Abstract
We dedicated this review to discuss Helicobacter pylori as one of the latest identified bacterial pathogens in humans and whether its role is mainly as a pathogen or a commensal. Diseases associated with this bacterium were highly prevalent during the 19th century and gradually have declined. Most diseases associated with H. pylori occurred in individuals older than 40 years of age. However, acquisition of H. pylori occurs mainly in young children inside the family setting. Prevalence and incidence of H. pylori has had a dramatic change in the last part of the 20th century and beginning of the 21th century. In developed countries there is a clear interruption of transmission and the lowest prevalence is observed in children younger than 10 years in these countries. A similar decline is observed but not at the same level in developing countries. Here we discuss the impact of the presence or absence of H. pylori in the health status of humans. We also discuss whether it is necessary or not to establish H. pylori eradication programs on light of the current decline in H. pylori prevalence.
Keywords: H. pylori, pathogen, commensal, microbiome, peptic ulcer, gastric cancer
Introduction
Looking back at the history of medical bacteriology, during the last 20 years of the 19th century and for most of the first half of the 20th century, bacteriologists and infectious disease specialists have isolated and identified most of the pathogenic bacteria affecting humans. Helicobacter pylori probably represented one of the latest infectious bacteria isolated from humans.
The earliest description of spiral bacteria dates back to 1893 in the stomachs of dogs (Steer, 2002). Thirteen years later the same findings were discovered in human stomachs (Bizzozero, 1893; Krienitz, 1906). Despite the high prevalence of the microorganism demonstrated later, its discovery occurred only at the beginning of the 1980’s. There are several reasons that may explain the delay in identifying and characterizing this prevalent microorganism. Bacteriologists and physicians assumed the high acidity present in stomachs did not provide the ideal condition for microbial colonization, but it turned out the human stomach provided the ideal niche for H. pylori. Furthermore, before 1970 gastric biopsies were not routinely obtained for medical analysis and endoscopies were not a common medical practice (Egan and O’Morain, 2007).
The isolation and characterization of H. pylori did not occur until 1983 (Marshall, 2002) and this event produced a major multidisciplinary interest including gastroenterologists, microbiologists, and infectious disease specialists among others. After 1983 it was evident that H. pylori was colonizing more than half of the world population. As a result of this, H. pylori is among the five most studied bacterial organisms in the last 20 years of the 20th century.
To explain this phenomenal global interest in H. pylori, we need to remember that as a result of vaccine use, the introduction and globalization use of antibiotics, and the progress and improvement in public health policies, an impressive and steady decline in morbidity and mortality associated with infectious diseases during most of the second half of the 20th century have been documented (Armstrong et al., 1999). At the time of H. pylori’s discovery, most of the others infectious diseases, have been controlled or prevented and only few infectious diseases such as tuberculosis, influenza, malaria and HIV remain as major unresolved infectious diseases, mainly in developing countries.
Historical Perspective
The publication of the presence of H. pylori in the gastric human stomach generated radical changes in the medical concepts established before 1980 by gastroenterologists and bacteriologists (Warren and Marshall, 1983). The presence of H. pylori in the gastric mucosa was initially associated with an inflammatory process named gastritis, but more relevant from the clinical point of view was the association of this bacterium with peptic ulcer diseases (NIH, 1994). This etiological association was first insinuated by Steer in 1975 and followed by Warren in 1979 and finally confirmed by Warren and Marshall in 1984 (Steer, 2002; Egan and O’Morain, 2007).
Peptic ulcer diseases were very prevalent during the 19th century and the beginning of the 20th century (Susser and Stein, 2002). The only recognized etiologic agent associated with gastric and duodenal ulcers was the production of gastric acid. The conventional concept of “no acid-no ulcer” was very well established in the medical community at the time. After the isolation of H. pylori in 1983, it took several years for health professionals to accept the etiologic role of this microorganism in peptic ulcer diseases.
