Abstract
The exteroceptive somatosensory system is important for reflexive and adaptive behaviors and for the dynamic control of movement in response to external stimuli. This review outlines recent efforts using genetic approaches in the mouse to map the spinal cord circuits that transmit and gate the cutaneous somatosensory modalities of touch, itch and pain. Recent studies have revealed an underlying modular architecture in which nociceptive, pruritic and innocuous stimuli are processed by distinct molecularly defined interneuron cell types. These include excitatory populations that transmit somatosensory information and inhibitory populations that serve as a gate to prevent innocuous stimuli from activating nociceptive and pruritic transmission pathways within the spinal cord. Ongoing efforts to map dorsal horn networks are therefore beginning to reveal the intricate computational logic of somatosensory transformation in health and disease.
Keywords: somatosensory system, dorsal horn, interneuron, mechanosensation, nociception, gate control
Introduction
The somatosensory system endows animals with the ability to monitor their external and internal environment. It is composed of interoceptive (proprioceptive and autonomic) and exteroceptive (cutaneous) receptors and their associated afferent pathways that relay information about the body to the spinal cord, medulla and to brain structures including the cerebellum, parabrachial nucleus, thalamus and cortex. The interoceptive arm is made up proprioceptive and visceral receptors and their associated afferents. Muscle spindle and Golgi tendon afferents embedded in striated muscles and their associated tendons provide proprioceptive information about muscle tension and body position, which is essential for the sense of self and self-agency (Windhorst, 2007). Visceral mechanoreceptors and nociceptors regulate multiple body functions, including blood pressure, heart rate, micturition and gastrointestinal movements (Janig, 2006) (Figure 1). The exteroceptive arm of the somatosensory system comprises specialized skin receptors and their dedicated sensory neurons whose role it is to relay information about the body’s interaction with the immediate environment, such as conveying information on temperature, touch, pruritic and noxious stimuli to sensory centers in the CNS (Abraira and Ginty, 2013; Lallemend and Ernfors, 2012; Bautista et al., 2014).
Figure 1. The spinal cord receives and processes information about the internal and external environment.
Left: autonomic afferents monitor stretch within smooth muscle to provide information about the activity of internal organs. In the example shown, mechanoreceptors within the bladder wall activate afferent nerves as the bladder fills; in turn, a putative interneuronal pathway (dashed) within the dorsal horn mediates afferent input onto the sympathetic preganglionic nucleus (SPN), which controls bladder filling. Center: proprioceptive afferents monitor tension and stretch within striated muscle to provide information about the position of the body in space. In the example shown, Ia afferents originating in muscle spindles monitor the length and velocity of muscle fibers; in turn, Ia afferents activate agonist alpha motoneurons (MNs) as well as Ia inhibitory interneurons (INs), which suppress activity of antagonist MNs. Right: cutaneous afferents innervate the hairy and glabrous skin and are activated by mechanical, thermal, noxious or pruritic stimuli to provide information about the external environment. In the example shown, low-threshold mechanoreceptors (LTMRs) and high-threshold mechanoreceptors (HTMRs), which exhibit tuning to the intensity of mechanical stimulation of the skin, activate distinct cohorts of INs within the dorsal horn.
The afferent pathways that carry each of the three streams of somatosensory information terminate in the spinal cord and medulla, where they display a high degree of anatomical and functional segregation. Cutaneous afferents project to, and synapse with, interneurons in the upper layers of the dorsal horn, while proprioceptive afferents converge onto interneurons and motor neurons throughout the intermediate spinal cord and ventral horn. Autonomic afferents carrying visceral sensory information predominantly target the intermediolateral spinal cord and the deep dorsal horn. Consequently, the laminar position of an interneuron in the dorsal horn provides some indication of its role in somatosensation. This review will briefly discuss the important features of the interoceptive arm of the somatosensory system before focusing on the exteroceptive arm and the functional organization of the dorsal horn circuits that process and gate cutaneous noxious and innocuous sensory stimuli.
The Interoceptive Somatosensory System
The interoceptive somatosensory system provides sensory feedback from skeletal muscle and organs, permitting the control of the contractile activity of skeletal and smooth muscle, respectively. Visceral sensory afferents, which form part of the autonomic nervous system, regulate a variety of homeostatic body functions by monitoring the stretch and tension of smooth muscle in the viscera. The cell bodies of autonomic sensory neurons are located in the sensory ganglia and relay visceral sensory information to medullary and spinal sympathetic and parasympathetic preganglionic networks. Sensory information carried by these visceral afferents is essential for a variety of reflexes, including micturition (urination), defecation, ejaculation and the baroreflex (Janig, 2006). Although the general pathways that underlie these reflexes have been mapped, we still know very little about the interneurons that are interposed in these reflex pathways and the processing of visceral information within the spinal cord. A number of thinly myelinated group III and unmyelinated group IV afferents that respond to mechanical and chemical stimuli innervate skeletal muscle, and thus appear to share some features of visceral afferents. Activation of these fibers during exercise prevents fatigue by inhibiting motoneuron activity and enhancing cardiac and ventilatory responses (Amann et al. 2015).
The second major group of interoceptive somatosensory afferents are the specialized mechanoreceptors that are present in skeletal muscles, tendons and their associated joints, which are commonly referred to as proprioceptors. Proprioceptive afferents provide an organism with the sense of self by informing the nervous system about the location of the body and limbs in time and space. As such, proprioception is critical for posture, motor control and compensatory motor reflexes. Although the basic patterns of locomotor activity can be maintained in the absence of proprioceptive feedback, proprioception is required for the proper phasing of the joints during locomotion and for smooth and graded motor movements (Pearson, 2004; Rossignol, 2006; Akay et al., 2014; Fink et al., 2014). Moreover, the interneurons in the deep dorsal horn that integrate proprioceptive input are known to have essential roles in shaping and maintenance of a coherent motor rhythm (Jankowska, 1998; Windhorst, 2007). Proprioceptive input can be divided into several classes according to the peripheral receptor activated and the character of the stimulus. Group I afferents detect dynamic and static changes in muscle length, while Ib Golgi tendon afferents detect changes in muscle tension and are important for posture and the transition from stance to swing during walking (Pearson, 2004). Group II afferents are largely slow firing and non-adapting, and respond to large amplitude changes in muscle stretch during stance, joint movement, and skin deformation, allowing for adaptation to local unilateral ground conditions during standing. Interestingly, many of the spinal interneurons that are innervated by proprioceptors also receive multimodal input from cutaneous afferents, either monosynaptically or polysynaptically. In this way, information from the external environment is integrated with central and proprioceptive pathways that are important for postural control, locomotion and volitional movements such as grasping. This integration ensures that adaptive motor responses and reflexes elicited by cutaneous afferents are executed in a task-appropriate manner that takes into account the ongoing motor program and position of the body and limbs (Rossignol, 2006, Hasan and Stuart, 1988).
The Exteroceptive Somatosensory System
Cutaneous afferents convey exteroceptive information about the surrounding environment and the body’s interaction with it. This information is used to prevent tissue damage from noxious stimuli and to adjust ongoing centrally driven motor programs to environmental perturbations. Exteroceptive stimuli are generally classified as being innocuous, noxious or pruritoceptive, with distinct cutaneous primary afferent fiber subtypes transmitting a given stimulus type (Abraira and Ginty, 2013; Lallemend and Ernfors, 2012; LaMotte et al., 2014). While innocuous, noxious and pruritoceptive sensory modalities are conveyed by largely distinct sensory neuron populations, certain sensory afferent subtypes, particularly pruritoceptive afferents, are polymodal. Primary afferent fibers can be broadly characterized into three main subgroups according to the thickness of their myelin sheath and their conduction velocities. These are thickly myelinated, fast conducting Aβ fibers, thinly myelinated, fast Aδ fibers, and unmyelinated, slow conducting C fibers. While classification schemes have assigned each subgroup of fibers to different cutaneous sensory modalities- e.g. Aβ fibers transmit light touch and C fibers convey noxious pain-this is an oversimplification. For example unmyelinated C low threshold mechanoreceptors (C-LTMRs) transmit innocuous affective touch stimuli. Increasingly, sensory neuron subtypes are being defined by molecular markers that include developmental markers, notably the tropomyosin receptor kinases A, B and C (TrkA, TrkB, TrkC), c-Ret, Runx1 and Runx3, and postnatal sensory neuron molecular markers, such as calbindin, calretinin (CR) and tyrosine hydroxylase (TH) (Lallemend and Ernfors, 2012; Abriara and Ginty, 2013).
Light (innocuous) touch is transmitted by LTMR afferents that are each tuned a specific range of mechanical stimuli. These LTMRs project to the “LTMR recipient zone” and synapse with interneurons in laminae IIi–IV (reviewed in detail in (Abraira and Ginty, 2013)). Sensory neurons of the LTMR lineage are defined by early expression of TrkB and TrkC. Recent studies have established detailed molecular profiles for each LTMR subtype. Sensory neurons expressing c-Ret and MafA/c-Maf exhibit the fast firing properties of rapidly adapting LTMRs (Aβ RAI and RAII LTMRs) (Bourane et al., 2009, Luo et al., 2009; Wende et al., 2012). They innervate Meissner and Pacinian corpuscles and hair follicles, which respond to movement, vibration and hair deflection, respectively (Abraira and Ginty, 2013; Lallemend and Ernfors, 2012). These c-Ret and MafA/c-Maf neurons can be subdivided into three subgroups: Ret+ MafA+, Ret+ MafA+ TrkC+, and Ret+ MafA+ TrkB+ Shox2+. The role of each of these subgroups in peripheral coding have not yet been established. The second branch of innocuous primary afferents are defined by expression of TrkB or TrkC receptors in the absence of Ret expression. Of this subgroup, TrkB+ Shox2+ neurons that have the characteristic features of Aβ slowly adapting LTMRs (Aβ SAI and SAII LTMRs), which peripherally innervate Merkel cells, Ruffini endings and Aδ LTMRs and respond to skin indentation, stretch and hair deflection, respectively, whereas those expressing TrkC+ Runx3+ and parvalbumin+ (PV) have proprioceptive characteristics. An independent class of peripheral neurons involved in the transmission of low threshold touch are the unmyelinated C-LTMRs that are defined by their expression of c-Ret, vGluT3 and TH. These neurons convey slow dynamic touch, which is important for affective behaviors involved in social communication, and may also be involved in mechanical itch (McGlone et al, 2014). The selective coding of mechanical stimuli in the periphery appears to be maintained in part by the central projections of these LTMRs, in so far as each LTMR subtype displays a distinct morphology and laminar termination pattern (Brown, 1981; Abraira and Ginty, 2013).
