Abstract
A 52-year-old man who had been taking omeprazole, a proton pump inhibitor (PPI), for 25 years developed iron deficiency anemia. An evaluation of the entire gastrointestinal tract did not reveal any possible causes of gastrointestinal blood loss. The cause of the iron deficiency was considered to be a reduction in gastrointestinal iron absorption in association with the reduced secretion of gastric acid due to PPI use. This case demonstrates that long-term PPI use for as long as 25 years may cause iron deficiency anemia and should be considered in the differential diagnosis of iron deficiency anemia in long-term PPI users.
Keywords: iron deficiency anemia, proton pump inhibitor, long-term use
Introduction
Proton pump inhibitors (PPIs) are the most potent medications currently available to reduce gastric acid secretion and are widely prescribed in the treatment of peptic ulcer disease and gastroesophageal reflux disease (GERD) (1). Although the long-term use of PPIs is considered safe, there are several reported cases of iron deficiency anemia due to PPI use (2-4) and a community-based case control study reported that the risk of iron deficiency was increased among long-term PPI users (5). However, the association between PPI use and iron deficiency anemia remains controversial and it is not yet known whether the extended use of PPIs is associated with iron deficiency anemia after a long latency period. We herein report the case of a patient who developed iron deficiency anemia after 25 years of continuous PPI use.
Case Report
The patient, a 52-year-old man, had been diagnosed with GERD in the United States in 1986 and had been taking omeprazole continuously since then. He had taken annual health-checks in our institute since 2006; neither anemia nor fecal occult blood had been detected. A health-check in February 2011 showed a hemoglobin (Hb) level of 13.8 g/dL with a mean corpuscular volume (MCV) 82.3 fL. However, the next health-check in December 2011 showed microcytic anemia and he was referred to us for evaluation.
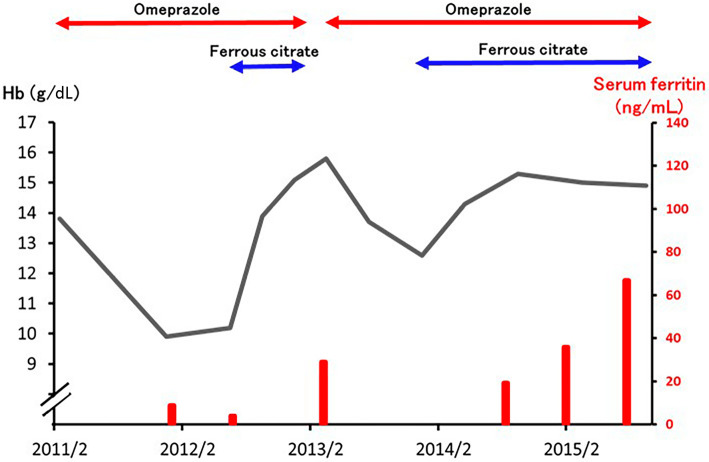
The physical examination revealed no abnormal findings. The laboratory findings were as follows: red blood cell (RBC) count, 4.51×1012/L with 0.82 % reticulocytes; Hb, 9.9 g/dL; hematocrit (Ht), 32.5 %; and MCV, 72.1 fL. His white blood cell count was 3.4×109/L with normal differentials and morphology and his platelet count was 301×109/L. The other findings were as follows: total bilirubin, 0.4 mg/dL; lactate dehydrogenase (LDH), 187 U/L (reference range: 118-223); serum iron, 21 μg/dL; total iron binding capacity, 518 μg/dL; and ferritin, 8.7 ng/mL. Based on these findings, the patient was diagnosed with iron deficiency anemia. Fecal occult blood tests were negative as they had been at the previous health-checks. An upper gastrointestinal (GI) endoscopic examination showed reflux esophagitis with a normal gastric mucosa. Colonoscopy and small intestine capsule endoscopy detected no evidence of bleeding. The patient was negative for anti-Helicobacter pylori IgG antibodies and his serum gastrin level was 222 pg/mL (reference range, 37-172). These findings strongly suggested that the patient's iron deficiency anemia was caused by the decreased absorption of dietary iron, which was associated with the suppression of the gastric acid secretion by omeprazole. As he insisted on taking omeprazole, oral ferrous citrate was started without the interruption of omeprazole (Figure). His anemia improved with an elevation in his MCV and serum ferritin values and ferrous citrate was stopped. Although he temporarily stopped taking omeprazole, the Hb level started to decrease again when he resumed taking omeprazole. His Hb and serum ferritin levels recovered after ferrous citrate was restarted.
Figure.
The clinical course. The polygonal line indicates the hemoglobin (Hb) level and the bars indicate the serum ferritin levels.