There are two different theories regarding the role of H. pylori in peptic ulcer diseases. One theory proposes the role of H. pylori as the etiological agent of a modern disease: peptic ulcer disease and as a major risk factor for the development of distal gastric cancer. A series of studies suggested that peptic ulcer disease and gastric cancer are diseases related to modern times. The prevalence and incidence of these diseases rose at the end of the 18th century and peaked by the 19th century. By the beginning of the 20th century there was a steady decline in the number of new and existing cases of peptic ulcer disease and gastric cancer (Susser and Stein, 2002; Sonnenberg et al., 2002). At the same time, the incidence and prevalence of these diseases were not mentioned or associated with a major health human problem before the second half of the 18th century (Sonnenberg and Baron, 2010; Sonnenberg, 2011).
The other theory recognizes that peptic ulcer disease and gastric cancer have been major human health problems since the beginning of human civilization. The evidence to support this idea is based on historic clinical case descriptions that resemble these diseases. The first human peptic ulcer classification dates back to the Western Han Dynasty of a man who died in 167 BCE (Graham, 2014). However, descriptions of this disease were rare and there were no descriptions of gastric cancer.
There is a potential explanation that may account for both conflicting points of view of H. pylori’s etiological role in peptic ulcer disease and as the major risk factor for the development of gastric cancer. Peptic ulcer disease and gastric cancer are indeed very ancient diseases, but because the number of cases related to these diseases was insignificant until the second half of the 18th century they have been suggested to be modern diseases. However, increased life expectancy in new industrialized countries, specifically the UK and US, may explain the escalation of peptic ulcer disease and gastric cancer cases. Several studies indicated major changes in life expectancy, from less than 45 years in the 18th century to more than 60 years by the 19th century in the same countries (Colchero et al., 2016) (Table 1). This jump in longevity among the human population provides sufficient time for H. pylori to express its latent pathogenic capabilities in the development of these major clinical outcomes in colonized individuals. This explanation is in agreement with Graham who suggested that these are not new diseases, but that they have recently become a popular diagnosis caused by increased prevalence due to longer life expectancies and/or change in diagnosis from changing clinical manifestations with time (Graham, 2014). Diagnostic methods for the detection of peptic ulcer disease and gastric cancer were not available prior to the 20th century when medical descriptions of lesions were based exclusively on autopsy.
Table 1.
Life expectancy according to time and location in the world for human populations∗.
| Country | Year | Life expectancy∗∗ | Comment |
|---|---|---|---|
| Liberia | 1820–1843 | 3 years | High mortality |
| Ukraine | 1933 | 18 years | High mortality |
| Trinidad | 1813–1816 | 19 years | High mortality |
| Iceland | 1882 | 20 years | High mortality |
| Sweden | 1751–1859 | 39–43 years | Longitudinal study |
| Sweden | 1900–1909 | 58 years | Longitudinal study |
| Sweden | 1925–1934 | 63 years | Longitudinal study |
| Sweden | 1950–1950 | 75 years | Longitudinal study |
| Sweden | 2000–2009 | 84 years | Longitudinal study |
| England | 1600–1725 | 38 years | Pre-modern era |
| India | 2013 | 68 years | Developing country |
| Russia, China, United States, and Japan | 2013 | 75–83 years | Developed countries |
∗Colchero et al. (2016); ∗∗Defined as the mean age at death.
The Role of H. pylori as a Pathogen
Despite the multiple studies on H. pylori, there are still unanswered questions about the role of this bacterium as a true pathogen or as a commensal organism when colonizing the human stomach.
The role of H. pylori as a true pathogen has been the center of major discussions for many years. Most of the well-known human pathogens such as Vibrio cholera, Streptococcus pyogenes, E. coli among others are capable of producing an acute infectious disease process with nearly identical symptoms in both pediatric and adult populations. In addition, virulence factors have been identified and exact mechanisms involved in disease progression have been determined. In contrast, the infectious process in H. pylori is chronic and only one in ten colonized individuals, most commonly the elderly, develop clinical manifestations years after. Virulence factors have been described in H. pylori, but the presence or absences of these factors are not a critical factor in disease development despite that multiple reports indicated that some virulence factors such as CagA are relevant in disease progression and also confirmed using animal models. (Cover and Peek, 2013; Backert et al., 2016; Cover, 2016). This is very important point because we know that most of the world population is colonized with H. pylori and that colonization occurs at early age. Furthermore, colonization occurs with H. pylori carrying or not carrying those virulence factors, but disease is not expressed until more than 40 years later and the disease only occur in less than 10% of those colonized individuals. This low incidence of disease clearly indicated that H. pylori is not a true pathogen and the virulence factors play a small role in disease outcome.