In contrast to LTMRs that innervate laminae IIi–IV, noxious afferents and high threshold mechanoreceptors (HTMRs) terminate predominantly in laminae I–IIo. Nociceptive information has been shown to be transmitted via a range of primary afferent fiber subtypes, which unlike the low threshold mechanoreceptors described above, are often polymodal; this allows for detection of, and subsequent aversion from, a wide range of chemical, thermal and mechanically noxious events (Basbaum et al., 2009; Julius and Basbaum, 2001; Lallemend and Ernfors, 2012). Nociceptors include myelinated Aδ fibers, defined by early expression of TrkA and calcitonin gene-related peptide (CGRP) or substance P (SP), and unmyelinated C fibers, defined by expression of either TrkA, identifying heat-sensitive and mechanically sensitive peptidergic nociceptors, or by expression of Ret, isolectin B4 (IB4) and of one of many Mas-related G protein-coupled receptors (Mrgprs). Although Aδ fibers transmit fast noxious stimuli (chemical, mechanical and thermal), and C fibers transmit slow mechanical and thermal pain or itch, Aδ fibers and C fibres are made up of multiple sensory neuron subclasses; the properties of which are conferred by their combinatorial expression of channels and G-protein coupled receptors (Julius and Basbaum, 2001, Lallemend and Ernfors, 2012). Members of the transient receptor potential (TRP) channel family confer a wide range of modality sensitivities to nociceptors (both Aδ and C), most notably high threshold thermal sensitivity and sensitivity to the noxious chemical capsaicin through expression of TRPV1 in peptidergic nociceptors. TRPA1 has also been proposed as a noxious cold sensor as well as a chemical receptor for mustard oil and icillin, although selective expression of TRPA1 by a subclass of peripheral neurons (nociceptive or otherwise) has not been demonstrated. A subset of dorsal root ganglion (DRG) neurons express the cold receptor TRPM8, which contributes to both innocuous and noxious cold detection in mice as well as detection of the chemical cooling agent menthol (Dhaka et al., 2007). Whether TRPM8+ cells represent nociceptors is unclear, as TRPM8 is expressed in non-peptidergic peripheral neurons which do not express IB4. Mrgrprs, by contrast, have been shown to confer unique selectivity to non-peptidergic IB4+ C nociceptors. Of the identified Mgrpr subfamily, only the MrgprD+ subgroup are true nociceptors, exhibiting mechanical sensitivity (Zylka et al., 2005).
Itch is transmitted by diverse peripheral receptors and afferents sensitive to mechanical and chemical stimuli. Pruritogens, substances that generate itch, and mechanical stimuli can activate subpopulations of these afferents or overlapping afferent populations depending on the stimulus and its intensity (see (Bautista et al., 2014, LaMotte et al., 2014)). There is to date no evidence of an itch-specific primary sensory neuron; instead, itch is conveyed either mechanically by LTMRs, or by a subgroup of polymodal nociceptors expressing one or more receptor subtypes. Of the identified peripherally expressed pruritic receptors, the Mrgpr family has received the most attention. MrgprA3 has been identified as the receptor for the pruritogen chloroquine, and is expressed in a subpopulation of non-peptidergic C fibers; it also confers pruritic sensitivity to histamine. MrgprC11 confers sensitivity to the pruritogens BAM8–22, its natural ligand, SLIGRL-NH2, capsaicin and serotonin. It should be noted, however, that histamine can also signal through TRPV1, and BAM8–22 and chloroquine through TRPA1, highlighting that the sensitivities of these pathways are not mutually exclusive. The mechanical itch pathway is not as well described, although C-LTMRs and Aδ LTMRs have been suggested to play a role in transmitting the sensation of mechanical itch (Fukuoka et al., 2013). Recent developments in the identification of peripheral itch circuits have substantially enhanced understanding of how itch is processed and controlled within the dorsal horn, including interactions between the circuitry for pain and itch resulting in the inhibition of itch by painful stimuli.
Cellular organization of the dorsal spinal cord: development and interneuron cell types
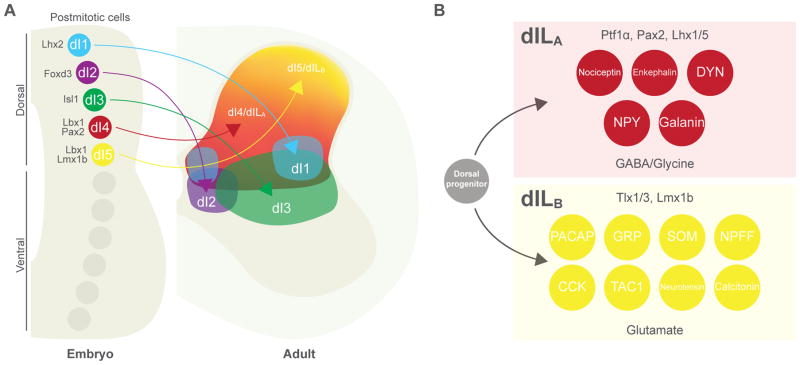
The anatomical and functional organization of the cutaneous somatosensory circuits in the spinal cord are a reflection of the lamina-specific sensory innervation and the provenance and patterning of the spinal interneurons that make up the dorsal horn. Neurons in the dorsal spinal cord display a characteristic dorsoventral (DV) laminar organization that is based in part on the early DV patterning of the caudal neural tube (Figure 2A). Neural progenitors in the hindbrain (medulla) and spinal cord are specified by the morphogenetic activities of Sonic hedgehog (Shh) ventrally (Jessell, 2000; Goulding et al., 2002) and BMPs and Wnts that are produced by dorsal midline and roof plate cells (Lai et al., 2016; Lee and Jessell, 1999; Le Dreau and Marti, 2012). Neural progenitors in the dorsal neural tube give rise to six cardinal dorsal interneuron cell types (dI1–6 interneurons), that can be further subdivided into Class A (dI1–3) neurons, which are dependent on BMPs and Wnts signaling and are marked by the expression of Olig3 (Muller et al, 2002, 2005), and the more ventrally-derived Class B interneurons (dI4–dI6) that arise in a BMP-independent manner (Muller et al., 2002). The interneurons that make up the dorsal horn proper (laminae I–V) are derived from Lbx1-expressing dI4 and dI5 interneurons and their later born counterparts, the dILA and dILB interneurons (Gross et al., 2002). dI4/dILA neurons differentiate as inhibitory interneurons while the dI5/dILB interneurons constitute the progenitors of all the excitatory local circuit neurons that transmit cutaneous somatosensory modalities. Inhibitory GABA/glycinergic dI4/dILA interneurons, which are marked by the expression of Pax2, Lhx1/5 and Gbx1, arise from Ptf1α+ progenitors (Glasgow et al., 2005), whereas excitatory glutamatergic dI5/dILB interneurons arise independently of Ptf1α and express the Lmx1b and Tlx1/3 transcription factors (Cheng et al., 2004; Guo et al., 2012) (Figure 2B). These two broad populations of interneurons play complementary and opposing roles in transmitting somatosensory information: excitatory Lmx1b+-derived cells are essential for cutaneous sensory transmission, while Ptf1α+-derived inhibitory cells types function to gate sensory transmission, via both postsynaptic and presynaptic mechanisms. Second order polymodal reflex encoder (RE) neurons that are located in lamina V (Schouenborg, 2002) are also likely to be derived from the Lbx1+ progenitors. However, the molecular identity of these RE neurons and whether they include inhibitory as well as excitatory cell types remains to be determined.
Figure 2. Neuronal diversity is established during embryonic development of the dorsal horn.
A: (left) schematic cross section of the embryonic spinal cord showing transcription-factor (TF) specification and migration of dorsal horn interneurons (INs). At embryonic day 11 (E11), five early classes of postmitotic neuron (dI1–5) are present within distinct domains along the dorsoventral axis of the dorsal horn; these subsequently migrate to their positions within the mature spinal cord (right). Between E12 and E14, two further classes (dILA and dILB) are born from dorsal progenitors across a large domain occupied by dI4 and dI5 cells. B: many molecularly defined neuronal populations within the mature dorsal horn arise from late-born dILA and dILB classes: (upper panel) dILA cells are distinguished by expression of Ptf1α, Pax2 and Lhx1/5 TFs and are glycine/GABAergic; (lower panel) dILB cells are distinguished by Tlx1/3 and Lmx1b TFs and are glutamatergic. Abbreviations: CCK, cholecystokinin; DYN, dynorphin; GRP, gastrin-releasing peptide; NPY, neuropeptide Y; NPFF, neuropeptide FF; PACAP, pituitary adenylate cyclase-activating polypeptide; SOM, somatostatin; TAC1, tachykinin 1.
Ongoing efforts have begun to define with finer granularity the interneuron cell types that make the spinal somatosensory circuitry. Multiple studies have identified molecular markers that delineate subpopulations of dI4/dILA and dI5/dILB interneurons (Brohl et al., 2008; Del Barrio et al., 2013; Wildner et al., 2013, Xu et al., 2013; Abraira et al., 2017). dI4/dILA fate mapping studies have revealed these interneurons differentiate into several subgroups of deep dorsal inhibitory interneurons, marked in part by expression of neuropeptide Y (NPY), galanin, and dynorphin (DYN). Conversely, excitatory dI5/dILB interneurons are positioned in the superficial dorsal horn and may be involved in the amplification of nociceptive signals. Some identified neurochemical markers for these neurons include cholecystokinin (CCK), gastrin releasing peptide (GRP), and somatostatin (SOM) (Figure 2B). Over the past five years there has been a leap in the advancement of molecular and genetic tools, allowing unprecedented access to these identified spinal sensory interneurons and an insight into the processing of modality selective information of touch, pain and itch (summarized in Tables 1 and 2). The following sections will cover the recent developments in the field of spinal dorsal circuits and the outstanding questions in the field of sensory processing.
Table 1.
Molecularly defined populations of inhibitory dorsal-horn neurons for which a physiological or behavioral role has been proposed.
