Abstract
Background
We hypothesized that upregulation of inducible cyclooxygenase 2 (COX-2) contributes to altered coronary arteriolar reactivity early after cardioplegic arrest and cardiopulmonary bypass (CP/CPB) in patients with diabetes mellitus who are undergoing cardiac surgery.
Methods
The right atrial tissue samples of non-diabetes (ND), controlled diabetes (CDM), and uncontrolled diabetes (UDM) patients undergoing cardiac surgery were harvested before and after CP/CPB. Coronary arterioles (80 to 150 mm) were dissected from the harvested atrial tissue samples, cannulated, and pressurized. The changes in diameter were measured with video microscopy. The protein expression and localization of COX-1 and COX-2 were assayed by Western blot and immunohistochemistry.
Results
In the diabetes arterioles, bradykinin-induced relaxation response was inhibited by the selective COX-2 inhibitor NS398 at baseline (p < 0.05). This effect was more pronounced in UDM arterioles than CDM (p < 0.05). After CP/CPB, bradykinin-induced responses in all groups were inhibited by NS398, but this effect was more pronounced in the UDM patients (p < 0.05). The intensities of COX-2 staining of coronary arterioles and COX-2 protein levels in myocardium were higher in diabetes than nondiabetes at baseline (p < 0.05). The post-CP/CPB protein levels of the inducible COX-2 were significantly increased compared with pre-CP/CPB values in all groups (p < 0.05), whereas this increase was higher with diabetes than with ND (p < 0.05). Furthermore, these effects were more profound in UDM than CDM (p < 0.05).
Conclusions
Diabetes and CP/CPB are associated with upregulation in COX-2 expression in human coronary vasculature. Upregulation of COX-2 expression may contribute to bradykinin-induced coronary arteriolar relaxation in diabetic patients undergoing cardiac surgery.
Diabetes mellitus is associated with increased morbidity and mortality in patients after cardiac surgery [1, 2]. That is especially true after heart operations involving ischemic cardioplegic arrest (CP) and cardiopulmonary bypass (CPB) [1–6]. Recently, we have demonstrated that diabetes is associated with increased vascular permeability [4, 6], decreased microvascular function [7–9], increased tissue edema, and prolonged postoperative hospital stay [4, 6].
We have found previously that CP/CPB is associated with cyclooxygenase (COX)-2 overexpression in patients undergoing cardiac surgery [10, 11]. Serotonin-induced constriction partially occurs through activation of COX-2 in patients after CP/CPB and cardiac surgery [10, 11]. Interestingly, a recent study reported that diabetes is associated with an increase in COX-2 expression and prostaglandin-mediated bradykinin-induced dilation in human coronary arterioles [12]. However, it is not known whether CP/CPB may differently affect COX-2 expression and microvascular reactivity between diabetes patients and nondiabetes patients or between patients with well-controlled diabetes (CDM) and uncontrolled diabetes (UDM) after cardiac surgery. Therefore, we hypothesized that diabetes and cardiac surgery may significantly affect COX-2 expression early after CP/CPB. We further hypothesized that COX-2 overexpression induced by diabetes may contribute to the altered coronary arteriolar reactivity early after CP/CPB in patients with diabetes.
Material and Methods
Human Subjects and Tissue Harvesting
Samples of right atrial appendage were harvested from patients undergoing cardiac surgery before and after exposure of the heart to blood CP and short-term reperfusion under conditions of CPB [9, 13–16]. The first sample of atrial appendage was harvested before CP/CPB. The second sample of atrial appendage was harvested after CP/CPB. The standard pump-prime solution (total 1,700 mL) was combined 1,200 mL Plasma-Lyte A (Baxter Healthcare, Deerfield, IL) and 10 mL heparin (1,000 U/mL), 50 mL NaHCO3 (8.4%), 250 mL albumin (5%), 60 mL mannitol (20%), and 10 mL lidocaine (2%). The final ionic concentrations (mmol/L) were 140 Na+, 4.6 K+, 2.8 Mg, 76.6 Cl−, 21 acetate 10 HCO3−, and 18 gluconate. Cardiopulmonary bypass was established with a membrane oxygenator (Medtronic, Minneapolis, MN) with target flow rates of 2.4 to 2.8 L · min−1 · m−2 for all patients.
