Abstract
Objective
Achromobacter xylosoxidans (A. xylosoxidans) has been recently reported to have an association with the development of pulmonary mucosa-associated lymphoid tissue (MALT) lymphoma in patients from European countries. However, the prevalence rates for A. xylosoxidans may vary significantly from country to country. To assess this association, the prevalence of A. xylosoxidans was analyzed in Japanese patients with pulmonary B-cell lymphoma.
Methods
DNA samples were obtained from formalin-fixed, paraffin-embedded sections of pulmonary MALT lymphomas (n=52), diffuse large B-cell lymphomas (DLBCLs, n=18), and benign pulmonary lesions (n=19). All samples were histopathologically reviewed by experienced hematopathologists, and the clonality of all MALT lymphoma cases was confirmed by a polymerase chain reaction (PCR)-based IGH rearrangement clonality assay. They were also tested for the API2-MALT1 fusion transcript. The presence of bacterial DNA was assessed with a nested PCR, and DNA sequencing was performed to confirm the PCR specificity.
Results
A. xylosoxidans DNA was detected in 1/52 cases of pulmonary MALT lymphoma, 2/18 cases of DLBCL, and 0/19 cases of benign pulmonary lesions. The prevalence of A. xylosoxidans in pulmonary lymphoma was not significantly higher than in benign lesions.
Conclusion
The present study shows that A. xylosoxidans infection may not be associated with pulmonary B-cell lymphoma in a Japanese case series. Large-scale international studies are needed to clarify the role of A. xylosoxidans in pulmonary lymphoma.
Keywords: achromobacter xylosoxidans, geographical variability, lung, lymphoma
Introduction
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is a distinct B-cell lymphoma that develops in extranodal sites and usually has an indolent clinical course as a localized disease (1). Histologically, MALT lymphoma is classified as a low-grade lymphoma. The tumor cells are small in size, grow in extranodal lymphoid tissue, and infiltrate epithelial tissues, forming lymphoepithelial lesions. It is often difficult to distinguish MALT lymphoma from benign inflammatory conditions. Accumulating clinicopathological studies suggest that the development of MALT lymphoma is often associated with chronic inflammation caused by infections or autoimmune diseases (2). The latter link has been reported in MALT lymphoma associated with Sjögren's syndrome in the salivary gland and with Hashimoto's disease in the thyroid. The best known example of the former link is the association between gastric MALT lymphoma and chronic gastritis caused by Helicobacter pylori (3). The growth and maintenance of gastric MALT lymphoma may be dependent on stimulation by H. pylori antigen; malignant lymphoid cells may respond specifically to H. pylori antigen (4), and the lymphoma is known to regress with eradication of the infectious agent (5). Interestingly, gastric MALT lymphomas possessing the API2-MALT1 fusion, a MALT-lymphoma-specific gene alteration, show resistance to H. pylori eradication therapy (6). Other suspected links include those of B. burgdorferi in cutaneous MALT lymphoma, hepatitis C virus in hepatic MALT lymphoma, and C. psittaci in ocular adnexal MALT lymphoma (7-9).
Recently, a European research group examined pulmonary MALT lymphoma cases and reported that approximately half were positive for Achromobacter xylosoxidans (A. xylosoxidans) DNA, suggesting that A. xylosoxidans has an etiological role in the development of pulmonary MALT lymphoma (10). A. xylosoxidans is a Gram-negative, aerobic, and oxidase-positive bacillus (11). It is an opportunistic pathogen that can cause a wide variety of infections in immunocompromised patients (12-15) but is mainly recovered from the airways of cystic fibrosis patients (16). The association between pulmonary MALT lymphoma and A. xylosoxidans has attracted a considerable attention. However, the association showed potential geographical variability and needs to be confirmed by independent series across different countries (17,18). To our knowledge, the significance of this infectious agent in Asian cases of pulmonary MALT lymphoma has not been studied.