| Marker | Proposed function | Dorsal laminar distribution |
Primary afferents | Outputs | Neurotransmitter profile |
Other co-expression |
Morphology | Behavioral phenotype | Electrophysiological profile |
Other | Citations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bhlhb5 (B5-I subset) | Modulate itch and pain sensitization | L I–II | Aβ and Aδ; putative inputs from fibers expressing TRPV1, TRPA1, TRPM8 and MrgprD | Pax2 | SST2AR; nNOS; galanin; DYN; PrP | Ablation: elevated chemical itch; heightened response to formalin, indicating pain or itch. Inhibition: evokes itch behaviors. | Tonic firing | 110; 54; 111; 112 | |||
| DYN | Gate acute touch | L I–II (majority); L III–V (minority) | MS Aβ | SOM (proposed) | GAD67 (86%); GlyT2 (28%); vGluT2 (12%) | Largely coexpressed with galanin in L I–IIo and with nNOS in L III; Bhlhb5/B5-I (49–50%) | Fusiform (33%); flattened (49%); pyramidal (18%) | Ablation: spontaneous allodynia | Tonic firing (majority); bursting (minority) | 50; 46; 51; 54 | |
| Galanin | L I–II (majority); L III (minority) | GABA (100%) | DYN (~60%), nNOS in L III; SST2AR; CGRP | Upregulation of c-Fos and pERK in response to noxious stimulation | 51; 53 | ||||||
| nNOS | Modulate inflammatory pain | L I–III | NK1R | GABA; GlyT2; other | Excitatory nNOS: PKCγ in L III. Inhibitory nNOS: SST2AR in L I–II; DYN (~40%) | Global KO/inhibition: elevated response to inflammatory and neuropathic pain | Upregulation of c-Fos and pERK in response to noxious stimulation | 57; 58; 59; 53; 60; 61; 68 | |||
| NPY | Gate mechanical itch; may modulate acute, neuropathic and inflammatory pain | L III–IV (70%); L I–II (30%) | MS and PS Aβ, Aδ and C | Gad1/GlyT2 (>98%) | nNOS (0–5%); galanin (0–5%); DYN (8%); PV (2–6%); SST2AR | Non-canonical inhibitory; not islet | Ablation/silencing: elevated itch in response to mechanical stimuli. Global inhibition or genetic inactivation of NPY receptors: elevated neuropathic pain. | Tonic (81%) firing; intermittent bursting (14%); tonic firing with gap (5%) | Ablation: hyperactivity of dorsal neurons to repeated brushing of the hairy skin. pERK expression after noxious stimulation or inflammatory insult; NPY is upregulated in the dorsal horn and DRG following neuropathic or inflammatory stimuli and in the dorsal horn during inflammation | 103; 125; 115; 126; 113; 116 | |
| PV | Modulate hypersensitivity in inflammatory and neuropathic pain | L IIi–III (majority) | MS Aβ, Aδ and C | Other PV (postsynaptic); low threshold primary afferents (presynaptic); PKCγ | vGAT (majority); vGluT2 (minority) | Ret; Cdh3 | Islet (majority); central | Activation: elevated mechanical von Frey hair thresholds and reduced mechanical allodynia in inflammatory and neuropathic pain. Ablation/inhibition: mechanical allodynia. | Tonic firing (67%); initial bursting (33%). Ih subthreshold voltage-gated currents (94%). High rheobase | PV contacts onto PKCγ cells decrease following nerve injury, without PV cell loss | 40; 70; 71; 69 |
| Ret | Modulate dynamic touch and mechanical pain as well as inflammatory and neuropathic pain | L III–V (majority); L IIi (minority) | MS and PS Aβ (majority) and Aδ (minority), including vGluT1-expressing myelinated fibers; MS and PS C (50%). PS inputs are excitatory and inhibitory glycinergic. MS inputs are inhibitory glycinergic. | Presynaptic GABAergic Aβ and C; postsynaptic PKCγ and SOM; inhibit other Ret | vGAT (93%; 50% with GlyT2, 80% with GAD1/2) | PV (25–30%) | Islet (29%); radial (22%); vertical (16%); inverted stalked (8%); unclassified (25%) | Ablation: static and dynamic allodynia; mechanical, thermal and cold hyperalgesia; aggravation of neuropathic and inflammatory pain. Activation: reduced basal and chronic pain. | Tonic firing (80%); delayed, phasic or single firing (minority) | Activation: reverses disinhibition of L I–II neurons receiving Aβ and C fiber inputs | 69 |
| TRPV1 | Modulate mechanical allodynia and hypersensitivity | L I–II | L II INs | GAD65 (majority); vGluT2 (minority) | KO: attenuated hypersensitivity after nerve injury. Activation: reduced mechanical sensitivity after nerve injury. | Long-lasting tonic or phasic firing | 89; 91; 96 |
Abbreviations: Bhlhb5, basic helix-loop-helix domain containing, class B5; Cdh3, cadherin 3; CGRP, calcitonin gene-related peptide; DYN, dynorphin; GRPR1, gastrin-releasing peptide receptor 1; MrgprD, Mas-related G-protein coupled receptor member D; L, Rexed’s lamina; MS, monosynaptic; NK1R, neurokinin 1 receptor; nNOS, neuronal nitric oxide synthase; NPY, neuropeptide Y; PKCγ, protein kinase C gamma; PrP, prion promotor; PS, polysynaptic; PV, parvalbumin; RET, retinin; SOM, somatostatin; SST2AR, somatostatin receptor 2A; TRPA1, transient receptor potential cation channel subfamily A member 1; TRPM8, transient receptor potential cation channel subfamily M member 8; TRPV1, transient receptor potential cation channel subfamily V member 1; vGluT3, vesicular glutamate transporter 3.
Table 2.
Molecularly defined populations of excitatory dorsal-horn neurons for which a physiological or behavioral role has been proposed.
| Marker | Proposed function | Dorsal laminar distribution |
Primary afferents | Outputs | Neurotransmitter profile |
Other co- expression |
Morphology | Behavioral phenotype | Electrophysiological profile |
Other | Citations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCK | Mediate light touch | L II–III | MS Aβ (<15%) and Aδ (<15%) | vGluT2 (92%) | RORα; NeuD4 (4%) | Ablation: deficits in texture discrimination and responses to air puffs | Abraira et al., 2017; Bourane et al., 2015a | ||||
| CR/Calb2 | Mediate thermal and mechanical pain | L I–II (majority); L III (minority) | Putative TRPV1-expressing fibers | PAX2 (12%); remaining moiety proposed to be excitatory | SST2AR (48%); nNOS (27%); SOM; vGluT3 (8–45%); NPY (2%) | Central; radial; vertical; islet | Ablation: increased mechanical and thermal pain thresholds. Stimulation: mechanical hypersensitivity | Delayed firing (majority); single or tonic firing; initial bursting; reluctant. Iar, Ih and ICa,T currents. Profiles differ between preparations; proposed to differ between excitatory and inhibitory subsets. | A subset of excitatory and inhibitory CR/Calb2INs expresses pERK following pinch or capsaicin. Mechanical stimulation elevated c-FOS in L I–II, including CR/Calb2cells not directly activated. | Duan et al. 2014; Smith et al. 2015; Peirs et al., 2015; Cheng et al., 2017 | |
| GRP | Transmit itch and weak pain | L I–II | Fibers express CGRP (64%), IB4 (39%), MrgprA3 (22%), TRPV1 (50%) and NF200 (2%) | Enkephalin | vGluT2 (>90%); GAD1 (<10%) | NPRA | Vertical (100%) | Ablation: reduced chemical itch. Stimulation: pain and itch responses; at high frequency enkephalin-expressing INs recruited to inhibit pain | Strong firing in response to capsaicin, weak firing in response to pruritogens | Mishra and Hoon, 2013; Sun et al., 2017; Li et al., 2006 | |
| GRPR | Transmission of chemical itch | L I | vGluT2 (probable) | Ablation/null mutant/receptor antagonism: reduced responses to histamine-dependent and independent pruritogenic stimuli | Sun and Chen 2007; Sun et al. 2009; Sun et al., 2017 | ||||||
| NPRA | Transmit chemical itch | L I | Fibers express Nppb, TRPV1, PLCβ3 | vGluT2 (probable) | GRP (100%) | Vertical (100%) | Ablation: reduced chemical itch | Mishra and Hoon, 2013; Sun et al., 2017 | |||
| NK1R | Mediate responses to noxious and inflammatory agents and allodynia in neuropathic pain | L I (majority); L III–V (minority) | MS C and Aδ; PS Aβ (majority, L I). MS Aβ; PS Aδ and C (majority, L III) | Supraspinal sites: parabrachial nucleus; periaqueductal gray; thalamus; nucleus tractus solitarius; ventrolateral medulla | GABA/glycine negative (90%) | Ablation: reduced responses to noxious stimuli; reduced mechanical and thermal hyperalgesia in inflammatory pain; reduced mechanical allodynia in neuropathic pain | Expression of pERK in response to noxious stimuli | Littlewood et al. 1995; Mantyh et al. 1997; Nichols et al., 1999; Torsney and MacDermott 2006; Baseer et al. 2012; Polgár et al., 2007 | |||
| PKCγ | May mediate mechanical allodynia | L I–IIi | MS Aδ (~25%), C (>10%), Aβ (<10%), including vGluT1-positive myelinated | vGluT2 (>95%); vGAT (<5%) | NeuD4 (91%); vGluT3 (25%) | Global KO: mice fail to develop neuropathic pain and exhibit reduced mechanical and thermal sensitivity after nerve injury; reduced inflammatory pain behavior. Pharmacological inhibition: reduced mechanical allodynia after nerve injury | Delayed firing (60%); initial bursting (40%) | Inhibition: reduced c-Fos expression in dorsal horn interneurons following gentle brushing in nerve injury; reduced allodynia following CR/Calb2ablation. | Malmberg et al., 1997; Neumann et al., 2008; Abraira et al., 2017; Petitjean et al. 2015; | ||
| RORα | Integrate light touch information and descending motor commands | L IIi/III (majority) | MS Aβ and Aδ; fibers expressing CGRP | MS connections to MNs and premotor INs | vGluT2 (92%); GAD1 (3%) | CCK (60%); cMaf (49%); MafA (48%); PKCγ (22%); | Radial (33%); vertical (33%); central (~22%); unclassified (12%) | Ablation/silencing: deficits in dynamic and static touch | Bourane et al., 2015a; Li et al., 2006; Del Barrio et al., 2013 | ||
| SOM | Mediate mechanical pain and itch; role in light touch | L II (majority); L I, L III–V (minority) | MS and PS Aδ/C | vGluT2 (94%); GAD67 (2%); GlyT2 (5%) | CR/Calb2; Tac2; vGluT3 (22%) | Central; radial; vertical | Ablation: mechanical pain and thermal deficit; touch deficit. Activation: spontaneous pain and decreased mechanical pain thresholds | Delayed bursting; single firing | Duan et al., 2014; Christensen et al., 2016; Cheng et al., 2017 | ||
| vGluT3 | Mediate transmission of innocuous touch; mediate dynamic mechanical hypersensitivity and affective or cognitive component of associated allodynia | L II–III | MS Aβ; PS Aδ and C | PS CR/Calb2 and PKCγ; NK1R in L I | vGluT2 (96%) | CR/Calb2(10–45%); PKCγ (16–42%); SOM (24%) | Central; radial; vertical | Ablation: reduced sensitivity to light punctate touch; deficits in acute, inflammatory and neuropathic pain; reduced dynamic mechanical hypersensitivity and aversive behavior induced by nerve injury/inflammation. Silencing: reduced dynamic mechanical hypersensitivity induced by nerve injury. Activation: mechanical hypersensitivity/allodynia | Tonic (53%); phasic (39%); single (8%); delayed | Activation: c-FOS induction in excitatory and inhibitory cells, including PKCγ and CR/Calb2. Induction of c-FOS in the dorsal horn by brushing following nerve injury is reduced following vGLUT3 ablation. | Peirs et al., 2015; Cheng et al., 2017 |
Abbreviations: CCK, cholecystokinin; CGRP, calcitonin gene-related peptide; CR, calretinin/calb2; GRP, gastrin-releasing peptide; GRPR, gastrin-releasing peptide receptor; IB4, isolectin B3; L, Rexed’s lamina; MrgprA3, Mas-related G-protein coupled receptor member A3; MS, monosynaptic; NF200, neurofilament 200; NK1R, neurokinin 1 receptor; nNOS, neuronal nitric oxide synthase; NPY, neuropeptide Y; NPRA, natriuretic peptide receptor A; PKCγ, protein kinase C gamma; PS, polysynaptic; PV, parvalbumin; RORα, RAR-related orphan receptor alpha; SOM, somatostatin; SST2AR, somatostatin receptor 2A; TAC2, tachykinin 2; TRPM8, transient receptor potential cation channel subfamily M member 8; TRPV1, transient receptor potential cation channel subfamily V member 1; vGluT3, vesicular glutamate transporter 3.
Spinal processing of innocuous touch
LTMRs sensitive to light touch synapse onto first order sensory interneurons located in laminae IIi, III and IV, which in turn play critical roles in processing and transmission of innocuous stimuli. The deep dorsal horn is heterogeneous, consisting of multiple excitatory and inhibitory cell types. Two recent studies explored the molecular identity and diversity of the sensory interneurons in the LTMR recipient zone. Del Barrio et al. (2013) analyzed the expression of multiple transcription factors that are expressed postnatally in laminae III–IV and identified nine distinct populations of neurons. Four of these are excitatory and marked by the expression of Lmx1b; the remaining five are inhibitory populations that express GAD1/GAD67 and/or Gbx2 or Pax2. Further to this study, Abraira et al. (2017) performed an extensive analysis of 11 subpopulations of interneurons within the LTMR recipient zone, seven of which are excitatory and four are inhibitory. Each of these subpopulations, defined by expression of transcription factors or other molecules, was found to exhibit a distinct physiological and morphological profile. For example, interneurons that are defined by expression of protein kinase C gamma (PKCγ) display delayed firing properties and have complex dendritic arborization patterns, whereas Cerebellin-2+ interneurons have an initial burst pattern of firing upon current injection and less ramified dendrites. The patterns of LTMR inputs to sensory interneuron cell types appear to be complex, with each population receiving inputs from at least two LTMR subtypes, and with individual neurons often receiving LTMR input from more than one source (Abraira et al., 2017). Moreover, these sensory interneurons within the LTMR recipient zone receive synaptic inputs from other deep dorsal horn neurons within the LTMR as well as from corticospinal neurons (Bourane et al., 2015a; Abriara et al., 2017), and likely from other laminae allowing for polysynaptic input from nociceptive afferents, indicating a high degree of neuronal diversity and convergence within this region. Clearly, the excitatory interneurons in laminae IIi–IV do not function as simple linear relays for specific touch modalities, but instead play important roles in encoding and integrating multiple touch modalities with descending signals from supraspinal sensorimotor centers. A further finding from both studies is that a number of molecular markers, including PV, RORβ, MafB and c-Maf, are expressed in both excitatory and inhibitory neurons, thus demonstrating that single molecular markers do not delineate functionally distinct populations of spinal interneurons. The apparent neuronal diversity that exists within the LTMR recipient zone complicates functional analyses that are only just beginning to probe the organization of these circuits and the functional contribution that defined neuronal cell types make to touch-driven behaviors.