Cold blood CP (4° to 8°C) consisted of a 4:1 mixture of oxygenated blood with a hyperkalemic crystalloid CP solution (CAPS, Lanham, MD). An initial 600 to 1,000 mL (introduction) of cold-blood (4° to 8°C) hyperkalemic (K+ 25 mmol/L) CP solution was delivered antegrade into the aortic root, followed by 200 to 500 mL (maintenance) of cold, low-potassium CP solution (K+ 12.5 mmol/L) every 15 to 20 minutes. Sections of atrial samples were immediately frozen in liquid nitrogen (immunoblotting), fixed in 10% formalin for 24 hours, followed by paraffinization and sectioning into 5-μm slices (immunofluorescent staining), or placed in cold (5° to 10°C) Krebs-Henseleit buffer (microvascular studies). All procedures were approved by the Institutional Review Board of Rhode Island Hospital, Alpert Medical School of Brown University, and informed consent was obtained from all enrolled patients.
The patients were then grouped as follows: (1) patients with a normal hemoglobin A1c and no history or treatment for diabetes (ND group); (2) patients with a history of diabetes with a hemoglobin A1c of 7 or less were considered controlled (CDM group); and (3) patients with a hemoglobin A1c of 8.5 or higher were considered poorly controlled (UDM group).
Micovessel Reactivity
Coronary arterioles (80 to 150 μm in internal diameter) were dissected from harvested right atrial appendage tissue-samples before and after CP/CPB. Microvessel studies were performed by in vitro organ bath video microscopy, as described previously [7–9, 13–16]. After a 60-minute stabilization period in the organ chamber, the microvessels were pre-constricted with the thromboxane A2 analog U46619 25% to 40% of the baseline diameter. The dose-dependent relaxation was measured in response to the application of the vasodilators endothelium-dependent bradykinin, 10−10 to 10−5 M. Six vessels each from pre- and post-CP/CPB were pretreated with the selective COX-2 inhibitor NS398, 10−5M, before the perfusion with bradykinin.
Immunoblot
Protein purification of atrial tissue samples and the method for Western blot have been previously described in detail [6, 9]. Membranes were incubated with individual rabbit polyclonal primary antibodies COX-1, COX-2, and nicotinamide adenine dinucleotide phosphate oxidase (NOX)-1 (ABCAM, Cambridge, MA). The membranes were then incubated with horseradish peroxidase-conjugated secondary antiimmunoglobulin. Peroxidase activity was visualized with enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA), and the images were captured with a digital camera system (G Box; Syngene, Cambridge, UK). The imaging bands were quantified with densitometry using ImageJ software (National Institute of Health, Bethesda, MD).
Immunoperoxidase Staining of COX-1 and COX-2
Atrial tissue sections from the three groups were deparaffinized in xylene, rehydrated in graded ethanol and phosphate-buffered saline (PBS) solution. The sections were incubated in 3% H2O2 for 20 minutes. The section was then incubated with anti-COX-1 and COX-2 antibody, 1 μg/mL, for 60 minutes at room temperature and washed with PBS. The sections were then incubated with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA). The chromogen 3,3°-diaminobenzidine was then used. Tissue was visualized using a Nikon E800 microscope system (Nikon, Melville, NY). Six image photos per slide were taken at the same magnification resolution (×20) for optical density (intensity) analysis (Spot RT3; Diagnostic Instruments, Sterling Heights, MI) [6, 9, 15, 16].