In this study, we examined the presence of A. xylosoxidans DNA in a large cohort of Japanese patients with pulmonary lymphomas, including MALT lymphomas and diffuse large B-cell lymphomas (DLBCLs). We confirmed the diagnosis of MALT lymphoma via a polymerase chain reaction (PCR)-based IGH monoclonality assay. In these lymphoma cases, we also detected the API2-MALT1 fusion transcript, which is specific to MALT lymphoma and more frequent in pulmonary MALT lymphoma than in MALT lymphoma at any other site.
Materials and Methods
Case selection
We retrieved 70 Japanese cases of primary pulmonary lymphoma from the pathology files of Nagoya City University, Okayama University, Kurume University, and Nagoya University. All tumors were reviewed by experienced hematopathologists, and diagnoses of DLBCL (n=18) and MALT lymphoma (n=52) were obtained. Lymphoma cases were staged according to the modified Ann Arbor classification system (19). We also retrieved 19 Japanese cases of non-neoplastic pulmonary tissues for use as reactive controls. These tissues were obtained during surgery for lung cancer and showed either minimal inflammation (n=10) or moderate acute or chronic inflammation due to bronchial obstruction by the tumor mass (n=9). We purchased a lyophilized pellet of A. xylosoxidans (Polysciences, Warrington, USA) to use as a positive control. This study was approved by the Nagoya City University Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
To identify primary pulmonary lymphoid lesions, we used the following pathological and clinical criteria: the lymphoid lesion involves only the lung or the lung and its regional lymph nodes, there is no evidence of dissemination of the tumor for at least three months after the diagnosis is established, no lesions extend into the lung from the mediastinum, and the patient has no history of lymphoma and no clinical or pathological evidence of a tumor outside the thorax at the time of the pulmonary lesion diagnosis (20-22).
DNA extraction and nested polymerase chain reaction
All human materials were fixed in formalin and embedded in paraffin. Paraffin sections were deparaffinized, and DNA was extracted by incubating the tissues in proteinase K digestion buffer overnight and then treating them with RNase A. Amplification of a beta-globin DNA fragment (198 bp) was used to indicate satisfactory preservation of the DNA in the sample. In accordance with the protocol described by Adam et al. (10), we amplified A. xylosoxidans DNA via a nested PCR strategy using genomic DNA extracted from the samples. In the first-round PCR, 16s rDNA primers AX-F1 (5'GCAGGAAAGAAACGTCGCGGGT) and AX-B1 (5'ATTTCACATCTTTCTTTCCG), corresponding to the nucleotide positions 427-448 and 576-595, respectively, were used, and the expected length of the PCR product was 163 base pairs. A 45-cycle amplification by PCR was performed in a 20-μL reaction mixture after an initial denaturation step. To further increase the sensitivity and specificity of the PCR assay, the second-round nested PCR was performed using the following primers: AX-F1 nest (5'AACTGACGGTACCTGCAGAATAA) and AX-B1 nest (5'CACGCTTTACGCCCAGTAAT), corresponding to the nucleotide positions 462-484 and 545-564, respectively. In the nested PCR, 35 cycles of amplification were carried out after the initial denaturation step. The final PCR products (103 base pairs) were analyzed by electrophoresis on 2% agarose gels. As a negative control, reaction mixture samples without template DNA were interspersed between the test samples to detect possible cross-contamination. For all cases, the PCR assay was performed in triplicate, and only cases that showed positive results in at least two of three separate reactions were regarded as positive. To confirm the PCR specificity, all positive cases were subjected to DNA sequencing. A portion of the A. xylosoxidans lyophilized pellet was directly subjected to the digestion buffer for DNA extraction and used as a positive control.