Important insights into the organization of touch circuits in the spinal cord has come from the characterization of a population of sensory interneurons that expresses the nuclear orphan receptor transcription factor RORα. These excitatory neurons are located in laminae IIi/III and are innervated by LTMRs but not prioprioceptive or nociceptive afferents. They also display selectively with regard to the types of LTMR input that they receive, being predominantly innervated by Meissner corpuscles in the glabrous skin, and Merkel cells and Aδ D-hair afferents in the hairy skin. In keeping with the profile of their sensory afferent input, the targeted ablation of RORα+ interneurons in the caudal spinal cord results in selective deficits in both dynamic and static touch, without any overt nociceptive phenotype (Bourane et al., 2015a). Previous studies in the cat have suggested that innocuous LTMR pathways are coupled to the spinal motor circuitry and contribute to the dynamic control of movement. In support of this, corrective foot movements were degraded in mice lacking excitatory spinal RORα+ interneurons, which were found to form synaptic contacts with premotor interneurons and motor neurons (Bourane et al., 2015a). This circuit configuration presumably allows LTMR inputs to the spinal cord to engage the spinal locomotor circuitry with high acuity in order to generate compensatory movements in response to small perturbations in the environment.
The convergent innervation of the RORα+ interneuron population by multiple LTMR subtypes raises the question to how LTMR information is encoded by interneurons in the LTMR recipient zone. In keeping with what Del Barrio et al. (2013), and Abraira et al. (2017) have described for other LTMR interneuron populations, the demonstration that the RORα+ population comprises at least two molecularly distinct cells types, a dorsal PKCγ+ population, and a ventral MafA/cMaf+ population, in addition its apparent to morphological diversity, argues for a high degree of complexity in the processing of light touch in the context of motor behavior. A large proportion of the RORα+ interneurons (60%) expresses the excitatory neuropeptide CCK, which is also involved in innocuous touch sensation. A significant number of CCK+ interneurons are located within laminae IIi–III, where they receive afferent input from Aβ RA-LTMRs and Aδ LTMRs. Accordingly, chronic silencing of caudal CCK+ interneuronal activity impairs tactile discrimination without affecting responses to noxious heat (Abraira et al., 2017). Nonetheless, it should be noted that the CCK+ interneuron population covers a large fraction of the excitatory interneurons within the dorsal horn, including those located in more superficial laminae that receive nociceptive as opposed to innocuous mechanosensory input. The molecular identity and contribution that these dorsally located CCK+ neurons have in nociceptive or pruriceptive transmission remains to be determined.
Spinal circuits for mechanical and thermal nociception
Mechanical and thermal nociception are largely processed within the superficial laminae I and II of the dorsal horn, in the terminal end zones of nociceptive Aδ and C fibers, although deeper dorsal horn neurons also receive nociceptive input via a polysynaptic pathway mediated by relay excitatory interneurons. Afferent input from nociceptors can be processed within the dorsal horn before being relayed to projection neurons within lamina I for transmission to higher brain centers, and is also directly submitted to ventral motor centers to initiate protective nociceptive withdrawals (Todd, 2010). Inhibitory and excitatory interneurons serve to modulate this nociceptive information before its relay to to supraspinal sites, and the interplay between inhibition and excitation is crucial in setting the tone for spinal sensitivity; however, the sensory circuits for nociceptive perception are still poorly understood. Early efforts to classify individual neuronal populations within lamina II did so according to their dendritic morphology. Four classes of interneurons were described: islet cells, central cells, radial cells and vertical cells (Grudt and Perl, 2002; Light et al., 1979). Whereas some characteristics of these classifications were found to correlate with other neuronal properties- islet cells display tonic firing and have an inhibitory phenotype, whereas radial cells display delayed firing and are excitatory, for example- morphological characteristics were not found to be selective enough to attribute sensory roles to interneuronal subgroups (Grudt and Perl, 2002). Subsequently, populations were defined by their expression of neurochemical and neuropeptidergic markers, including SOM, CR, SP, NPY and PV (Todd, 2010). One such population of interneurons are the excitatory SOM+ interneurons, which are located in laminae IIo–III (Christensen et al., 2016; Duan et al., 2014). These neurons are recruited within a mechanical pain circuit by two distinct mechanisms: more superficial SOM+ interneurons receive monosynaptic Aδ and C fiber input, and are involved in the processing of acute mechanical pain, while a second population located in laminae IIi–III receives polysynaptic Aβ fiber input via an intermediate inhibitory interneuron. As a result of this interplay, caudal ablation of SOM+ interneurons leads to both mechanical and thermal pain deficits, as well as a deficit in light touch. Conversely, direct activation of SOM+ interneurons results in both spontaneous pain states and decreased mechanical pain thresholds (Christensen et al., 2016; Duan et al., 2014), consistent with the existence of multiple functionally distinct populations.
The broad population of SOM+ interneurons also partially colocalizes with the excitatory markers CR and vGluT3, which have also been implicated in the amplification of nociceptive signaling. Calretinin+/Calbindin 2+ (CR/Calb2) interneurons are located in laminae I and II and are morphologically, neurochemically and electrophysiologically diverse, with 85% of recorded neurons displaying properties characteristic of excitatory interneurons, and the remaining subset displaying predominantly inhibitory properties (Smith et al., 2015). Dorsal spinal ablation of CR/Calb2 results in a deficit in von Frey hair threshold as well as an increased latency to withdraw from heat without the development of an innocuous touch phenotype (Duan et al., 2014), marking them as putative amplifiers of nociceptive specific input. Although located more ventrally in laminae II–III, vGluT3+/Lbx1+ interneurons have been shown to be involved in the processing of similar nociceptive signals as CR+/Calb2+ interneurons, as vGluT3+ interneuron ablated mice also display selective deficits in von Frey hair detection (Cheng et al., 2017). VGluT3+ cells can be divided into two subpopulations according to their afferent input, with the more ventral vGluT3+ interneurons receiving Aβ LTMR input. It is suggested that these Aβ-receiving vGluT3+ interneurons, which co-express SOM, and presumably the more superficial cells, some of which coexpress CR+/Calb2+, contribute to the nociceptive transmission of punctate von Frey hair stimuli.
Although classically thought of as pruritoceptors, GRP+ interneurons have recently been suggested to gate nociceptive information in the superficial dorsal horn and so act as polymodal nociceptors (Sun et al., 2017). These neurons have been shown to receive afferent input from nociceptive IB4+, CGRP+ and TRPV1+ primary afferent fibers and fire at high frequency to nociceptive input in slice preparations. Accordingly, ablation of GRP+ interneurons results in a hypersensitivity to thermal stimuli and to inflammatory mediators. Although these neurons respond to nociceptive afferent input, high intensity nociceptive stimulation of GRP+ interneurons leads to the postsynaptic recruitment of enkephalinergic interneurons in the superficial dorsal horn, resulting in the inhibition of nocifensive behaviors. This internal gate mechanism is pain-specific, as delta opioid receptor antagonists can block nociceptive behaviors in GRP+ interneuron ablated mice, but not pruritoceptive behaviors (Sun et al., 2017). Such polymodal activation of identified populations highlights the plasticity of dorsal interneuronal populations, and the adaptability of a single sensory circuit. It also raises the question of the polymodal nature of other identified dorsal populations, and the need for careful experimental design to dissect the broad integrative properties of dorsal sensory circuits.
Inhibitory interneurons and sensory gating
Although the position of interneurons in the spinal dorsal horn is an indication of their role in sensory transmission, the selectivity of an interneuronal population to a given sensory modality is defined not only from its periperhal afferent input, but also by the neuronal input it receives from other local circuit interneurons. According to the gate theory proposed by Melzack and Wall, low threshold primary afferent input to superficial interneurons is gated by inhibitory interneurons positioned in the deep dorsal horn to prevent aberrant activation of projection neurons to innocuous stimuli, hence preventing modality crosstalk between touch and pain under baseline naïve conditions (Melzack and Wall, 1965). These inhibitory interneurons thus function as a ‘gate’ by inhibiting excitatory activity either presynaptically at the level of the primary afferent, or postsynaptically, onto local circuit interneurons, which are themselves activated by low threshold afferent stimulation. This gate circuit is proposed to be under constant inhibitory control, however, under conditions of pathological pain and allodynia, whereby touch is perceived as painful, this inhibitory control is lost, leading to crossmodal activation and the aberrant spread of excitation.
Several recently identified and genetically accessed inhibitory populations have been proposed to function as the inhibitory gate that excludes low threshold input to the superficial dorsal horn. These include the Dynorhin positive (DYN+) interneurons found both in the superficial dorsal horn and laminae III–IV (Duan et al., 2014; Lima et al., 1993). DYN+ interneurons are of a mixed phenotype, but are predominanatly inhibitory and express the inhibitory transmitters GAD67 and GlyT2. The dorsally located and more ventral DYN+ interneurons have been shown to send dendrites to laminae III–V and receive monosynaptic input from Aβ afferents, and are thus positioned to gate Aβ input onto superficial dorsal horn neurons. As such, mice in which the DYN+ interneurons have been ablated in the dorsal spinal cord develop an allodynic phenotype, characterized by increased sensitivity to both static and dynamic low threshold inputs, but do not exhibit mechanical or thermal hyperalgesia (Duan et al., 2014). This pathway was suggested to be in part mediated through the recruitment of more ventrally positioned SOM positive excitatory interneurons, which themselves receive Aβ input (Duan et al., 2014). DYN+ interneurons would therefore postsynaptically inhibit the aberrant activation of SOM+ interneurons by LTMRs. DYN+ interneurons largely coexpress galanin in superficial laminae I–IIo and nNOS in lamina III (Sardella et al., 2011a). Galanin interneurons have been shown to upregulate cfos in response to noxious stimulation (Noguchi et al., 1991) and pERK after formalin or heat (Polgár et al., 2013), and a subpopulation of these, presumably DYN negative, persist after DYN+ interneuron ablation, which could explain the lack of any detectable pain phenotype in DYN+ ablated mice or in the presence of an antagonist to the dynorphin receptor (Duan et al., 2014; Kardon et al., 2014). A second, deeper population overlapping with DYN+ interneurons are the nNOS+ interneurons, which are located in laminae I–III and include excitatory and inhibitory subtypes (Liuzzi et al., 1993; Polgár et al., 2013). nNOS+ inhibitory interneurons express both glycine and GABA inhibitory neurotransmitters and have been shown to make direct synaptic contact to lamina I neurokinin 1 receptor positive (NK1R) projection neurons, although the functional relevance of this connectivity has yet to be tested (Puskar et al., 2001), whereas the excitatory nNOS+ population colocalize with PKCγ in lamina III (Hughes et al., 2008; Sardella et al., 2011b; Spike et al., 1993). The reported effects of NOS and NO on basal pain states are controversial, but NOS inhibitors and global knockout studies have shown that nitrous oxide synthase is required for basal maintenance of hyperalgesia, and not thermal hypersensitivity, in both inflammatory and neuropathic pain states (Boettger et al., 2007; Chu et al., 2005; Guan et al., 2007; Iwamoto and Marion, 1994; Luo and Cizkova, 2000; Meller et al., 1992; Osborne and Coderre, 1999; Tao et al., 2004; Zeitz et al., 2002), implicating a role for nNOS+ neurons in gating low threshold input to superficial interneurons in pathological states of disinhibition.