It is widely accepted that colonization with H. pylori induces an inflammatory response at the gastric mucosa level (O’Morain, 2006). This natural immune reaction does not produce any symptomatology and it is persistent and variable in intensity. Chronic gastritis represents the latent and potential risk for more severe complications, including peptic ulcer disease, gastric MALT–lymphoma and distal gastric cancer that represent the most relevant clinical manifestations of H. pylori pathogenesis (Chey et al., 2017). However, it is important to remember that those severe diseases or complications occur mainly in adults older than 40 years of age and are rare in pediatric populations. Because of this age specific incidence of diseases, it is apparent the colonization with H. pylori requires a long time of establishment and the continuous stimulation of the inflammatory response to produce enough histological deterioration for disease expression. Serious complications of H. pylori colonization are observed in only 10% of the infected individuals, indicating that H. pylori is more of an opportunistic or latent pathogen rather than a true pathogenic bacterium, particularly in adults.
The Role of H. pylori as a Commensal
Because of its low virulence and the fact that disease expression is observed mostly in elderly infected individuals as mentioned above, H. pylori could be considered a commensal organism and only an opportunistic pathogen. H. pylori’s colonization of the human stomach occurs early in life and transmission is mainly in a family setting. In addition, there is evidence that the association between H. pylori and the human host existed for more than 3,000 years based on the detection of this bacterium in the feces from a mummified human (Allison et al., 1999). However, some investigators have suggested that this association is even older. Recent reports of the co-evolution of the gut microbiota in primates (human and non-human) indicate that the colonization of gastric and intestinal bacteria has occurred since the development and evolution of Homo sapiens (Ochman et al., 2010). The sophisticated and specific association of the intestinal microbiota with a specific host clearly indicated a co-evolution between these organisms and their host (Moeller et al., 2016). This new evidence of a specific and unique intestinal microbiota in each primate species support the previous finding of phylogeographic differences that were described in H. pylori strains isolated from different regions of the world and from different ethnic groups (Falush et al., 2003; Olbermann et al., 2010).
Interruption in the Transmission of H. pylori
The exact mechanism of transmission of H. pylori has not been determined despite almost 35 years of research in this area. However, an interesting epidemiological phenomenon has been occurring, a gradual decrease in the prevalence of H. pylori infection and the consequent decline in the incidence and prevalence of peptic ulcer diseases and distal gastric cancer (El-Serag and Sonnenberg, 1998; Sonnenberg, 2011). The decrease in H. pylori prevalence is more apparent in developed countries, but is also expected to occur in developing countries.
The decline in H. pylori prevalence in relation with time was first reported in 1997 (Haruma et al., 1997), and later confirmed in two large population based studies in the United States (Chen and Blaser, 2008) (Figure 1) Similar results of the decline of H. pylori prevalence have been reported in the Netherlands (den Hollander et al., 2015) and more recently in Japan (Urita et al., 2013). As a result of H. pylori’s gradual decline, a series of negative consequences have been described. There has been an alarming increase in asthma (Eder et al., 2006; Holster et al., 2012), a notable increment in body weight that is reaching pandemic proportion of obesity (Blaser, 2012), as well as a potential increase in the susceptibility to diarrheal diseases (Rothenbacher et al., 2000). However, a more serious consequence in the decline of H. pylori is the escalation of esophageal diseases such as, GERD, Barrett’s esophagus and adenocarcinoma of the esophagus (Hunt and Yaghoobi, 2017) (Figure 2), which have been contributing the surge in Gastroenterology consultations. At the same, time the decline of H. pylori prevalence has been directly correlated with a decline in distal gastric cancer and peptic ulcer disease (El-Serag and Sonnenberg, 1998) (Figure 3).
FIGURE 1.