Sensitivity assessment of the nested PCR for A. xylosoxidans DNA
DNA extracted from a lyophilized pellet of A. xylosoxidans was subjected to PCR amplification using an outer primer pair, and the PCR product thus obtained was purified and had its DNA sequence confirmed. The PCR product was then quantified using a spectrophotometer, and its copy numbers were estimated. The PCR product was serially diluted in sterile water, ranging from 106 to 10-2 copies/μL, and the sensitivity of the present nested PCR assay was determined. The lowest copy number showing a visible band was taken as the detection limit. The dilution assay was performed in triplicate.
PCR-based IGH rearrangement clonality assay
A PCR-based IGH clonality assay was performed in all cases of MALT lymphoma (n=52) included in this study. As we previously described (23), a semi-nested strategy was employed for the PCR amplification of the VH genes using the FR2A consensus primer for the framework-2 segment of the VH gene and the LJH and VLJH consensus primers for the JH region. In brief, the DNA template was amplified by first-round PCR using the primers FR2A and LJH, followed by a semi-nested PCR using the primers FR2A and VLJH. The PCR products were visualized on 10% polyacrylamide gels stained with ethidium bromide. When definite bands were obtained, the cases were judged to be positive for clonality.
Multiplex RT-PCR for the API2-MALT1 fusion transcript
All lymphoma cases, including both MALT lymphoma and DLBCL, were subjected to RT-PCR probing for the API2-MALT1 fusion transcript, which was performed in accordance with a method we previously described (24). Briefly, the tissue sections were deparaffinized and incubated in digestion buffer overnight. Total RNA was extracted with concentrated phenol/guanidine isothiocyanate (Trizol LS; Life Technologies, Tokyo, Japan), after proteinase K digestion of the tissues, followed by RNase-free DNase I treatment and a final resuspension in 50 μL of RNase-free water. RNA was subjected to first-round multiplex one-tube RT-PCR, then to second-round nested multiplex PCRs (three times in parallel), as we previously described (21). RNA samples known to possess the API2-MALT1 fusion were used as positive controls. The PCR products were visualized on 10% polyacrylamide gels stained with ethidium bromide, and the presence of fusion transcripts was determined. As an internal RNA quality control, the beta-actin mRNA fragment (190 base pairs) was amplified.
Statistical analyses
The statistical evaluation of the data from two groups was performed using Fischer's exact test. All analyses were two-tailed, and a probability value of p <0.05 was regarded as statistically significant.
Results
Pulmonary lymphoma cases
Characteristics of the B-cell lymphoma patients are summarized in Table 1. For MALT lymphoma cases, only those whose clonality was confirmed with the PCR-based IGH clonality assay were included. The MALT lymphoma group (n=52) consisted of 23 men and 29 women ranging from 31 to 81 years of age (median, 65 years of age). Nineteen (37%) patients were classified as clinical stage III/IV, and 6 (12%) patients presented with B symptoms. Two patients had an autoimmune disease (both with Sjögren's syndrome). Of 52 MALT lymphoma cases, 27 (52%) were positive for the API2-MALT1 fusion transcript. The DLBCL group (n=18) consisted of 11 men and 7 women ranging from 22 to 78 years of age (median, 65 years of age). Eight (44%) patients were classified as clinical stage III/IV, and 4 (22%) patients presented with B symptoms. Three patients had an autoimmune disease (one with Sjögren's syndrome and two with rheumatoid arthritis). In all DLBCL cases, marked nuclear atypia was evident, and the API2-MALT1 fusion transcript was negative. In our B-cell lymphoma cohort, none of the patients suffered from cystic fibrosis.
Table 1.