Immunofluorescent Staining of COX-2, NOX-2, and NOX-4
Atrial tissue sections were deparaffinized in xylene, rehydrated in graded ethanol and PBS, and antigen-unmasked with sodium citrate (10 mmol/L, pH = 6.0) followed by PBS wash and blocking with 2% bovine serum albumin in PBS at room temperature for 2 hours [6–9, 11, 12]. After the PBS wash, overnight incubation with anti-COX-2, NOX-2, and NOX-4 (ABCAM) was performed at 4°C. Antimouse, smooth muscle actin (Sigma-Aldrich, St Louis, MO) was used to detect microvascular smooth muscle. Sections were then washed in PBS and incubated with the appropriate Alexa Fluor secondary antibody (Thermo Fisher Scientific) and mounted using fluorescent mounting medium (Vector Laboratories). Tissue was visualized using a Nikon E800 epifluorescent microscope system (Nikon). Six image photos per slide were taken at the same magnification resolution (×20 Plan Fluor [Nikon] objective) for optical density analysis (Spot RT3; Diagnostic Instruments).
Protein Oxidation
Total protein oxidation in atrial tissue samples (n = 6 per group) was measured according to the manufacturer’s recommendation (OxyBlot; Chemicon International, Temecula, CA) [7].
Chemicals
Bradykinin, U46619, and NS398 were obtained from Sigma-Aldrich.
Data Analysis
Data are presented as mean and standard error of the mean. Microvessel responses are expressed as percent relaxation of the preconstricted diameter. Microvascular data were analyzed using two-way repeated-measures analysis of variance with a post hoc Bonferroni test. Clinical and Western blot data were analyzed by Kruskal-Wallis tests, followed with the Dunn multiple comparison test (Graph-Pad Software, San Diego, CA). Categoric data of patient characteristics were analyzed with Fisher’s exact test. All p values less than 0.05 were considered significant.
Results
Patient Characteristics
Patient characteristics are listed in Table 1. All patients (n = 12 per group) with preoperative hypertension were receiving antihypertensive medication (aspirin and beta-blocker, or calcium-channel blocker, or statins, or angiotensin-converting enzyme inhibitors). The preoperative hemoglobin A1C levels were 5.5 ± 0.13 in the ND patients, 6.7 ± 0.2 in the CDM patients, and 9.9 ± 0.33 in the UDM patients.
Table 1.
Patient Characteristics
| Patient Characteristics | ND | CDM | UDM | p Value |
|---|---|---|---|---|
| Age, yearsa | 69 ± 2 | 63 ± 5 | 66 ± 3 | 0.3 |
| Hemoglobin A1c, %a | 5.5 ± 0.13 | 6.7 ± 0.20 | 9.9 ± 0.33 | 0.0001 |
| Male/female | 3/9 | 2/10 | 2/10 | 0.96 |
| Blood glucose before CP/CPB, mg/dLa | 112 ± 4 | 130 ± 5 | 209 ± 9b | 0.0001 |
| Blood glucose during CP/CPB, mg/dLa | 138 ± 6 | 168 ± 7 | 190 ± 6b | 0.0001 |
| Obesity, BMI >30 kg/m2 | 4 | 5 | 6 | 0.59 |
| Hypertension | 9 | 10 | 10 | 0.86 |
| Atrial fibrillation | 0 | 0 | 0 | 1.0 |
| Hypercholestesterolemia | 6 | 7 | 7 | 0.82 |
| CPB duration, minutesa | 115 ± 4 | 108 ± 6 | 117 ± 4 | 0.64 |
| Cross-clamp time, minutesa | 95 ± 4 | 91 ± 7 | 98 ± 3 | 0.47 |
| CABG only | 8 | 7 | 8 | 1.0 |
| CABG plus AVR or MVR | 4 | 5 | 4 | 0.9 |
| Number of grafts | 2.9 ± 0.8 | 3.0 ± 0.9 | 3.2 ± 0.8 | 0.60 |
| Preoperative insulin | 0 | 3 | 12b | 0.003 |
| Intraoperative insulin | 0 | 10b | 12b | 0.003 |
| Oral antidiabetic agents | 0 | 9b | 12b | 0.005 |
| Aspirin | 10 | 12 | 12 | 0.78 |
Values are mean SD or n.
AVR = aortic valve replacement; BMI = body mass index; CABG = coronary artery bypass grafting; CDM = controlled diabetes mellitus; CP = cardioplegic arrest; CPB = cardiopulmonary bypass; MVR = mitral valve replacement; ND = no diabetes; UDM = uncontrolled diabetes mellitus.