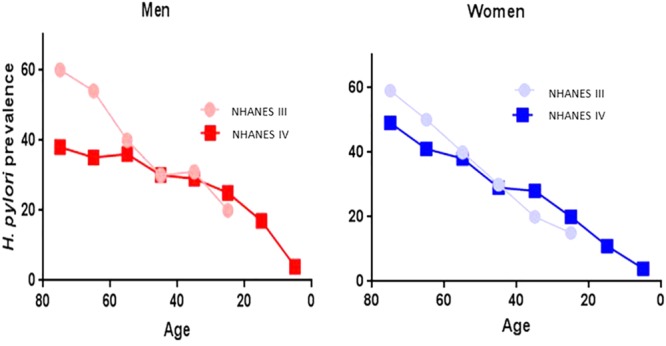
Decline in H. pylori prevalence observed in two population studies NHANES III Phase I (1988–1991) (○) and NHANES IV 1999–2000 (□), according to age and gender (blue-women and red-men) in the US (Chen and Blaser, 2008).
FIGURE 2.
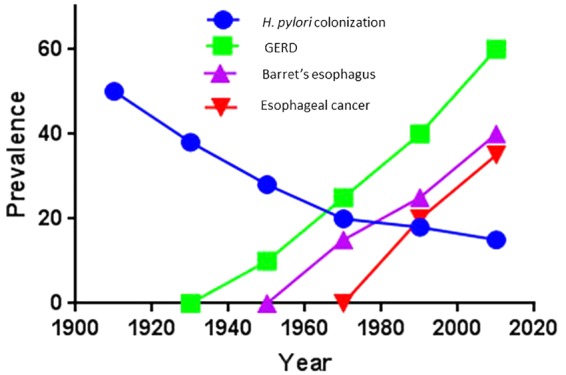
Current trends in esophageal diseases in relation to the gradual decline in H. pylori colonization specific for Caucasian populations in developed countries (Hunt and Yaghoobi, 2017).
FIGURE 3.
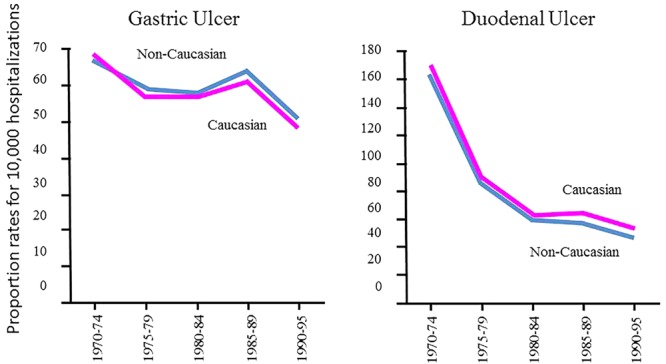
Gradual decline in gastric and duodenal ulcer diseases associated to H. pylori infection in Caucasian and non-Caucasian men using the US Department of Veterans Affairs database from 1970 to 1995, prevalence shown as the proportional rate per 10,000 hospitalizations and the Vital Statistics of the United States obtained by the National Center of Health Statistics (NCHS) (El-Serag and Sonnenberg, 1998).
The increasing prevalence of obesity among human populations is an intriguing worldwide health problem that has been occurring in the last 30 years. This problem has become pandemic with no indication of improvement. In particular, the CDC has clearly documented in the US a dramatic increase in obesity in all 50 states from 1990 to 2015 (Centers for Disease Control and Prevention [CDC], 2017) (Figure 4). Originally, it was proposed that H. pylori prevalence was associated with the increment in obesity. Early reports indicated that successful eradication of H. pylori was associated with an increase in body mass index (BMI) (Azuma et al., 2002). Further studies confirmed the role of H. pylori in the regulation of hormones related to appetite like ghrelin and leptin Francois et al. (2011). However, the fast increment in obesity rates around the world is not comparable with the speed of H. pylori’s decline. The fact that obesity is also affecting populations of low socio-economic level, where H. pylori prevalence is still very high, may indicate that the decrease in H. pylori infections and the current problem of obesity are not related.
FIGURE 4.
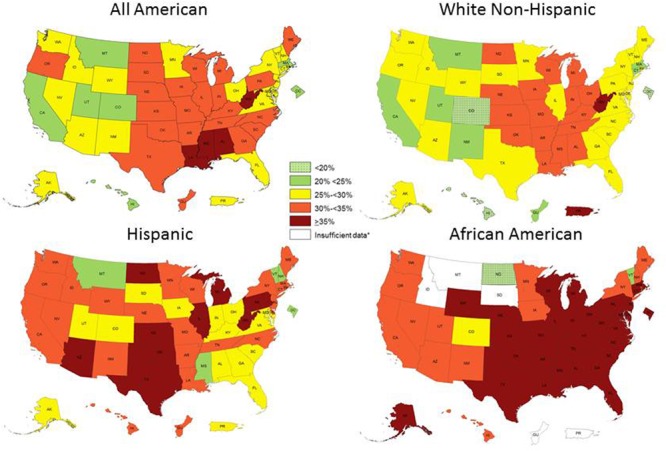
Obesity prevalence according to ethnicity in the US in 2015. Modified from reference 35.