An additional study identified tyrosine kinase receptor Ret as a marker of 30% of the inhibitory interneurons in laminae III–V. Neurons that express Ret in the early postnatal period (early Ret+ interneurons) are located in the deep dorsal horn (ddh) (Cui et al., 2016) and are inhibitory; Ret expression spreads to the superficial dorsal horn by the second postnatal week and subsequently decreases by the third week of life. Neurochemically, 25–30% Ret+ neurons co-express PV, but otherwise represent a distinct subpopulation of interneurons that do not overlap with the DYN+ interneurons. Early Ret+ ddh interneurons receive prominent local excitatory input from neighboring interneurons in the deep dorsal horn, as well as a complex network of afferent and local inputs, segregating them into two subpopulations: Ret+ neurons which receive monosynaptic input from Aβ fibers and convergent C fiber afferent input via neighbouring Ret+ glycinergic inhibitory synapses, and Ret+ neurons which receive monosynaptic Aβ and C fiber input, as well as convergent polysynaptic Aβ fiber afferent input via Ret+ glycinergic inhibitory synapses. In contrast to the DYN+ inhibitory interneurons, Ret+ interneurons control the flow of afferent information through both presynaptic and postsynaptic mechanisms, thereby placing them in the classic gate inhibitory circuit. Presynaptic targeting of Aβ and C fibers is achieved via GABAergic inhibition onto primary afferents and postsynaptic control is achieved via glycinergic control of both PKCγ+ interneurons, as well as via SOM+ interneurons. Two independent pathways for transduction of sensory information have been presented: an inhibitory gate to PKCγ+ for dynamic touch, and inhibition to SOM+ for mechanical pain (Cui et al., 2016), although it remains to be determined if these two mechanisms are achieved by independent subgroups of Ret+ interneurons. As such, genetic ablation or silencing of early Ret+ ddh interneurons results in a static and dynamic allodynic phenotype as well as a hyperalgesic pain phenotype, which is absent in DYN+ interneuron ablated mice. Interestingly, early Ret+ neurons also appear to have a role in the severity and duration of both inflammatory and neuropathic pain conditions, as ablation or silencing of early Ret+ ddh interneurons during these pain states precipitates existing pain conditions (Cui et al., 2016). Activation of these neurons in pathological pain states can ameliorate these symptoms, and can decrease withdrawal frequency to von Frey hair and to thermal stimuli under baseline conditions.
PV expression in the postnatal dorsal horn defines a major population of inhibitory interneurons, some of which overlap with the Ret+ interneuronal population. The PV+ interneurons are located in laminae IIi–III and form axoaxonic presynaptic inhibitory synapses with presumed non-nociceptive Aδ down hair afferents and larger myelinated afferent fibers, which suggests a microcircuitry that is primarily tuned for low threshold input (Abraira et al., 2017; Hughes et al., 2012; Petitjean et al., 2015). PV also demarcates a small population of excitatory interneurons (Abraira et al., 2017; Antal et al., 1991). PV+ interneurons have an islet or central cell-like morphology with an elongated rostrocaudal axis, which is consistent with an inhibitory phenotype, and arborize extensively in laminae IIi and III. Importantly, they fire high frequency action potentials upon somatic current injection (Hughes et al., 2012), a property likely to be necessary for the inhibition of fast firing myelinated afferents. Morphological analysis in the rat has identified three possible subtypes within the PV+ population according to their axon trajectory, cell body location and dendritic morphology (Yamamoto et al., 1989). The role of the PV interneurons in the control of acute pain is still not fully appreciated. Petitjean et al. (2015) reported that chemogenetic activation of these interneurons increases mechanical von Frey hair thresholds without affecting heat sensitivity, albeit only upon strong PV+ interneuron activation. After onset of neuropathic or inflammatory pain states, activating the PV+ interneurons partially reverses the mechanical hypersensitivity that arises as a result of spinal nerve injury, and both mechanical and thermal hypersensitivity in capsaicin-induced inflammatory pain (Petitjean et al., 2015). The authors suggest this effect is largely due to postsynaptic glycinergic connectivity to PKCγ+ interneurons in lamina II, with which they make considerable contact. Interestingly, as with many identified neuropeptides and calcium-binding proteins, expression of PV is dynamic and has been shown to decrease in the dorsal horn during inflammatory pain states such as arthritis (Mineta et al., 1996; Zacharova et al., 2009). The functional relevance of this is yet to be determined.
Spinal pathways for inflammatory and neuropathic pain
Many of the neurons outlined above are implicated in pathological pain states, namely inflammatory pain and neuropathic pain. These states are more enduring than acute pain, and most likely arise from distinct mechanisms, including disinhibition or degradation of inhibitory gate pathways by which both touch and pain signals are normally muted. Yet other neuronal populations, which are normally not associated with a somatosensory phenotype in healthy animals, appear to now be recruited and active in pathological pain states. Importantly, the function of each neuronal subtype in the initiation or maintenance of a pathological pain state is likely to be more complex than may be apparent from the evidence and tools currently available, not least because many studies have utilized neuronal ablation to examine the role of individual classes on nociceptive processing. This in itself is likely to modify circuit connectivity and function, obscuring the role of neurons in organic conditions (Kuner, 2010; Sandkuhler, 2009; Scholz and Woolf, 2007).
NK1R is the principal receptor for substance P, which is released within the spinal cord by a subset of primary afferents in response to noxious stimulation (Duggan et al., 1991; Jessell and Iversen, 1977; Schaible et al., 1990). NK1R is expressed broadly in the dorsal horn, but most notably distinguishes a major fraction of spinothalamic and spinobrachial projection neurons in lamina I, which are thought to transmit nociceptive information to higher brain centers (Al-Khater et al., 2008; Spike et al., 2003; Todd et al., 2000). Lamina I NK1R+ neurons receive monosynaptic inputs from C and Aδ fibers conducting nociceptive signals, as well as polysynaptic input from Aβ low threshold afferents, which is revealed upon spinal disinhibition (Torsney and MacDermott, 2006). Thus, NK1R+ neurons are proposed to act as a convergent site for pathological pain, in which release of Aβ fibers from inhibition could give rise to allodynia/hyperalgesia. Lamina I NK1R+ interneurons are targeted by nNOS+ interneurons (Puskár et al., 2001) whereas those located in lamina III receive inputs from DYN+ interneurons (Baseer et al., 2012). The NK1R+ neurons have not successfully been genetically targeted due to the early broad expression of NKR1; however, selective ablation of lamina I NK1R+ neurons with saporin-conjugated SP has been shown to attenuate heat and mechanical hyperalgesia produced by intradermal capsaicin, without affecting baseline nociceptive or sensory behavior (Mantyh et al., 1997).
Through their polysynaptic excitatory connectivity to NK1R+ neurons in lamina I, vGluT3+ interneurons are proposed to transmit the sensation of touch in allodynic pain states, when strong inhibitory control of their postsynaptic partners is relieved; in support of this, selective activation of this subset of interneurons results in a spontaneous pain phenotype (Peirs et al., 2015; Chen et al., 2017). In addition to their role in the processing of von Frey hair punctate stimuli, vGluT3+ interneurons are also suggested to be actively involved in the amplification of allodynia and hypersensitivity during inflammation and neuropathic pain states following spinal disinhibition. Interestingly, the authors suggest two separate pathways to allodynia depending on the nature of the pathology: vGluT3+ excitation of CR+/Calb2+ interneurons in inflammatory pain states, and vGluT3+ excitation of PKCγ+ interneurons in neuropathic conditions, although this remains to be fully elucidated. The lack of brush-evoked sensitivity following vGluT3+ interneuron ablation or silencing suggests that there are other key players in the transmission of brush-evoked activity in the dorsal horn.
Dorsal horn inhibitory interneurons expressing the TRPV1 in laminae I and II are proposed to modulate nociceptive signaling (Doly et al., 2004; Ferrini et al., 2010; Kim et al., 2012; Valtschanoff et al., 2001; Zhou et al., 2009) and have been shown to be implicated in mechanical allodynia and hypersensitivity. TRPV1 antagonists alleviate allodynia caused by nerve injury not only via afferent fibers that express the receptor, but also by inhibiting a subset of TRPV1-expressing dorsal horn interneurons (Costigan et al., 2009; Coull et al., 2003; Kim et al., 2012; Patapoutian et al., 2009; Torsney and MacDermott, 2006; Woolf et al., 1992). Interestingly, TRPV1 knockout mice do not display altered responses to baseline mechanical pain stimuli, suggesting that TRPV1+ interneurons are involved in a pathology-specific circuit (Caterina et al., 2000; Kim et al., 2012). After nerve injury, inhibitory TRPV1+ interneurons are proposed to reduce presynaptic expression of the glutamatergic receptor GluR2 in excitatory dorsal neurons through a mechanism involving long term depression, leading to a decrease in excitatory drive to GABAergic interneurons and disinhibition of spinothalamic tract projection neurons (Kim et al., 2012).
PKCγ, and the dorsal interneurons that express PKCγ, have been implicated in maintaining mechanical and thermal pain after neuropathic injury (Malmberg et al., 1997; Petitjean et al., 2015; Pham Dang et al., 2016). Interneurons expressing PKCγ are likely to be a common convergent output for many of the neurons described above. PKCγ+ interneurons are restricted to lamina IIi and III, and represent a morphologically diverse subpopulation of glutamatergic interneurons that receive myelinated afferent input (Abraira et al., 2017; Alba-Delgado et al., 2015; Miraucourt et al., 2009; Pham Dang et al., 2016). These neurons have been linked to many disinhibitory pathways involving touch-evoked pain; consistent with this, both pharmacological manipulation of PKCγ and global deletion of PKCγ disrupts allodynic pain behavior (Duan et al., 2014; Miraucourt et al., 2009; Peirs et al., 2015; Petitjean et al., 2015; Pham Dang et al., 2016). Despite evidence for cfos activation in PKCγ+ interneurons after walking on a rotarod in naïve rats, global deletion did not affect acute pain thresholds in any sensory tests (Neumann et al., 2008). A direct role for the PKCγ+ interneurons in the initiation or maintenance of pain states remains to be established.