Microvessel Characteristics
There was no significant difference in the baseline diameters of the microvessels among the three groups (n = 12 per group) in two conditions: ND pre-CP/CPB 118 ± 5 μm; ND post-CP/CPB 121 ± 4.5 μm; CDM pre-CP/CPB 116 ± 6.5 μm; CDM post-CP/CPB 109 ± 5 μm; UDM pre-CP/CPB 119 ± 3.5 μm; and UDM post-CP/CPB 115 ± 4.9 μm; p > 0.05). The degrees of precontraction by the thromboxane A2 analog U46619 (10−6 to 10−5M) were 34% ± 3% for ND pre-CP/CPB, 32% ± 4% for ND post-CP/CPB, 35% ± 4% for CDM-pre-CPB, 31% ± 2% for CDM post-CP/CPB, 30% ± 4% for pre-CP/CPB, and 33% ± 3% UDM for UDM post-CP/CPB group (p > 005).
Microvascular Reactivity
There was no significant difference in relaxation response to the endothelium-dependent vasodilator bradykinin between the UDM and ND or CDM patients at baseline (pre-CP/CPB; Fig 1A). After CP/CPB, the arteriolar relaxation response to bradykinin was significantly reduced compared with that of pre-CP/CPB in the three groups (Fig 1B–D). However, this effect was more pronounced in the UDM group than in the ND or CDM groups (Fig 1B). At baseline (pre-CP/CPB), the selective COX-2 inhibitor NS398 significantly suppressed the response to bradykinin in diabetes vessels, but not in the ND vessels (Fig 2A, C, E). This effect was more pronounced for UDM versus CDM (p < 0.05). In contrast, the post-CP/CPB responses to bradykinin were significantly inhibited by the selective COX-2 inhibitor NS398 in all three groups (Fig 2B, D, F); but this inhibition was more pronounced for UDM than for ND or CDM (p < 0.05).
Fig 1.
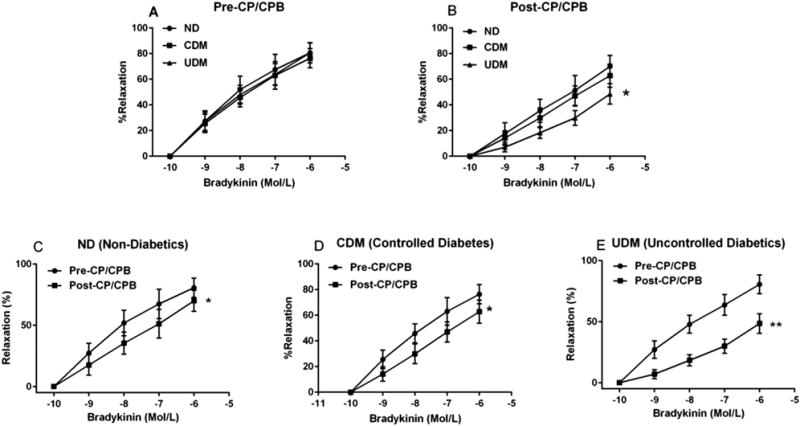
(A) The relaxation responses of nondiabetes mellitus (ND [circles]), controlled diabetes (CDM [squares]), and uncontrolled diabetes (UDM [triangles]) coronary arterioles to the dose-dependent, endothelium-dependent vasodilator bradykinin at baseline, before cardioplegia arrest and cardiopulmonary bypass (pre-CP/CPB). (B) The relaxation response of ND, CDM, and UDM coronary arterioles to bradykinin after CP/CPB (post-CP/CPB). (C) The relaxation response of ND coronary arterioles to bradykinin between pre-CP/CPB (circles) and post-CP/CPB (squares). (D) The relaxation response of CDM coronary arterioles to bradykinin between pre-CP/CPB and post-CP/CPB. (E) The relaxation response of UDM coronary arterioles to bradykinin between pre-CP/CPB and post-CP/CPB. Data are mean ± standard error of the mean; n = 12 per group. *p < 0.05 versus ND or CDM or pre-CP/CPB. **p < 0.001 versus pre-CP/CPB.