Clinicopathological Characteristics of the Lymphoma Cases.
| MALT (%) | DLBCL (%) | ||
|---|---|---|---|
| Number of cases | n | 52 | 18 |
| Age (yr) | Median | 65 | 65 |
| >60 | 35 (67) | 10 (56) | |
| <60 | 17 (33) | 8 (44) | |
| Sex | Male | 23 (44) | 11 (61) |
| Female | 29 (56) | 7 (39) | |
| Stage | I/II | 33 (63) | 10 (56) |
| III/IV | 19 (37) | 8 (44) | |
| B symptoms | Absent | 46 (88) | 14 (78) |
| Present | 6 (12) | 4 (22) | |
| Autoimmune disease | Pos | 2* (4) | 3** (17) |
| Neg | 50 (96) | 15 (83) | |
| API2-MALT1 fusion | Pos | 27 (52) | 0 (0) |
| Neg | 25 (48) | 18 (100) | |
| A. xylosoxidans | Pos | 1 (2) | 2 (11) |
| Neg | 51 (98) | 16 (89) | |
| Cystic fibrosis | Pos | 0 (0) | 0 (0) |
| Neg | 52 (100) | 18 (100) |
MALT: mucosa-associated lymphoid tissue lymphoma, DLBCL: diffuse large B-cell lymphoma, *: Sjögren’s syndrome (n=2), **: Sjögren’s syndrome (n=1) and rheumatoid arthritis (n=2).
Detection of A. xylosoxidans DNA
The detection sensitivity of the present PCR assay for A. xylosoxidans DNA was estimated using a serial dilution test. Visible PCR bands were obtained when one or more copies of A. xylosoxidans DNA were subjected to a tube of first-round PCR (Fig. 1A). The detection limit of one DNA copy per tube seemed to be comparable to the limit (4-10 copies per sample) shown by Adam et al. (10).
Figure 1.
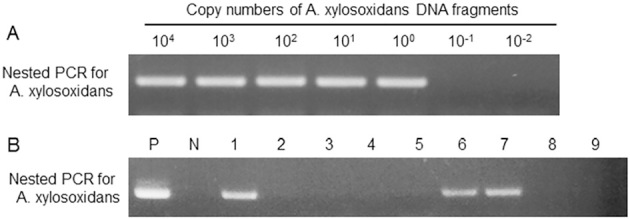
A: The sensitivity of the nested PCR assay for A. xylosoxidans DNA. A total of 104 to 10-2 copy numbers of A. xylosoxidans DNA per tube in the first-round PCR. Visible PCR bands were obtained when one or more copies of A. xylosoxidans DNA, indicating that the detection limit is one DNA copy per tube. B: Nested PCR assay for the detection of A. xylosoxidans in pulmonary lymphomas. P: positive control, N: negative control, #1-5: pulmonary MALT lymphomas, and #6-9: pulmonary DLBCLs. Note that cases #1, 6, and 7 are positive for A. xylosoxidans DNA, and cases #2-5, 8 and 9 are negative for the DNA.
The PCR assay showed that 1/52 (2%) cases of MALT lymphoma and 2/18 (11%) cases of DLBCL were positive for A. xylosoxidans DNA (Table 1 and Fig. 1B). The specificity of the PCR assay for A. xylosoxidans DNA was confirmed by DNA sequencing (Fig. 2). In all three positive cases, the PCR products showed DNA sequences identical to those of A. xylosoxidans (GeneBank accession number AF411019). The clinicopathological features of the three cases positive for A. xylosoxidans DNA are summarized in Table 2. One case had MALT lymphoma, and the other two cases had DLBCL. These three cases were negative for the API2-MALT1 fusion transcript and appeared to be typical pulmonary MALT lymphoma or pulmonary DLBCL, with no particular clinicopathological features noted. In the present pulmonary lymphoma series, no significant association was found between the presence of A. xylosoxidans DNA and the absence of the API2-MALT1 fusion transcript.
Figure 2.
Results of direct sequencing of the nested PCR products positive for A. xylosoxidans.
Table 2.