Spinal pathways for itch
Itch and itch-induced scratching are often elicited in response to skin born parasites and insect bites that are a source of pathogens and endoparasites, indicating that itch-induced scratching represents an acute nocifensive and/or protective behavior. Nonetheless, maladaptive and pathogenic states that lead to chronic itch can develop in the absence of insects or other irritants. There are two widely recognized forms of chemical itch, histamine-dependent and histamine–independent itch, both of which are transmitted centrally by gastrin-releasing peptide receptor (GRPR)+ interneurons (Sun et al., 2009). Additionally, there is growing evidence for a mechanical itch pathway that is independent of GRPR+ interneurons (Fukuoka et al., 2013; Bourane et al, 2015b). With regards to chemical itch, signaling from pruritic nociceptors, which respond to a variety of pruritogens (reviewed in Bautista et al. 2014, LaMotte et al., 2014; Barry et al., 2017) converges on excitatory GRPR+ neurons that are located predominantly in laminae I–II, where they are intermixed with nociceptive neurons. GRPR+ neurons are seemingly itch-specific, as deletion of the receptor and associated neurons attenuates pruritogen-induced scratching without affecting nocifensive or locomotor behaviors (Mishra and Hoon, 2013). GRP+ interneurons in laminae I–II are also implicated in itch transmission, although the nature of the GRP-GRPR neuron circuit is still the subject of much debate. GRP-expressing neurons are present in lamina II (Fleming et al., 2012; Solorzano et al., 2015; Sun et al., 2017) and expression of the receptor in the dorsal horn overlaps with that of NPRA, the receptor for Brain Natriuretic Peptide (NBP), which is also a pruritogen (Mishra and Hoon, 2013). Furthermore, ablation of GRP-expressing interneurons or NPRA-expressing interneurons significantly reduces pruritogen-induced scratching (Mishra and Hoon, 2013; Sun et al., 2017), arguing that lamina II GRP/NPRA neurons are essential elements of the itch transmission pathway. Opiates that induce itch also appear to signal via the GRPR pathway, with the mu opioid receptor 1 (MORD1) forming a heterodimeric complex with GRPR (Liu et al., 2011) that activates the downstream itch pathway.
It is clear that inhibition also plays an important role in regulating the central transmission pathways for itch. Inhibitory neurons that are derived from dI4/dILA progenitors play a preeminent role in gating multiple sensory modalities including itch. This regulation is most likely mediated by both postsynaptic and presynaptic inhibitory mechanisms (Akiyama et al., 2011; Fink et al., 2014). The relative contribution that postsynaptic versus presynaptic inhibition makes to the gating of itch remains unclear, and while the increased scratching that arises following GAD2Cre-dependent ablation of dI4/dILA interneurons points to presynaptic inhibition playing a role; many GAD2+ neurons also form postsynaptic inhibitory synapses within the dorsal horn. One population of dI4/dILA interneurons that have been directly implicated in gating itch are the basic helix-loop-helix (bHLH) transcription factor Bhlhb5 inhibitory (B5-I) interneurons (Ross et al., 2010). Deletion of the Bhlhb5 gene results in a spontaneous scratching phenotype and elevated scratching in response to various pruritogens. B5-I interneurons are thought to suppress itch via cross-activation by nociceptive afferents, such that the priming of B5-I interneurons by a nociceptive stimulus inhibits itch transmission. Accordingly, B5-I neurons receive input from nociceptive afferents sensitive to mechanical and chemical stimuli (Chiang et al., 2016; Hachisuka et al., 2016; Kardon et al., 2014). The B5-I population includes interneurons that additionally express DYN, the kappa opioid receptor agonist, which has also been implicated in itch. Kappa opioid agonists inhibit itching behavior and can abate scratching in Bhlhb5 knockout mice, consistent with a reduced kappa opioid tone in these animals. These results, in addition to the lack of itch phenotype in DYN+ ablated mice, suggest an intriguing role for the enkephalin dynorphin in itch modulation (Kardon et al., 2014; Duan et al., 2014). Most B5-I neurons express the inhibitory SOM receptor SST2AR and show strong hyperpolarization in response to activation. Activation of the receptor elicits or exacerbates itch responses in control but not Bhlhb5 knockout mice, without altering nociceptive reflexes (Kardon et al., 2014). In accordance with this, a separate analysis found that direct activation of SOM+ interneurons by channelrhodopsin enhanced spontaneous itch behavior in awake mice, which was blocked by the SST2R antagonist CYN-154806 (Christensen et al., 2016).
While significant progress has been made in defining the peripheral and central pathways that transmit chemical itch modalities, evidence for a dedicated spinal mechanical itch pathway has only recently been forthcoming. In a recent study, Bourane et al, (2015b) identified a subpopulation of NPY-expressing interneurons enriched in laminae II–IV that are critical in the inhibitory gating of mechanical itch, but not touch or pain. These inhibitory interneurons are marked by expression of NPY::Cre and are distinct from the B5-I interneurons that gate chemical itch (Kardon et al., 2014). Interestingly, these NPY+ interneurons are selectively innervated by low threshold myelinated afferents from the hairy skin, namely Aδ LTMRs and Aβ LTMRs, and exhibit tonic firing patterns characteristic of inhibitory interneurons. Ablation of the NPY::Cre+ interneurons leads to a spontaneous scratching phenotype, which is characterized by increases in dorsal neuron afterdischarge activity in response to repeated brushing of the hairy skin. The increase in behavioral scratching is GRPR-independent, with the NPY::Cre+ ablated mice displaying no change in sensitivity to known chemical pruritogens. The spontaneous scratching phenotype that is seen in these mice suggests the deep dorsal NPY+ interneurons exert strong tonic inhibition of low threshold input from hairy skin within the dorsal horn under basal conditions, such that removal of this inhibition leads to runaway excitation. Interestingly the neuropeptide NPY has also been implicated in the inhibition of neuropathic and inflammatory pain (Duggan et al., 1991; Naveilhan et al., 2016; Intondi et al., 2008; Iwagaki et al., 2016; Solway et al., 2011), suggesting two independent roles for the NPY+ interneurons in the dorsal horn. It remains to be determined whether this indicates the existence of two functionally distinct NPY+ interneuron populations, or the differential actions of the neuropeptide and the interneurons that express it on nociceptive and mechanosensory transmission.
The central processing of cutaneous somatosensory modalities
One of the major challenges for the field is understanding how cutaneous sensory modalities are encoded within the dorsal horn and the ascending pathways that relay sensory information supraspinally, so as to update the ongoing motor program and provide a real time picture of the environment and the body’s interactions with its surroundings. Much of this information, particularly that which is used to update the motor system, is processed locally in the spinal cord and hindbrain, where it engages local motor circuits. One thing that is becoming increasingly clear is the high level of neuronal diversity with regards to the sensory afferents that transmit cutaneous information and the sensory interneurons that process this information within the dorsal horn. Indeed, it is not clear how many functionally distinct cell types are present in the dorsal horn, since many of the molecular markers identified to date are expressed in overlapping mosaic populations of neurons, which may represent multiple functional subtypes. It is also unclear whether the spinal coding of an individual modality occurs at the level of individual subsets of dorsal horn interneurons within a given spinal laminar locale, or between multiple interneuron populations. Likewise, the contribution that supraspinal pathways make to sensory coding remains to be determined. Several models have been proposed for the coding of nociceptive information (reviewed in Ma, 2010; Prescott et al., 2014)). These include the labeled line theory, intensity theory, pattern theory, gate control theory and combinatorial theory. In brief, specificity theory states that information about a given modality is carried in a so called “labeled line” by a series of dedicated neurons from the periphery, through the spinal cord and to the brain. The intensity theory proposes that pain is not a discrete modality, but instead results from temporal summation of subthreshold stimulations, whereas the pattern theory excludes cellular tuning, proposing that each modality is encoded by a specific spatiotemporal pattern of firing. Finally, population coding retains the concepts of central summation of somatosensory information advanced by gate control theory, but emphasizes tuning within peripheral afferents (Ma, 2010, 2012). Although a degree of selectivity towards one modality has certainly been demonstrated in a number of identified interneuronal populations and circuits (eg. Bourane et al., 2015a; Duan et al., 2014; Sun and Chen, 2007)), this is likely an oversimplification of the circuit, since there is evidence for integration and tuning of convergent input at the spinal level (e.g. Abriara et al., 2017; Sun et al., 2017). There is also a growing stream of evidence that first order sensory interneurons receive a range of afferent input from the periphery, as well as from local circuit neurons and descending pathways. How then is discrimination between different modalities achieved in the spinal dorsal horn? Whereas the intensity code theory suggests convergent input is weighted within an individual interneuron, such that a stronger stimulus from an individual fiber will evoke a more salient response than a weak stimulus of a second modality, the convergence of local circuits also points towards the pattern of inputs or combinatorial coding being important for salience. The pattern and combinatorial theories also allow for localized processing of sensory modalities, as well as plasticity within the circuit. Importantly, each of the proposed theories place a heavy emphasis on the laminar localization of the interneuron in question, which is a primary determinant of the range of afferent inputs a first order interneuron receives.
Cutaneous stimuli, both noxious and innocuous are able to elicit stereotypical motor reflexes, examples being the nociceptive withdrawal reflex and the stumbling corrective reflex, the latter of which is elicited only during the swing phase of stepping. A key to understanding how cutaneous sensory information is encoded and transformed within the spinal cord lies in determining how different sensory stimuli engage with the locomotor CPG to produce stimulus-specific reflexes. This approach has been used with varying degrees of success in the cat and turtle (Burke, 1999; Berkowitz, 2010). With the advent of genetic tools we can now interrogate these circuits in the mouse in order to understand the cellular logic that underlies sensorimotor transformation in the spinal cord. Moreover, by untangling the coding mechanisms that operate in the somatosensory system we can begin to understand how the nervous system allows animals adapt to their ever-changing environment.
Figure 3. Molecularly defined dorsal-horn interneurons form modality-specific circuits.
Composite circuit diagrams showing proposed interactions between molecularly defined classes of dorsal-horn neurons in (A) static and dynamic touch, (B) mechanical and thermal nociception, (C) itch and (D) inflammatory and neuropathic pain (see text and Tables 1 and 2 for detailed description). Dashed line: connectivity not directly demonstrated. Abbreviations: Bhlhb5, basic helix-loop-helix domain containing, class B5; CR, calretinin/calb2; DYN, dynorphin; GRP, gastrin-releasing peptide; GRPR1, gastrin-releasing peptide receptor 1; IN, interneuron; MN, motoneuron; NK1R, neurokinin 1 receptor; NPRA, natriuretic peptide receptor A; NPY, neuropeptide Y; PKCγ, protein kinase C gamma; PN, projection neuron; PV, parvalbumin; Ret, retinin; RORα, RAR-related orphan receptor alpha; SOM, somatostatin; TRPV1, transient receptor potential cation channel, subfamily V, member 1; vGluT3, vesicular glutamate transporter 3.
Acknowledgments
Work in the Goulding lab is supported by grants from the National Institutes of Health (NS 080586, NS 086372, NS090919 and a Marie Curie Fellowship to SK. MG holds the Frederick W. and Joanne J. Mitchell Chair in Molecular Biology.