Is It Really Necessary to Eradicate H. pylori?
Based on the steady decline in incidence and prevalence of H. pylori in developed countries as well as in developing countries, one of the most interesting questions in the last 5 years is whether or not is necessary to eradicate H. pylori from those asymptomatic colonized individuals to prevent future clinical complications. Despite the evidence in H. pylori prevalence decline, clinicians, public health personnel and researchers have not reached a consensus. In some countries a national program to eradicate H. pylori has been established. The main argument for the establishment of these programs is that existing populations with a high risk to develop severe diseases associated to H. pylori, like Japan, that justify its implementation due the high prevalence of gastric cancer (Sugano, 2016). However, before applying this massive eradication policy we need to document the interesting changes in H. pylori’s epidemiology and tendencies.
A gradual and consistent decline in the incidence and prevalence of peptic ulcer disease and gastric cancer has been reported in nearly all developed countries (El-Serag and Sonnenberg, 1998; Sonnenberg, 2011) (Figure 3). It is expected that a similar decline will take place in developing countries. The most logical and feasible explanation for the decline in the prevalence of these diseases is the gradual and consistent decline in H. pylori’s prevalence, which has been occurring several years prior to the discovery of this organism and years prior to the use of antibiotics for its eradication.
An important and difficult question to answer is how to explain this steady decline in H. pylori prevalence in modern human populations. It is possible that we have not fully understood the transmission of this bacterium. Clearly the improvement of sanitary conditions, availability of clean drink water, changes in family size and many other factors have contributed to block H. pylori transmissibility in the same way that all those factors have contributed in the dramatic decline of mortality due to infectious diseases during the 20th century and part of 21th century (Armstrong et al., 1999) (Figure 5).
FIGURE 5.
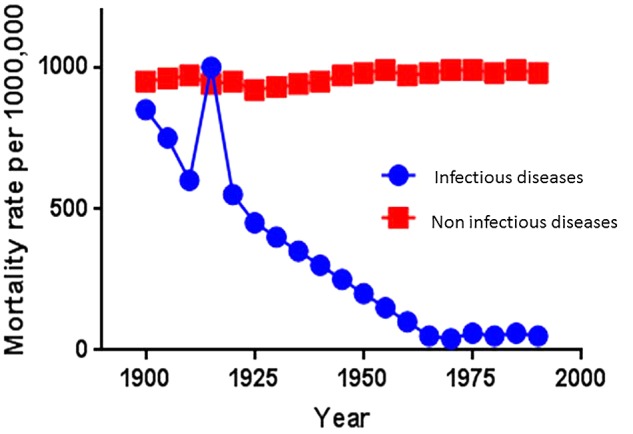
Tendency of human mortality rates associated with infectious diseases and non-infectious diseases in the US during the 20th century (Armstrong et al., 1999).
Conclusion
The sharp and dramatic decline of mortality attributed to infectious diseases during the 20th century is without a doubt one of the major achievements in modern medicine. As a result of the decline of the infectious disease processes, there is now a better opportunity to study chronic human diseases and their potential association with the normal human microbiota.
Most of the systematic studies of the human microbiota were initiated in early 2005, but the role of the human microbiome and its interaction with human immunology and physiology has been known for many years. Its relevance was more clearly define when the hygiene hypothesis was proposed in 1989 (Stiemsma et al., 2015) to explain the sudden increase in certain chronic or immunological diseases that correlated with the notable decline in bacterial, viral and parasitic diseases.