Fig 2.
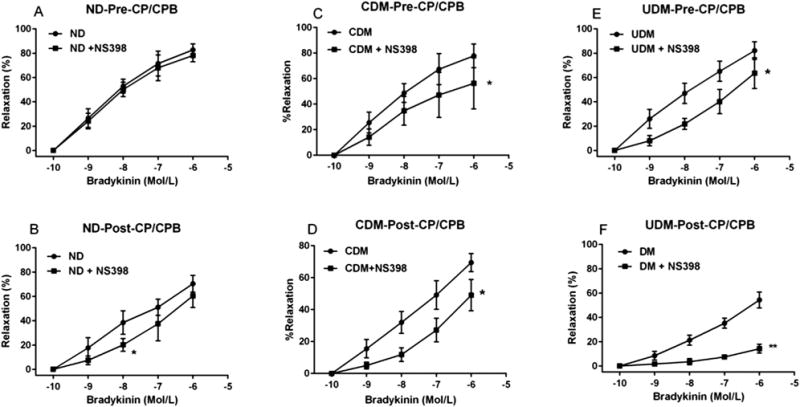
Relaxation response of arterioles to bradykinin before cardioplegia arrest and cardiopulmonary bypass (pre-CP/CPB) with (squares) and without (circles) the selective cyclooxygenase (COX)-2 inhibitor NS398 from (A) no diabetes mellitus (ND) patients, (C) controlled diabetes (CDM) patients, and (E) uncontrolled diabetes (UDM) patients. The relaxation response of post-CP/CPB arterioles to bradykinin with (squares) and without (circles) the selective COX-2 inhibitor NS398 from (B) ND, (D) CDM, and (F) UDM patients. Data are mean ± standard error of the mean; n = 6 per group. *p < 0.05 versus bradykinin alone. **p < 0.001 versus bradykinin alone.
Effect of Diabetes on COX-1, COX-2, and NOX-1 Expression
There were no significant differences in COX-1 expression between diabetic and nondiabetic hearts or between pre-CP/CPB and post-CP/CPB (Fig 3A, B). There was significant increase in COX-2 (Fig 3C, D) and NOX-1(Fig 3E, F) protein levels in the UDM myocardium as compared with ND or CDM at baseline (pre-CP/CPB, p < 0.05). The post-CP/CPB protein levels of the inducible COX-2 (Fig 3D) and NOX-1 (Fig 3F) were significantly increased compared with pre-CP/CPB values in the three groups (p < 0.05), whereas this increase was higher in UDM patients than in ND or CDM patients (p < 0.05).
Fig 3.
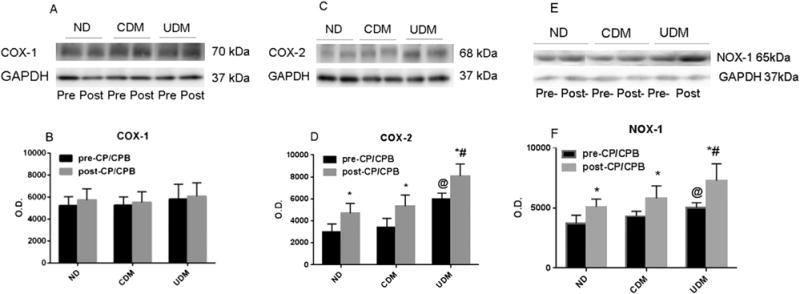
(A, C, E) Representative immunoblots of human atrial tissue samples from no diabetes mellitus (ND) patients, controlled diabetes (CDM) patients, and uncontrolled diabetes (UDM) patients. Lanes 1 to 6 loaded with 40 μg protein were developed for cyclooxygenase (COX)-1, COX-2, and nicotinamide adenine dinucleotide phosphate oxidase (NOX)-1 polypeptides. (GAPDH = glyceraldehyde 3-phosphate dehydrogenase.) (B) Densitometric evaluation of immunoblot band intensity shows no significant differences in the levels of COX-1 among three groups, or between pre-cardioplegia arrest and cardiopulmonary bypass (pre-CP/CPB [black bars]) and post-CP/CPB (gray bars). (D) Densitometric evaluation of immunoblot band intensity shows significant differences in the levels of COX-2 between UDM and ND or CDM groups, and between pre- and post-CP/CPB. (F) Densitometric evaluation of immunoblot band intensity shows significant differences in the levels of NOX-1 between UDM and ND or CDM groups, or between before and after CP/CPB. Data are mean ± standard error of the mean; n = 6 per group. *p < 0.05 versus pre-CP/CPB. @p < 0.05 versus ND pre-CP/CPB or CDM pre-CP/CPB. #p < 0.05 versus ND post-CP/CPB or CDM= post-CP/CPB. (O.D. = optical density.)