Pulmonary Lymphoma Cases Positive for A. Xylosoxidans DNA.
| Case | 1 | 2 | 3 |
|---|---|---|---|
| Lymphoma type | MALT | DLBCL | DLBCL |
| Age | 63 | 66 | 54 |
| Sex | F | F | F |
| Clinical stage | I | II | I |
| B symptoms | - | + | + |
| Lymph node status | - | + | - |
| Serum LDH | Normal | Normal | Normal |
| Autoimmune disease | Absent | Absent | Present |
| Cystic fibrosis | Absent | Absent | Absent |
| API2-MALT1 | Negative | Negative | Negative |
MALT: mucosa-associated lymphoid tissue lymphoma, DLBCL: diffuse large B-cell lymphoma, LDH: lactate dehydrogenese
None of the non-neoplastic pulmonary tissues (n=19) was positive for A. xylosoxidans DNA. The prevalence rate of this bacillus in our pulmonary MALT lymphoma or DLBCL cases was not significantly higher than that in non-neoplastic pulmonary tissues.
Discussion
A recent study conducted by Adam et al. showed that the prevalence of A. xylosoxidans in European cases of pulmonary MALT lymphoma was 46% on average and that the rate ranged from 33% to 67% among the European countries analyzed (10). The prevalence rate was significantly higher than that of control cases (18%), suggesting an oncogenetic role for A. xylosoxidans in pulmonary lymphomas. However, the prevalence rates for A. xylosoxidans varied significantly from country to country, and the rates need to be evaluated by independent series across different countries (17,18).
In this study of a Japanese cohort, we examined A. xylosoxidans DNA in 52 cases of pulmonary MALT lymphoma and 18 cases of pulmonary DLBCL and found that the incidence of cases positive for the DNA was very low in MALT lymphomas (1/52, 2%), DLBCL cases (2/11, 11%), and non-neoplastic controls (0/19, 0%). The statistical analysis showed that the prevalence of the DNA of this microorganism in MALT lymphomas and DLBCLs was not significantly higher than that of the control, suggesting that A. xylosoxidans may play a limited role in the lymphomatogenesis of pulmonary lymphomas in Japanese patients. We found that all three pulmonary lymphoma cases positive for A. xylosoxidans DNA were negative for API2-MALT1 fusions. As API2-MALT1 fusion-negative gastric MALT lymphomas are often dependent on underlying chronic inflammation, it would be intriguing to consider an etiological link between A. xylosoxidans infection and the occurrence of pulmonary MALT lymphoma. However, this link was not statistically significant.
It is difficult to explain the difference in prevalence rates of A. xylosoxidans DNA between European and Japanese MALT lymphoma patients. However, we can offer some possible explanations. The first consideration would be the actual diagnosis of pulmonary MALT lymphoma. It is often difficult to make a histopathological distinction between MALT lymphoma and reactive lymphoid hyperplasia. For this distinction, we performed a PCR-based IGH clonality assay and confirmed clonal proliferation in all MALT lymphomas included in this study. Unfortunately, the lymphoma cases presented by Adam et al. (10) had not had their clonal proliferation confirmed. The second point we need to concern ourselves with is the polymorphism of A. xylosoxidans genes. Although the details are still unknown, A. xylosoxidans genomes show substantial sequence diversity (25). Careful sequence interpretation is necessary in the design of PCR primers to ensure that A. xylosoxidans-specific sequences are targeted and non-specific gene amplification eliminated.
The last point to be considered derives from the prevalence of A. xylosoxidans in the general population. A clear regional difference has been reported between Europe and North America with respect to populations positive for antibodies against hepatitis C virus in patients with non-Hodgkin's lymphoma (26-28). A similar regional difference has been discussed in the context of the relationship between Borrelia burgdorferi infection and non-Hodgkin's lymphoma (29). Many studies have examined the association between C. psittaci and ocular adnexal lymphoma, and their results have shown striking variability across geographic regions and even between studies of cases from the same geographic regions (30). A. xylosoxidans is an opportunistic pathogen (12-15) and is mainly recovered from the airways of cystic fibrosis patients (16). Although not statistically significant, the prevalence rate in our series was somewhat higher in DLBCL patients (11.1%) than in MALT lymphoma patients (1.9%), possibly due to the immunocompromised status of the former patients. Cystic fibrosis is extremely rare in Asian countries, and it has been estimated that, in Japan, 1 in 350,000 newborns is diagnosed with this disease (31), while in European countries, 1 in 3,000 newborns is affected (32,33). This difference may partly explain the regional difference in the prevalence of A. xylosoxidans between Japanese and European patients.