One of the first associations noticed was between the rapid disappearance of H. pylori and the emergence of other diseases. H. pylori has been prevalent in human populations, however, as a result of the dramatic decline of prevalence in many developed countries other diseases have emerged. The gradual decline in H. pylori prevalence, particularly in Caucasian populations in developed countries has been associated as an attributed risk for the development of gastric esophageal disease (GERD), Barrett’s esophagus, and adenocarcinoma of the esophagus (Chen and Blaser, 2007; Rutsgi and El-Serag, 2014). This potential beneficial role of H. pylori infection has also been reported in atopy, allergy, and asthma (Shiotani et al., 2008; Miftahussurur et al., 2017). We do not know if the decline of H. pylori infections is the cause of these emerging diseases or if it is just an indicator of the hygiene hypothesis (Figure 6).
FIGURE 6.
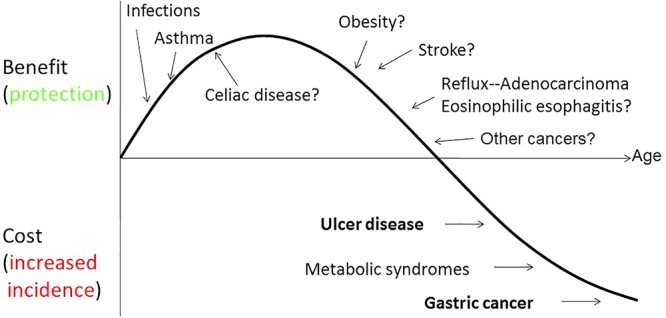
Model of the biphasic nature of H. pylori interaction with humans (Atherton and Blaser, 2009).
The increase of all these diseases coincided not only with the decline of H. pylori’s prevalence but also with improved socio-economic conditions, use of antibiotics as therapeutic agents, improved quality in drinking water and food. In addition to the decrease of H. pylori, humans have witnessed the decline in incidence and prevalence of many of the infectious diseases that were prevalent in the first half of the 20th century (Strachan, 1989).
Finally, one of the major arguments used to support the “test and treat” approach is the high cost of human lives as result of the major clinical consequence of H. pylori infection that is distal gastric cancer. We already mentioned that the incidence and prevalence of gastric cancer is gradually decline in both developed and developing countries. Despite this decrease it is estimated that the number of gastric cancer will be increase in the next decades as result of the increase in the expectancy of life in the human populations around the world. However, it is possible to treat only those individuals with high risk to develop gastric cancer or establish programs for early detection of gastric cancer without perform massive eradication programs of this bacterium that may be important to colonize the gastric stomach of young human beings.
Author Contributions
Both of the authors were involved in the conception, writing, and discussion of this review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Allison M. J., Bergman T., Gerszten E. (1999). Further studies on fecal parasites in antiquity. Am. J. Clin. Pathol. 112 605–609. 10.1093/ajcp/112.5.605 [DOI] [PubMed] [Google Scholar]
- Armstrong G. L., Conn L. A., Pinner R. W. (1999). Trends in infectious disease mortality in the United States during the 20th century. JAMA 281 61–66. 10.1001/jama.281.1.61 [DOI] [PubMed] [Google Scholar]
- Atherton J. C., Blaser M. J. (2009). Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J. Clin. Invest. 119 2475–2487. 10.1172/JCI38605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T., Suto H., Ito Y., Muramatsu A., Ohtani M., Dojo M., et al. (2002). Eradication of Helicobacter pylori infection induces an increase in body mass index. Aliment. Pharmacol. Ther. 16(Suppl. 2) 240–244. 10.1046/j.1365-2036.16.s2.31.x [DOI] [PubMed] [Google Scholar]
- Backert S., Neddermann M., Maubach G., Naumann M. (2016). Pathogenesis of Helicobacter pylori infection. Helicobacter 21(Suppl. 1) 19–25. 10.1111/hel.12335 [DOI] [PubMed] [Google Scholar]
- Bizzozero G. (1893). Sulle ghiandole tubulari del tubo gastroenterico e sui rapporti del loro coll’epitelio di rivestimento della mucosa. Arch. Mikr. Anat. 42:82 10.1007/BF02975307 [DOI] [Google Scholar]
- Blaser M. J. (2012). The Jeremiah Metzger lecture: global warming redux: the disappearing microbiota and epidemic obesity. Trans. Am. Clin. Climatol. Assoc. 123 230–241. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [CDC] (2017). Overweight & Obesity. Available at: http://www.cdc.gov/obesity/data/prevalence-maps.html [Google Scholar]