Literature Cited
- Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, Bashista KA, O’Neill TG, Zhuo J, Tsan C, Hoynoski J, Rutlin M, Kus L, Niederkofler V, Watanabe M, Dymecki SM, Nelson SB, Heintz N, Hughes DI, Ginty DD. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell. 2017;168:295–310. doi: 10.1016/j.cell.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akay T, Tourtellotte WG, Arber S, Jessell TM. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. PNAS. 2014;111:16877–16882. doi: 10.1073/pnas.1419045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khater KM, Kerr R, Todd AJ. A quantitative study of spinothalamic neurons in laminae I, III, and IV in lumbar and cervical segments of the rat spinal cord. J Comp Neurol. 2008;511:1–18. doi: 10.1002/cne.21811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba-Delgado C, El Khoueiry C, Peirs C, Dallel R, Artola A, Antri M. Subpopulations of PKCγ interneurons within the medullary dorsal horn revealed by electrophysiologic and morphologic approach. Pain. 2015;156:1714–28. doi: 10.1097/j.pain.0000000000000221. [DOI] [PubMed] [Google Scholar]
- Amann M, Sidhu SK, Weavil JC, Mangum TS, Venturelli M. Autonomic responses to exercise: Group III/IV muscle afferents and fatigue. Auton Neurosci. 2015;188:19–23. doi: 10.1016/j.autneu.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal M, Polgár E, Chalmers J, Minson JB, Llewellyn-Smith I, Heizmann CW, Somogyi P. Different populations of parvalbumin- and calbindin-D28k-immunoreactive neurons contain GABA and accumulate 3H-D-aspartate in the dorsal horn of the rat spinal cord. J Comp Neurol. 1991;314:114–24. doi: 10.1002/cne.903140111. [DOI] [PubMed] [Google Scholar]
- Aprison MH, Shank RP, Davidoff RA. A comparison of the concentration of glycine, a transmitter suspect, in different areas of the brain and spinal cord in seven different vertebrates. Comp Biochem Physiol. 1969;28:1345–55. doi: 10.1016/0010-406x(69)90571-4. [DOI] [PubMed] [Google Scholar]
- Aprison MH, Werman R. The distribution of glycine in cat spinal cord and roots. Life Sci. 1965;4:2075–83. doi: 10.1016/0024-3205(65)90325-5. [DOI] [PubMed] [Google Scholar]
- Araki T, Yamano M, Murakami T, Wanaka A, Betz H, Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988;25:613–24. doi: 10.1016/0306-4522(88)90263-1. [DOI] [PubMed] [Google Scholar]
- Basbaum AI. Distribution of glycine receptor immunoreactivity in the spinal cord of the rat: cytochemical evidence for a differential glycinergic control of lamina I and V nociceptive neurons. J Comp Neurol. 1988;278:330–36. doi: 10.1002/cne.902780303. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseer N, Polgár E, Watanabe M, Furuta T, Kaneko T, Todd AJ. Projection neurons in lamina III of the rat spinal cord are selectively innervated by local dynorphin-containing excitatory neurons. J Neurosci. 2012;32:11854–63. doi: 10.1523/JNEUROSCI.2707-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci. 2014;17:175–82. doi: 10.1038/nn.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A. Multifunctional and specialized spinal interneurons for turtle limb movements. Ann NY Acad Sci. 2010;1198:119–32. doi: 10.1111/j.1749-6632.2009.05428.x. [DOI] [PubMed] [Google Scholar]
- Boettger MK, Üceyler N, Zelenka M, Schmitt A, Reif A, Chen Y, Sommer C. Differences in inflammatory pain in nNOS-, iNOS- and eNOS-deficient mice. Eur J Pain. 2007;11:810–8. doi: 10.1016/j.ejpain.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Bourane S, Garces A, Venteo S, Pattyn A, Hubert T, Fichard A, Puech S, Boukhaddaoui H, Baudet C, Takahashi S, Valmier J, Carroll P. Low-Threshold Mechanoreceptor Subtypes Selectively Express MafA and Are Specified by Ret Signaling. Neuron. 2009;64:857–70. doi: 10.1016/j.neuron.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Bourane S, Grossmann KS, Britz O, Dalet A, Del Barrio MG, Stam FJ, Garcia-Campmany L, Koch S, Goulding M. Identification of a spinal circuit for light touch and fine motor control. Cell. 2015a;160:503–15. doi: 10.1016/j.cell.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, Goulding M. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science. 2015b;350:550–4. doi: 10.1126/science.aac8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG. Organization in the Spinal Cord. Springer-Verlag; Berlin, Heidelberg, New York: 1981. [Google Scholar]
- Burke RE. The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Exp Brain Res. 1999;128:263–77. doi: 10.1007/s002210050847. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cheng L, Duan B, Huang T, Zhang Y, Chen Y, Britz O, Garcia-Campmany L, Ren X, Vong L, Lowell BB, Goulding M, Wang Y, Ma Q. Identification of spinal circuits involved in touch-evoked dynamic mechanical pain. Nat Neurosci. 2017 doi: 10.1038/nn.4549. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Hachisuka J, Todd AJ, Ross SE. Insight into B5-I spinal interneurons and their role in the inhibition of itch and pain. Pain. 2016;157:544–5. doi: 10.1097/j.pain.0000000000000474. [DOI] [PubMed] [Google Scholar]
- Christensen AJ, Iyer SM, Francois A, Vyas S, Ramakrishnan C, Vesuna S, Deisseroth K, Scherrer G, Delp SL. In Vivo Interrogation of Spinal Mechanosensory Circuits. Cell Rep. 2016;17:1699–710. doi: 10.1016/j.celrep.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Guan Y, Skinner J, Raja S, Johns R, Tao Y-X. Effect of genetic knockout or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund’s adjuvant-induced persistent pain. Pain. 2005;119:113–23. doi: 10.1016/j.pain.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Carlton SM. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Res Rev. 1997;24:28–66. doi: 10.1016/s0165-0173(97)00010-6. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JAM, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, Koninck PD, Koninck YD. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–42. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Cui L, Miao X, Liang L, Abdus-Saboor I, Olson W, Fleming MS, Ma M, Tao YX, Luo W. Identification of Early RET+ Deep Dorsal Spinal Cord Interneurons in Gating Pain. Neuron. 2016;91:1137–53. doi: 10.1016/j.neuron.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y, Ribeiro-Da-Silva A, Henry JL, Cuello AC. Spinal neurons exhibiting a specific nociceptive response receive abundant substance P-containing synaptic contacts. PNAS. 1992;89:5073–7. doi: 10.1073/pnas.89.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 Is Required for Cold Sensation in Mice. Neuron. 2007;54:371–8. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Doly S, Fischer J, Conrath M. The vanilloid receptor-1 (TRPV1) is expressed in some rat dorsal horn NK1 cells. Brain Res. 2004;1004:203–7. doi: 10.1016/j.brainres.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross SE, Lowell BB, Wang Y, Goulding M, Ma Q. Identification of spinal circuits transmitting and gating mechanical pain. Cell. 2014;159:1417–32. doi: 10.1016/j.cell.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- Duggan AW, Hope PJ, Lang CW. Microinjection of neuropeptide-Y into the superficial dorsal horn reduces stimulus-evoked release of immunoreactive substance-P in the anesthetized cat. Neuroscience. 1991;44:733–40. doi: 10.1016/0306-4522(91)90092-3. [DOI] [PubMed] [Google Scholar]
- Ferrini F, Salio C, Lossi L, Gambino G, Merighi A. Modulation of inhibitory neurotransmission by the vanilloid receptor type 1 (TRPV1) in organotypically cultured mouse substantia gelatinosa neurons. Pain. 2010;150:128–40. doi: 10.1016/j.pain.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Fleming MS, Ramos D, Han SB, Zhao J, Son YJ, Luo W. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol Pain. 2012;8:52. doi: 10.1186/1744-8069-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster E, Wildner H, Tudeau L, Haueter S, Ralvenius WT, Jegen M, Johannssen H, Hösli L, Haenraets K, Ghanem A, Conzelmann KK, Bösl M, Zeilhofer HU. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron. 2015;85:1289–304. doi: 10.1016/j.neuron.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka M, Miyachi Y, Ikoma A. Mechanically evoked itch in humans. Pain. 2013;154:897–904. doi: 10.1016/j.pain.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Yaster M, Raja S, Tao Y-X. Genetic knockout and pharmacologic inhibition of neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in mice. Molecular Pain. 2007;3:29. doi: 10.1186/1744-8069-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachisuka J, Baumbauer KM, Omori Y, Snyder LM, Koerber HR, Ross SE. Semi-intact ex vivo approach to investigate spinal somatosensory circuits. Elife. 2016:5. doi: 10.7554/eLife.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantman AW, Pol AN, Perl ER. Morphological and physiological features of a set of spinal substantia gelatinosa neurons defined by green fluorescent protein expression. J Neurosci. 2004;24:836–42. doi: 10.1523/JNEUROSCI.4221-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinke B, Ruscheweyh R, Forsthuber L, Wunderbaldinger G, Sandkuhler J. Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. J Physiol. 2004;560:249–66. doi: 10.1113/jphysiol.2004.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdegen T, Rudiger S, Mayer B, Bravo R, Zimmermann M. Expression of nitric oxide synthase and colocalisation with Jun, Fos and Krox transcription factors in spinal cord neurons following noxious stimulation of the rat hindpaw. Mol Brain Res. 1994;22:245–58. doi: 10.1016/0169-328x(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Hossaini M, French PJ, Holstege JC. Distribution of glycinergic neuronal somata in the rat spinal cord. Brain Research. 2007;1142:61–9. doi: 10.1016/j.brainres.2007.01.078. [DOI] [PubMed] [Google Scholar]
- Hughes AS, Averill S, King VR, Molander C, Shortland PJ. Neurochemical characterization of neuronal populations expressing protein kinase C gamma isoform in the spinal cord and gracile nucleus of the rat. Neuroscience. 2008;153:507–17. doi: 10.1016/j.neuroscience.2008.01.082. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Sikander S, Kinnon CM, Boyle KA, Watanabe M, Callister RJ, Graham BA. Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: a likely source of axo-axonic inputs in the mouse spinal dorsal horn. J Physiol. 2012;590:3927–51. doi: 10.1113/jphysiol.2012.235655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto ET, Marion L. Pharmacologic evidence that spinal muscarinic analgesia is mediated by an L-arginine/nitric oxide/cyclic GMP cascade in rats. J Pharmacol Exp Ther. 1994;271:601–8. [PubMed] [Google Scholar]
- Janig W. The integrative action of the autonomic nervous system. Cambridge University Press; Cambridge, UK: 2006. [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1998;38:335–78. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jessell TM, Iversen LL. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977;268:549–51. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kardon AP, Polgár E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82:573–86. doi: 10.1016/j.neuron.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Back SK, Davies AJ, Jeong H, Jo HJ, Chung G, Na HS, Bae YC, Kim SJ, Kim JS, Jung SJ, Oh SB. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron. 2012;74:640–7. doi: 10.1016/j.neuron.2012.02.039. [DOI] [PubMed] [Google Scholar]
- Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–66. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- Lallemend F, Ernfors P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci. 2012;35:373–81. doi: 10.1016/j.tins.2012.03.006. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. 2014;15:19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M-Z, Wang J-S, Jiang D-J, Xiang C-X, Wang F-Y, Zhang K-H, Williams PR, Chen Z-F. Molecular mapping of developing dorsal horn-enriched genes by microarray and dorsal/ventral subtractive screening. Dev Biol. 2006;292:555–64. doi: 10.1016/j.ydbio.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Light AR, Kavookjian AM. Morphology and ultrastructure of physiologically identified substantia gelatinosa (lamina II) neurons with axons that terminate in deeper dorsal horn laminae (III-V) J Comp Neurol. 1988;267:172–89. doi: 10.1002/cne.902670203. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186:133–50. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Light AR, Trevino DL, Perl ER. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979;186:151–71. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- Lima D, Avelino A, Coimbra A. Morphological characterization of marginal (lamina I) neurons immunoreactive for substance P, enkephalin, dynorphin and gamma-aminobutyric acid in the rat spinal cord. J Chem Neuroanat. 1993;6:43–52. doi: 10.1016/0891-0618(93)90006-p. [DOI] [PubMed] [Google Scholar]