In summary, the findings from the present study suggested that A. xylosoxidans infection may not be associated with pulmonary lymphoma in our Japanese case series and that the inflammatory agents that induce the development of pulmonary lymphomas are still unknown. The low prevalence of A. xylosoxidans in Japanese cases of pulmonary B-cell lymphoma suggests geographical differences in the etiology of this entity. International studies are needed to clarify the role of A. xylosoxidans in pulmonary lymphoma.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Isaacson PG, Chott A, Nakamura S, et al. . Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In: World Health Organization Classification of Tumours. Pathology and Genetics: Tumours of Haemopoietic and Lymphoid Tissues. 4th ed. Swerdlow SH, Campo E, Harris NL et al. , Eds. IARC Press, Lyon, 2008: 214-217. [Google Scholar]
- 2.Inagaki H. Mucosa-associated lymphoid tissue lymphoma: molecular pathogenesis and clinicopathological significance. Pathol Int 57: 474-484, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 338: 1175-1176, 1991. [DOI] [PubMed] [Google Scholar]
- 4.Hussell T, Isaacson PG, Crabtree JE, Spencer J. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol 178: 122-127, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Wotherspoon AC, Doglioni C, Diss TC, et al. . Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 342: 575-577, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Inagaki H, Nakamura T, Li C, et al. . Gastric MALT lymphomas are divided into three groups based on responsiveness to Helicobacter pylori eradication and detection of API2-MALT1 fusion. Am J Surg Pathol 28: 1560-1567, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Ferreri AJ, Guidoboni M, Ponzoni M, et al. . Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst 96: 586-594, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Ascoli V, Lo Coco F, Artini M, Levrero M, Martelli M, Negro F. Extranodal lymphomas associated with hepatitis C virus infection. Am J Clin Pathol 109: 600-609, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Cerroni L, Zöchling N, Pütz B, Kerl H. Infection by Borrelia burgdorferi and cutaneous B-cell lymphoma. J Cutan Pathol 24: 457-461, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Adam P, Czapiewski P, Colak S, et al. . Prevalence of Achromobacter xylosoxidans in pulmonary mucosa-associated lymphoid tissue lymphoma in different regions of Europe. Br J Hematol 164: 804-810, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Reverdy ME, Freney J, Fleurette J, et al. . Nosocomial colonization and infection by Achromobacter xylosoxidans. J Clin Microbiol 19: 140-143, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed MS, Nistal C, Jayan R, Kuduvalli M, Anijeet HK. Achromobacter xylosoxidans, an emerging pathogen in catheter-related infection in dialysis population causing prosthetic valve endocarditis: a case report and review of literature. Clin Nephrol 71: 350-354, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Aisenberg G, Rolston KV, Safdar A. Bacteremia caused by Achromobacter and Alcaligenes species in 46 patients with cancer (1989-2003). Cancer 101: 2134-2140, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Tena D, González-Praetorius A, Pérez-Balsalobre M, Sancho O, Bisquert J. Urinary tract infection due to Achromobacter xylosoxidans: report of 9 cases. Scand J Infect Dis 40: 84-87, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Turel O, Kavuncuoglu S, Hosaf E, et al. . Bacteremia due to Achromobacter xylosoxidans in neonates: clinical features and outcome. Braz J Infect Dis 17: 450-454, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Baets F, Schelstraete P, Van Daele S, Haerynck F, Vaneechoutte M. Achromobacter xylosoxidans in cystic fibrosis: prevalence and clinical relevance. J Cyst Fibros 6: 75-78, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Sammassimo S, Pruneri G, Andreola G, et al. . A retrospective international study on primary extranodal marginal zone lymphoma of the lung (BALT lymphoma) on behalf of International Extranodal Lymphoma Study Group (IELSG). Hematol Oncol 34: 177-183, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Ponzoni M, Ferreri AJM. Bacteria associated with marginal zone lymphomas. Best Pract Res Clin Haematol 30: 32-40, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Musshoff K. Clinical staging classification of non-Hodgkin's lymphomas. Strahlentherapie 153: 218-221, 1977. [PubMed] [Google Scholar]