- Chen Y., Blaser M. J. (2007). Inverse associations of Helicobacter pylori with asthma and allergy. Arch. Intern. Med. 167 821–827. 10.1001/archinte.167.8.821 [DOI] [PubMed] [Google Scholar]
- Chen Y., Blaser M. J. (2008). Helicobacter pylori colonization is inversely associated with childhood asthma. J. Infect. Dis. 198 553–560. 10.1086/590158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey W. D., Leontiadis G. I., Howden C. W., Moss S. F. (2017). ACG clinical guideline: treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 112 212–239. 10.1038/ajg.2016.563 [DOI] [PubMed] [Google Scholar]
- Colchero F., Rau R., Jones O. R., Barthold J. A., Conde D. A., Lenart A., et al. (2016). The emergence of longevous populations. Proc. Natl. Acad. Sci. U.S.A. 113 E7681–E7690. 10.1073/pnas.1612191113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L. (2016). Helicobacter pylori diversity and gastric cancer risk. mBio 7:e01869-15. 10.1128/mBio.01869-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Peek R. M., Jr. (2013). Diet, microbial virulence, and Helicobacter pylori-induced gastric cancer. Gut Microbes 4 482–493. 10.4161/gmic.26262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander W. J., Holster I. L., Van Gilst B., van Vuuren A. J., Jaddoe V. W., Hofman A., et al. (2015). Intergenerational reduction in Helicobacter pylori prevalence is similar between different ethnic groups living in a Western city. Gut 64 1200–1208. 10.1136/gutjnl-2014-307689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder W., Ege M. J., von Mutius E. (2006). The asthma epidemic. N. Engl. J. Med. 355 2226–2235. 10.1056/NEJMra054308 [DOI] [PubMed] [Google Scholar]
- Egan B. J., O’Morain C. A. (2007). A historical perspective of Helicobacter gastroduodenitis and its complications. Best Pract. Res. Clin. Gastroenterol. 21 335–346. [DOI] [PubMed] [Google Scholar]
- El-Serag H. B., Sonnenberg A. (1998). Opposing time trends of peptic ulcer and reflux disease. Gut 43 327–333. 10.1136/gut.43.3.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D., Wirth T., Linz B., Pritchard J. K., Stephens M., Kidd M., et al. (2003). Traces of human migrations in Helicobacter pylori populations. Science 299 1582–1585. 10.1126/science.1080857 [DOI] [PubMed] [Google Scholar]
- Francois F., Roper J., Joseph N., Pei Z., Chhada A., Shak J. R., et al. (2011). The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol. 11:37. 10.1186/1471-230X-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y. (2014). History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J. Gastroenterol. 20:5191. 10.3748/wjg.v20.i18.5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruma K., Okamoto S., Kawaguchi H., Gotoh T., Kamada T., Yoshihara M., et al. (1997). Reduced incidence of Helicobacter pylori infection in young Japanese persons between the 1970s and the 1990s. J. Clin. Gastroenterol. 25 583–586. 10.1097/00004836-199712000-00006 [DOI] [PubMed] [Google Scholar]
- Holster I. L., Vila A. M. J., Caudri D., den Hoed C. M., Perez-Perez G. I., Blaser M. J., et al. (2012). The impact of Helicobacter pylori on atopic disorders in childhood. Helicobacter 17 232–237. 10.1111/j.1523-5378.2012.00934.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. H., Yaghoobi M. (2017). The esophageal and gastric microbiome in health and disease. Gastroenterol. Clin. 46 121–141. 10.1016/j.gtc.2016.09.009 [DOI] [PubMed] [Google Scholar]
- Krienitz W. (1906). Ueber das auftreten von spirochäten verschiedener form im mageninhalt bei carcinoma ventriculi. Dtsch. Med. Wochenschr. 32 872–872. 10.1055/s-0028-1142055 [DOI] [Google Scholar]
- Marshall B. J. (ed.) (2002). “The discovery that Helicobacter pylori, a spiral bacterium, caused peptic ulcer disease,” in Helicobacter Pioneers: First Hand Accounts from the Scientists Who Discovered Helicobacters (Melbourne, VIC: Blackwell; ) 165–202. [Google Scholar]