- Liu X-Y, Liu Z-C, Sun Y-G, Ross M, Kim S, Tsai F-F, Li Q-F, Jeffry J, Kim J-Y, Loh HH, Chen Z-F. Unidirectional Cross-Activation of GRPR by MOR1D Uncouples Itch and Analgesia Induced by Opioids. Cell. 2011;147:447–58. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi FJ, Wu W, Scoville SA, Schinco FP. Development of nitric oxide synthase expression in the superficial dorsal horn of the rat spinal cord. Exp Neurol. 1993;121:275–8. doi: 10.1006/exnr.1993.1096. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ. Anatomy of synaptic circuits controlling the activity of sympathetic preganglionic neurons. J Chem Neuroanat. 2009;38:231–9. doi: 10.1016/j.jchemneu.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, Xiong L. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest. 2013;123:4050–62. doi: 10.1172/JCI70026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD, Cizkova D. The role of nitric oxide in nociception. Curr Rev Pain. 2000;4:459–66. doi: 10.1007/s11916-000-0070-y. [DOI] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular Identification of Rapidly Adapting Mechanoreceptors and Their Developmental Dependence on Ret Signaling. Neuron. 2009;64:841–56. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Molecular Structure and Function of the Glycine Receptor Chloride Channel. Physiol Rev. 2004;84:1051–95. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 2010;120:3773–8. doi: 10.1172/JCI43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Population coding of somatic sensations. Neurosci Bull. 2012;28:91–9. doi: 10.1007/s12264-012-1201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone F, Wessberg J, Olausson H. Discriminative and Affective Touch: Sensing and Feeling. Neuron. 2014;82:737–5. doi: 10.1016/j.neuron.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Euchenhofer C, Tegeder I, Beck KF, Pfeilschifter J, Geisslinger G. Regulation and immunhistochemical localization of nitric oxide synthases and soluble guanylyl cyclase in mouse spinal cord following nociceptive stimulation. Neurosci Lett. 2000;290:71–5. doi: 10.1016/s0304-3940(00)01302-1. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–83. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–9. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Meller S, Pechman P, Gebhart G, Maves T. Nitric oxide mediates the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Neuroscience. 1992;50:7–10. doi: 10.1016/0306-4522(92)90377-e. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–9. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Mineta Y, Koyanagi H, Morimoto M, Harano K, Totoki T, Jacobowitz D. Immunocytochemical study of parvalbumin, calbindin D-28k, and calretinin in the superficial dorsal horn of the rat spinal cord following unilateral hindpaw inflammation. J Anesth. 1996;10:211–17. doi: 10.1007/BF02471393. [DOI] [PubMed] [Google Scholar]
- Miraucourt LS, Moisset X, Dallel R, Voisin DL. Glycine Inhibitory Dysfunction Induces a Selectively Dynamic, Morphine-Resistant, and Neurokinin 1 Receptor- Independent Mechanical Allodynia. J Neurosci. 2009;29:2519–27. doi: 10.1523/JNEUROSCI.3923-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–71. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol. 2001;281:R683–98. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Lucas G, Blakeman KH, Hao J-X, Xu X-J, Wiesenfeld-Hallin Z, Thorén P, Ernfors P. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature. 2001;409:513–517. doi: 10.1038/35054063. [DOI] [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKC gamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–44. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–61. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Kowalski K, Traub R, Solodkin A, Iadarola MJ, Ruda MA. Dynorphin expression and Fos-like immunoreactivity following inflammation induced hyperalgesia are colocalized in spinal cord neurons. Mol Brain Res. 1991;10:227–33. doi: 10.1016/0169-328x(91)90065-6. [DOI] [PubMed] [Google Scholar]
- Osborne MG, Coderre TJ. Effects of intrathecal administration of nitric oxide synthase inhibitors on carrageenan-induced thermal hyperalgesia. Brit J Pharmacol. 1999;126:1840–46. doi: 10.1038/sj.bjp.0702508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res. 2004;143:123–9. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Peirs C, Williams SP, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, Seal RP. Dorsal Horn Circuits for Persistent Mechanical Pain. Neuron. 2015;87:797–812. doi: 10.1016/j.neuron.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan LY, Ribeiro-da-Silva A, Braz JM, Basbaum AI, Sharif-Naeini R. Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell Rep. 2015;13:1246–57. doi: 10.1016/j.celrep.2015.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham-Dang N, Descheemaeker A, Dallel R, Artola A. Activation of medullary dorsal horn γ isoform of protein kinase C interneurons is essential to the development of both static and dynamic facial mechanical allodynia. Eur J Neurosci. 2016;43:802–10. doi: 10.1111/ejn.13165. [DOI] [PubMed] [Google Scholar]
- Polgár E, Sardella TCP, Tiong SYX, Locke S, Watanabe M, Todd AJ. Functional differences between neurochemically defined populations of inhibitory interneurons in the rat spinal dorsal horn. Pain. 2013;154:2606–15. doi: 10.1016/j.pain.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, Ma Q, De Koninck Y. Normal and abnormal coding of somatosensory stimuli causing pain. Nat Neurosci. 2014;17:183–91. doi: 10.1038/nn.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, Hayes RL, Ruda M, Dubner R. Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J Neurophysiol. 1978;41:933–47. doi: 10.1152/jn.1978.41.4.933. [DOI] [PubMed] [Google Scholar]
- Puskár Z, Polgár E, Todd AJ. A population of large lamina I projection neurons with selective inhibitory input in rat spinal cord. Neuroscience. 2001;102:167–76. doi: 10.1016/s0306-4522(00)00445-0. [DOI] [PubMed] [Google Scholar]
- Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952;96:414–95. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Da-Silva A, Coimbra A. Neuronal uptake of [3H]gaba and [3H]glycine in laminae I–III (substantia gelatinosa rolandi) of the rat spinal cord. An autoradiographic study. Brain Res. 1980;188:449–64. doi: 10.1016/0006-8993(80)90044-x. [DOI] [PubMed] [Google Scholar]
- Ross S, Mardinly A, McCord A, Zurawski J, Cohen S, Jung C, Hu L, Mok S, Shah A, Savner E. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–98. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S. Dynamic Sensorimotor Interactions in Locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Bennett GJ, Dubner R. Neurochemistry and neural circuitry in the dorsal horn. Prog Brain Res. 1986;66:219–68. doi: 10.1016/s0079-6123(08)64606-3. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Sardella T, Polgár E, Garzillo F, Furuta T, Kaneko T, Watanabe M, Todd A. Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Mol Pain. 2011a;7:76. doi: 10.1186/1744-8069-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardella TCP, Polgá E, Watanabe M, Todd AJ. A quantitative study of neuronal nitric oxide synthase expression in laminae I–III of the rat spinal dorsal horn. Neuroscience. 2011b;192:708–20. doi: 10.1016/j.neuroscience.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible HG, Jarrott B, Hope PJ, Duggan AW. Release of immunoreactive substance P in the spinal cord during development of acute arthritis in the knee joint of the cat: a study with antibody microprobes. Brain Res. 1990;529:214–23. doi: 10.1016/0006-8993(90)90830-5. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Smith KM, Boyle KA, Madden JF, Dickinson SA, Jobling P, Callister RJ, Hughes DI, Graham BA. Functional heterogeneity of calretinin-expressing neurons in the mouse superficial dorsal horn: implications for spinal pain processing. J Physiol. 2015;593:4319–39. doi: 10.1113/JP270855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano C, Villafuerte D, Meda K, Cevikbas F, Braz J, Sharif-Naeini R, Juarez-Salinas D, Llewellyn-Smith IJ, Guan Z, Basbaum AI. Primary afferent and spinal cord expression of gastrin-releasing peptide: message, protein, and antibody concerns. J Neurosci. 2015;35:648–57. doi: 10.1523/JNEUROSCI.2955-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solway B, Bose SC, Corder G, Donahue RR, Taylor BK. Tonic inhibition of chronic pain by neuropeptide Y. PNAS. 2011;108:7224–9. doi: 10.1073/pnas.1017719108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike RC, Puskár Z, Andrew D, Todd AJ. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci. 2003;18:2433–48. doi: 10.1046/j.1460-9568.2003.02981.x. [DOI] [PubMed] [Google Scholar]
- Spike RC, Todd AJ, Johnston HM. Coexistence of NADPH diaphorase with GABA, glycine, and acetylcholine in rat spinal cord. J Comp Neurol. 1993;335:320–33. doi: 10.1002/cne.903350303. [DOI] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res. 1988;455:187–91. doi: 10.1016/0006-8993(88)90132-1. [DOI] [PubMed] [Google Scholar]
- Sun S, Xu Q, Guo C, Guan Y, Liu Q, Dong X. Leaky Gate Model: Intensity-Dependent Coding of Pain and Itch in the Spinal Cord. Neuron. 2017;93:840–53. doi: 10.1016/j.neuron.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–3. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–34. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa T, MacDermott AB. Glycinergic and GABAergic tonic inhibition fine tune inhibitory control in regionally distinct subpopulations of dorsal horn neurons. J Physiol. 2010;588:2571–87. doi: 10.1113/jphysiol.2010.188292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F, Tao YX, Zhao C, Doré S, Liaw WJ, Raja SN, Johns RA. Differential roles of neuronal and endothelial nitric oxide synthases during carrageenan-induced inflammatory hyperalgesia. Neuroscience. 2004;128:421–30. doi: 10.1016/j.neuroscience.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Todd AJ. An electron microscope study of glycine-like immunoreactivity in laminae I–III of the spinal dorsal horn of the rat. Neuroscience. 1990;39:387–94. doi: 10.1016/0306-4522(90)90275-9. [DOI] [PubMed] [Google Scholar]
- Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC, Price RF, Neilson M. Immunocytochemical evidence that neurotensin is present in glutamatergic neurons in the superficial dorsal horn of the rat. J Neurosci. 1994;14:774–84. doi: 10.1523/JNEUROSCI.14-02-00774.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–36. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtschanoff JG, Rustioni A, Guo A, Hwang SJ. Vanilloid receptor VR1 is both presynaptic and postsynaptic in the superficial laminae of the rat dorsal horn. J Comp Neurol. 2001;436:225–35. [PubMed] [Google Scholar]
- van den Pol AN, Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. J Neurosci. 1988;8:472–92. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennett GJ. Effects of peripheral nerve injuries and tissue inflammation on the levels of neuropeptide Y-like immunoreactivity in rat primary afferent neurons. Brain Res. 1992;598:349–352. doi: 10.1016/0006-8993(92)90206-o. [DOI] [PubMed] [Google Scholar]
- Wende H, Lechner SG, Cheret C, Bourane S, Kolanczyk ME, Pattyn A, Reuter K, Munier FL, Carroll P, Lewin GR, Birchmeier C. The Transcription Factor c-Maf Controls Touch Receptor Development and Function. Science. 2012;335:1373–76. doi: 10.1126/science.1214314. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 2. Plenum; New York: 1991. [Google Scholar]
- Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull. 2007;73:155–202. doi: 10.1016/j.brainresbull.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–8. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Carr PA, Baimbridge KG, Nagy JI. Parvalbumin- and calbindin D28k-immunoreactive neurons in the superficial layers of the spinal cord dorsal horn of rat. Brain Res Bull. 1989;23:493–508. doi: 10.1016/0361-9230(89)90195-0. [DOI] [PubMed] [Google Scholar]
- Yonehara N, Takemura M, Yoshimura M, Iwase K, Seo HG, Taniguchi N, Shigenaga Y. Nitric oxide in the rat spinal cord in Freund’s adjuvant-induced hyperalgesia. Jpn J Pharmacol. 1997;75:327–35. doi: 10.1254/jjp.75.327. [DOI] [PubMed] [Google Scholar]
- Zacharova G, Sojka D, Palecek J. Changes of parvalbumin expression in the spinal cord after peripheral inflammation. Physiol Res. 2009;58:435–42. doi: 10.33549/physiolres.931513. [DOI] [PubMed] [Google Scholar]
- Zarbin MA, Wamsley JK, Kuhar MJ. Glycine receptor: light microscopic autoradiographic localization with [3H]strychnine. J Neurosci. 1981;1:532–47. doi: 10.1523/JNEUROSCI.01-05-00532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bosl MR, Fritschy JM. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol. 2005;482:123–41. doi: 10.1002/cne.20349. [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–19. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. The glutamatergic nature of TRPV1-expressing neurons in the spinal dorsal horn. J Neurochem. 2009;108:305–18. doi: 10.1111/j.1471-4159.2008.05772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]