Immunolocalization of COX-1, COX-2, NOX-2, and NOX-4
Immunoperoxidase staining of COX-1 in human atrial tissue sections displayed strong signals in the myocardium from diabetes and nondiabetes patients (brown, Fig 4A). There were no significant differences in COX-1 distribution between diabetes and nondiabetes, or between pre- and post-CP/CPB (Fig 4A, C). In contrast, the intensities of COX-2 were higher in diabetic myocardium than in ND myocardium at baseline (pre-CP/CPB; p < 0.05; Fig 4B, D). The intensities of COX-2 were much stronger in UDM myocardium than in CDM myocardium (p < 0.05). After CP/CPB, the intensities of COX-2 were further increased in the three groups compared with pre-CP/CPB, but this effect was more pronounced in UDM patients that in ND or CDM patients (p < 0.05; Fig 4B, D). Immunofluorescent staining of coronary microvessels from patients with diabetes and ND patients displayed a strong signal in the coronary microvessels (red [endothelium] or orange [smooth muscle]) for COX-2 (Fig 5E). COX-2 was stained mainly in endothelium and also in microvascular smooth muscles. There were significant increases in COX-2 intensities in the diabetic vessels compared with nondiabetic vessels at baseline (pre-CP/CPB, p < 0.05; Fig 4E, F). This increase in COX-2 intensities was greater in UDM vessels than in CDM vessels (p < 0.05). Furthermore, the distribution of microvascular COX-2 was significantly increased after CPB/CPB as compared with the values before CP/CPB among the three groups, but this increase was more pronounced in UDM vessels than in ND vessels (p < 0.05; Fig 4E, F). The NOX-2 and NOX-4 were localized in the microvascular endothelial cells (red) and smooth muscles (orange; Fig 5). There were significant increases in NOX-2 and NOX-4 intensities in the UDM vessels compared with the ND vessels before and after CP/CPB (p < 0.05; Fig 5E, F).
Fig 4.