- 20.Salzstein SL. Pulmonary malignant lymphomas and pseudo-lymphomas: classification, therapy, and prognosis. Cancer 16: 928-955, 1963. [DOI] [PubMed] [Google Scholar]
- 21.Koss MN, Hochholzer L, Nichols PW, Wehunt WD, Lazarus AA. Primary non-Hodgkin's lymphoma and pseudolymphoma of lung: A study of 161 patients. Hum Pathol 14: 1024-1038, 1983. [DOI] [PubMed] [Google Scholar]
- 22.Tashiro K, Ohshima K, Suzumiya J, et al. . Clonality of primary lymphoproliferative disorders; using in situ hybridization and polymerase chain reaction for immunoglobulin. Leuk Lymphoma 36: 157-167, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Okabe M, Inagaki H, Ohshima K, et al. . API2-MALT1 fusion defines a distinctive clinicopathologic subtype in pulmonary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Am J Pathol 162: 1113-1122, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inagaki H, Okabe M, Seto M, Nakamura S, Ueda R, Eimoto T. API2-MALT1 fusion transcripts involved in mucosa-associated lymphoid tissue lymphoma: Multiplex RT-PCR detection using formalin-fixed paraffin-embedded specimens. Am J Pathol 158: 699-706, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittmann J, Dreiseikelmann B, Rohde C, Rohde M, Sikorski J. Isolation and characterization of numerous novel phages targeting diverse strains of the ubiquitous and opportunistic pathogen Achromobacter xylosoxidans. PLoS One 9: e86935, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferri C, Caracciolo F, Zignego AL, et al. . Hepatitis C virus infection in patients with non-Hodgkin's lymphoma. Br J Haematol 88: 392-394, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Mazzaro C, Zagonel V, Monfardini S, et al. . Hepatitis C virus and non-Hodgkin's lymphomas. Br J Haematol 94: 544-550, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Waters L, Stebbing J, Mandalia S, et al. . Hepatitis C infection is not associated with systemic HIV-associated non-Hodgkin's lymphoma: a cohort study. Int J Cancer 116: 161-163, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Inagaki H, Kuo TT, Hu S, Okabe M, Eimoto T. Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathologic study of 24 asian cases. Am J Surg Pathol 27: 1061-1069, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Husain A, Roberts D, Pro B, McLaughlin P, Esmaeli B. Meta-analyses of the association between Chlamydia psittaci and ocular adnexal lymphoma and the response of ocular adnexal lymphoma to antibiotics. Cancer 110: 809-815, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Yamashiro Y, Shimizu T, Oguchi S, Shioya T, Nagata S, Ohtsuka Y. The estimated incidence of cystic fibrosis in Japan. J Pediatr Gastroenterol Nutr 24: 544-547, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Assael BM, Castellani C, Ocampo MB, Iansa P, Callegaro A, Valsecchi MG. Epidemiology and survival analysis of cystic fibrosis in an area of intense neonatal screening over 30 years. Am J Epidemiol 156: 397-401, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Balascaková M, Holubová A, Skalická V, et al. . Pilot newborn screening project for cystic fibrosis in the Czech Republic: defining role of the delay in its symptomatic diagnosis and influence of ultrasound-based prenatal diagnosis on the incidence of the disease. J Cyst Fibros 8: 224-227, 2009. [DOI] [PubMed] [Google Scholar]