- Miftahussurur M., Nusi I. A., Graham D. Y., Yamaoka Y. (2017). Helicobacter, hygiene, atopy, and asthma. Front. Microbiol. 8:1034 10.3389/fmicb.2017.01034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller A. H., Caro-Quintero A., Mjungu D., Georgiev A. V., Lonsdorf E. V., Muller M. N., et al. (2016). Cospeciation of gut microbiota with hominids. Science 353 380–382. 10.1126/science.aaf3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH (1994). Consensus conference Helicobacter pylori in peptic ulcer disease. JAMA 272 65–69. 10.1001/jama.1994.03520010077036 [DOI] [PubMed] [Google Scholar]
- Ochman H., Worobey M., Kuo C. H., Ndjango J. B. N., Peeters M., Hahn B. H., et al. (2010). Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biol. 8:e1000546. 10.1371/journal.pbio.1000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbermann P., Josenhans C., Moodley Y., Uhr M., Stamer C., Vauterin M., et al. (2010). A global overview of the genetic and functional diversity in the Helicobacter pylori cag pathogenicity island. PLoS Genet. 6:e1001069. 10.1371/journal.pgen.1001069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Morain C. A. (2006). Long Term Complications of Helicobacter pylori Infection. Berlin: International Congress Centre. [Google Scholar]
- Rothenbacher D., Blaser M. J., Bode G., Brenner H. (2000). Inverse relationship between gastric colonization of Helicobacter pylori and diarrheal illnesses in children: results of a population-based cross-sectional study. J. Infect. Dis. 182 1446–1449. 10.1086/315887 [DOI] [PubMed] [Google Scholar]
- Rutsgi A. K., El-Serag H. B. (2014). Esophageal carcinoma. N. Engl. J. Med. 371 2499–2509. 10.1056/NEJMra1314530 [DOI] [PubMed] [Google Scholar]
- Shiotani A., Miyanishi T., Kamada T., Haruma K. (2008). Helicobacter pylori infection and allergic diseases: epidemiology study in Japanese university students. J. Gastroenterol. Hepatol. 23(7 Pt 2) e29–e33. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A. (2011). Time trends of mortality from gastric cancer in Europe. Dig. Dis. Sci. 56 1112–1118. 10.1007/s10620-010-1553-2 [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Baron J. H. (2010). Rising trends of gastric cancer and peptic ulcer in the 19th century. Aliment. Pharmacol. Ther. 32 901–907. 10.1111/j.1365-2036.2010.04413.x [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Cucino C., Bauerfeind P. (2002). Commentary: the unresolved mystery of birth-cohort phenomena in gastroenterology. Int. J. Epidemiol. 31 23–26. 10.1093/ije/31.1.23 [DOI] [PubMed] [Google Scholar]
- Steer H. W. (2002). “The discovery of Helicobacter pylori in England in the 1970’s,” in Helicobacter Pioneers: Firsthand Accounts from the Scientists Who Discovered Helicobacters, 1892-1982 ed. Marshall B. (Oxford: Blackwell Publishing; ) 119–121. [Google Scholar]
- Stiemsma L. T., Reynolds L. A., Turvey S. E., Finlay B. B. (2015). The hygiene hypothesis: current perspectives and future therapies. Immunotargets Ther. 4 143–157. 10.2147/ITT.S61528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan D. P. (1989). Hay fever, hygiene, and household size. Br. Med. J. 299 1259–1260. 10.1136/bmj.299.6710.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano K. (2016). Strategies for prevention of gastric cancer: progress from mass eradication trials. Dig. Dis. 34 500–504. 10.1159/000445229 [DOI] [PubMed] [Google Scholar]
- Susser M., Stein Z. (2002). Civilization and peptic ulcer. Int. J. Epidemiol. 31 13–17. 10.1093/ije/31.1.13 [DOI] [PubMed] [Google Scholar]
- Urita Y., Watanabe T., Kawagoe N., Takemoto I., Tanaka H., Kijima S., et al. (2013). Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J. Paediatr. Child Health 49 394–398. 10.1111/jpc.12191 [DOI] [PubMed] [Google Scholar]
- Warren J. R., Marshall B. (1983). Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1 1273–1275. [PubMed] [Google Scholar]