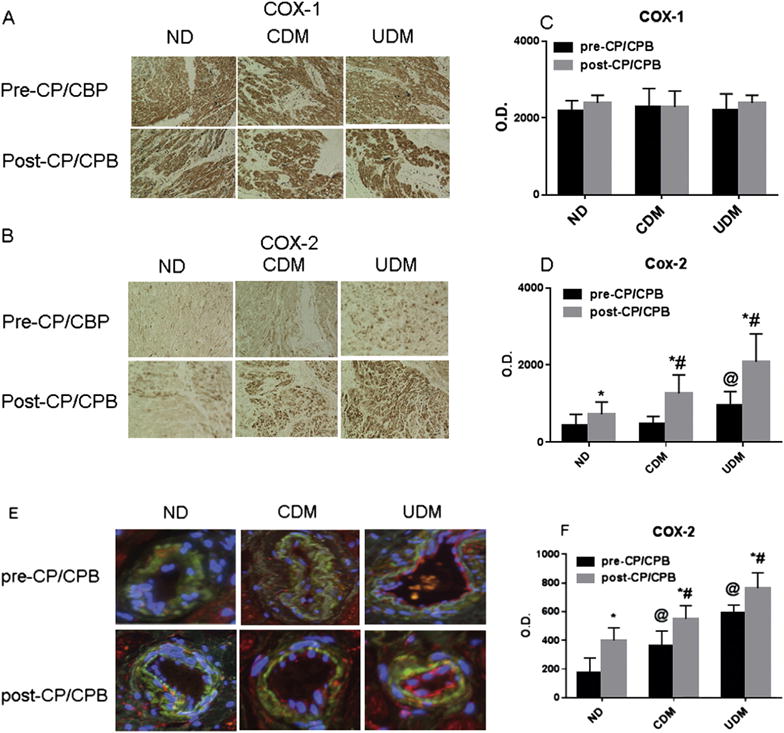
(A, B) Immunohistochemical image of COX-1 and COX-2 in paraffin-embedded human atrial tissues from no diabetes mellitus (ND) and diabetes mellitus (DM) patients. (CP/CPB = cardioplegia arrest and cardiopulmonary bypass.) (C) Densitometric analysis of signal intensities shows no significant differences in cyclooxygenase (COX)-1 distribution among the three groups or between pre-CP/ CPB (black bars) and post-CP/ CPB (gray bars). (D) Densitometric analysis of signal intensities shows differences in COX-2 distribution among the three groups or between pre- and post-CP/CPB. (E) Immunofluorescence staining of COX-2. Vessels were costained for cell nuclei (blue), smooth muscle α-actin (green), and COX-2 (red in endothelium and orange in smooth muscle). (F) Densitometric analysis of immunofluorescence intensities shows significant difference in COX-2 distribution in the coronary vessels among the three groups or between pre-CP/CPB (black bars) and post-CP/CPB (gray bars). Data are mean ± standard error of the mean; n = 6 per group. *p < 0.05 versus pre-CP/CPB. @p < 0.05 versus ND pre-CP/CPB or CDM pre-CP/CPB. #p < 0.05 versus ND post-CP/CPB or CDM post-CP/CPB. (CDM = controlled diabetes; O.D. = optical density; UDM = uncontrolled diabetes.)
Fig 5.
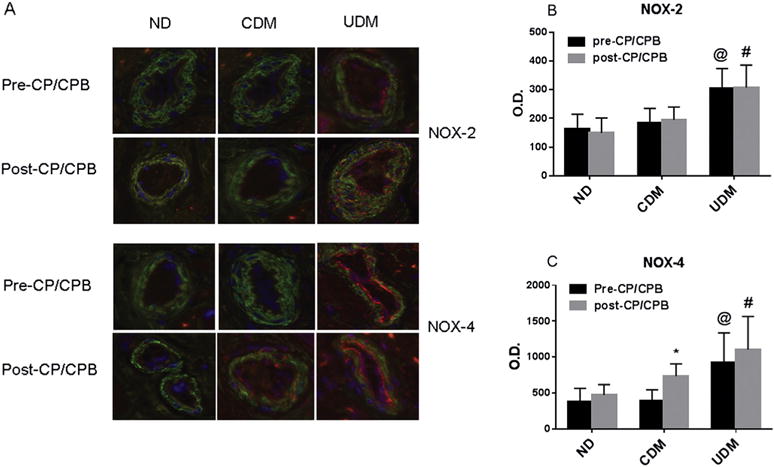
(A) Immunofluorescence staining of nicotinamide adenine dinucleotide phosphate oxidase (NOX)-2 and NOX-4 before cardioplegia arrest and cardiopulmonary bypass (pre-CP/CPB) and post-CP/CPB for no diabetes mellitus (ND), controlled diabetes (CDM), and uncontrolled diabetes (UDM) patients. Vessels were costained for cell nuclei (blue), smooth muscle α-actin (green), and NOX-2 or NOX-4 endothelium (red) and smooth muscle (orange). Bar graphs showing increased distribution of (B) NOX-2 and (C) NOX-4 in diabetes myocardium. Data are mean ± standard error of the mean; n = 6 per group. *p < 0.05 versus pre-CP/CPB. @p < 0.05 versus ND pre-CP/CPB. #p < 0.05 versus ND after CP/CPB or CDM post-CP/CPB. (O.D. = optical density.)
