? Otol Neurotol. 2014;35:1077–86. doi: 10.1097/MAO.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 10.Robertson NG, Resendes BL, Lin JS, et al. Inner ear localization of mRNA and protein products of COCH, mutated in the sensorineural deafness and vestibular disorder, DFNA9. Am J Hum Genet. 2001;69:348. doi: 10.1086/321280. [DOI] [PubMed] [Google Scholar]
- 11.Bom SJH, Kemperman MH, De Kok YJM, et al. Progressive Cochleovestibular Impairment Caused by a Point Mutation in the COCH Gene at DFNA9. Laryngoscope. 1999;109:1525–30. doi: 10.1097/00005537-199909000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Bischoff MLC, Huygen LMP, Kemperman HM, et al. Vestibular deterioration precedes hearing deterioration in the P51S COCH Mutation (DFNA9): an analysis in 74 mutation carriers. Otol Neurotol. 2005;26:918–25. doi: 10.1097/01.mao.0000185048.84641.e3. [DOI] [PubMed] [Google Scholar]
- 13.Yao J, Py BF, Bao J et al. Role of protein misfolding in DFNA9 hearing loss. J Biol Chem. 2010; 285, 14909-19. [DOI] [PMC free article] [PubMed]
- 14.Cho HJ, Park HJ, Trexler M, et al. A novel COCH mutation associated with autosomal dominant nonsyndromic hearing loss disrupts the structural stability of the vWFA2 domain. J Mol Med (Berl) 2012;90:1321–31. doi: 10.1007/s00109-012-0911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makishima T, Rodriguez CI, Robertson NG, et al. Targeted disruption of mouse Coch provides functional evidence that DFNA9 hearing loss is not a COCH haploinsufficiency disorder. Hum Genet. 2005;118:29–34. doi: 10.1007/s00439-005-0001-4. [DOI] [PubMed] [Google Scholar]
- 16.Gao J, Xue J, Chen L, Ke X, Qi Y, Liu Y. Whole exome sequencing identifies a novel DFNA9 mutation, C162Y. Clin Genet. 2013;83:477–81. doi: 10.1111/cge.12006. [DOI] [PubMed] [Google Scholar]
- 17.Jones SM, Robertson NG, Given S, et al. Hearing and vestibular deficits in the coch(−/−) null mouse model: comparison to the coch(G88E/G88E) mouse and to DFNA9 hearing and balance disorder. Hear Res. 2011;272:42–8. doi: 10.1016/j.heares.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda Masatsugu, Mutai Hideki, Arimoto Yukiko, et al. A novel frameshift variant of COCH supports the hypothesis that haploinsufficiency is not a cause of autosomal dominant nonsyndromic deafness 9. Biochem Biophys Res Commun. 2015;469:270–4. doi: 10.1016/j.bbrc.2015.11.106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
case details, mutation analyze, rna analysis