Discussion
Iron is essential for the production of RBCs and the iron balance is maintained by the absorption of dietary iron in the GI tract. Various factors and a number of surgical and medical conditions are known to impair iron absorption (6,7). There are two forms of dietary iron, heme iron and non-heme iron, in which iron is complexed as Fe2+ (ferrous iron) and Fe3+ (ferric iron), respectively (7). Dietary non-heme iron is much less well absorbed than heme iron and its absorption is markedly improved in the presence of gastric acid, which assists the absorption by dissociating the iron salts in the food and by increasing their solubility, allowing them to be reduced to ferrous form. However, it is uncertain whether the suppression of gastric acid by the long-term use of PPIs may actually lead to iron deficiency anemia.
No decrease in the body iron stores or iron deficiency was observed in patients with Zollinger-Ellison syndrome who received continuous omeprazole therapy for up to 12.5 years (8). On the contrary, in patients with hereditary hemochromatosis PPIs were shown to reduce the frequency of therapeutic phlebotomy and the absorption of dietary non-heme iron (9). However, these results cannot be applied to general PPI users.
A retrospective cohort study which reported a reduction in the levels of Hb, Ht, and MCV among individuals who took PPIs for more than one year suggests that iron deficiency would develop among long-term PPI users (10). A community-based case control study showed that the use of PPIs for a period of longer than two years was associated with iron deficiency; however, there was no consistent association between the use of PPIs for periods of more than two years and the development of iron deficiency (5). To the best of our knowledge, there have been four reported individual cases of iron deficiency anemia caused by PPI use; however, the entire GI tract, including the small intestine, was studied in only one of them (2-4). The present case is the second reported case in whom the entire GI tract was studied and GI blood loss was ruled out. The present case also revealed that very long-term PPI use, for as long as 25 years, can actually cause iron deficiency anemia after a long latency period. In the cohort and case control studies, the longest duration for which PPIs had been administered were more than one year (9) and longer than 10 years (5), respectively, and the duration of PPI administration was not specified in detail. All four reported cases developed iron deficiency anemia within three years after the start of PPI therapy (2-4). The present case clearly shows that iron deficiency anemia can develop in PPI users, even after 25 years of use. We hypothesize that the gastric acid-independent iron absorption of dietary heme iron and, possibly, the reduced secretion of gastric acid (which was still secreted) might have delayed the development of iron deficiency in the present case. This case also demonstrates that oral ferrous iron is effective in ameliorating iron deficiency without interrupting PPI use in such cases.
Vitamin B12 deficiency is another nutritional cause of anemia that is suggested to be associated with long-term PPI use, especially in elderly individuals (1,11). Although the vitamin B12 level was not evaluated in the present case, we think that it is unlikely that vitamin B12 deficiency contributed to the development of anemia because the MCV was low and because the RBCs never became macrocytic after the amelioration of iron deficiency and due to the absence of other characteristic findings of vitamin B12 deficiency, such as hypersegmented neutrophils and elevated bilirubin and LDH levels, throughout the clinical course. Nonetheless, it is possible that - in addition to iron deficiency - vitamin B12 deficiency will become more prevalent with the prolonged use of PPIs; thus, the vitamin B12 levels of long-term PPI users should be evaluated.
The long-term prescription of PPIs has been approved in Japan since as recently as in 2000. Accordingly, it is apparent that the number of long-term PPI users will increase and that the duration of PPI use will be extended. In addition, new PPIs with stronger gastric acid suppression are being developed and are becoming available. Thus, it is expected that the number of long-term PPI users with iron deficiency anemia will be increased in Japan and other countries worldwide in the near future. It is therefore necessary to limit long-term PPI use and to consider it in the differential diagnosis of iron deficiency anemia in long-term PPI users.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 122: 896-903, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Khatib MA, Rahim O, Kania R, Molloy P. Iron deficiency anemia induced by long-term ingestion of omeprazole. Dig Dis Sci 47: 2596-2597, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Sharma VR, Brannon MA, Carloss EA. Effect of omeprazole on oral iron replacement in patients with iron deficiency anemia. South Med J 97: 887-889, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto R, Matsuda T, Chonan A. Iron-deficiency anemia caused by a proton pump inhibitor. Intern Med 53: 2297-2299, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Lam JR, Schneider JL, Quesenberry CP, Corley DA. Proton pump inhibitor and histamine-2 receptor antagonist and iron deficiency. Gastroenterol 152: 821-829, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Camaschella C. Iron-deficiency anemia. N Engl J Med 372: 1832-1843, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anemia. Lancet 387: 907-916, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Stewart CA, Termanini B, Sutliff VE, et al. Iron absorption in patients with Zollinger-Ellison syndrome treated with long-term gastric acid antisecretory therapy. Aliment Pharmacol Ther 12: 83-98, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson C, Geissler CA, Powell JJ, Bomford A. Proton pump inhibitors suppress absorption of dietary non-haem iron in hereditary haemochromatosis. Gut 56: 1291-1295, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarzynski E, Putterajappa C, Xie Y, Grover M, Laird-Fick H. Association between proton pump inhibitor use and anemia: a retrospective cohort study. Dig Dis Sci 56: 2349-2353, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep 12: 448-457, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]