Protein Oxidation
Before and after CP/CPB, protein oxidation of atrial tissue samples was significantly increased in the UDM patients compared with the ND or CDM patients (p < 0.05; Fig 6).
Fig 6.
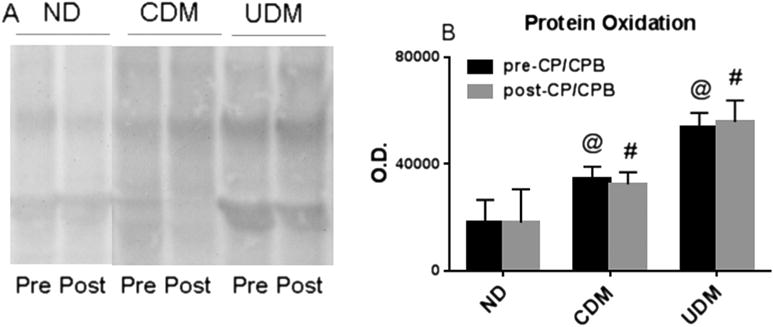
(A) Oxyblot protein oxidation detection for the no diabetes mellitus (ND), controlled diabetes (CDM), and uncontrolled diabetes (UDM) groups before (Pre) and after (Post) cardioplegia arrest and cardiopulmonary bypass (CP/CPB). (B) Protein oxidation of atrial tissue samples was significantly increased in the UDM and CDM groups. (Black bars = pre-CP/CPB; gray bars = post-CP/CPB.) Data are mean ± standard error of the mean; n = 6 per group. @p < 0.05 versus ND pre-CP/CPB or CDM pre-CP/CPB. #p < 0.05 versus ND post-CP/CPB or CDM post-CP/CPB. (O.D. = optical density.)
Comment
We have previously reported that CP/CPB is associated with impairment of coronary arteriolar endothelial function in patients after cardiac surgery [10, 11, 14]. In particular, the relaxation response to endothelium-dependent vasodilators adenosine diphosphate and substance P was significantly decreased after CP/CPB and cardiac surgery [10, 11, 14]. The response to nitric oxide donor sodium nitroprusside was also impaired early after CP/CPB [10, 11, 14]. In the present study, we found that the relaxation response of the coronary arterioles to bradykinin was also impaired in patients after CP/CPB and cardiac surgery. Recently, our study demonstrated that impaired relaxation responses to adenosine diphosphate and substance P were more pronounced in UDM patients than in ND or CDM patients before and after CP/CPB [7, 8, 13]. In this study, we observed that impaired relaxation response to bradykinin was more pronounced in UDM patients than in ND or CDM patients after CP/CPB. These findings are consistent with our previous studies suggesting that uncontrolled diabetes causes endothelial dysfunction in human coronary microcirculation early after CP/CPB and cardiac surgery.
A recent study showed that the coronary arteriolar relaxation response to bradykinin was significantly increased in diabetes patients at baseline [12]. In contrast, there were no significant changes in the response to bradykinin between patients with diabetes and patients not having diabetes or between CDM and UDM at baseline. This discrepancy between the present study and the other may be due to the different enrollment of diabetes patients [12]. For example, the diabetes patients enrolled in the present study were controlled (hemoglobin A1C greater than 7) or poorly controlled (HgA1C greater than 8.5). Consistent with the previous study, we observed that bradykinin-induced vasodilation was partially through COX-2 activation in diabetes patients, but not in nondiabetes patients at baseline. Interestingly, this effect is more pronounced in UDM than CDM. Importantly, after CP/CPB, the bradykinin-induced relaxation response was inhibited by the selective COX-2 inhibitor NS398 in all groups, with this effect more pronounced in the UDM group. These novel findings suggest that diabetes markedly enhances COX-2 activation in the coronary microvasculature, and COX-2 may play a compensatory role in bradykinin-induced vasodilatation early after CP/CPB.
We have previously found that CP/CPB significantly upregulates COX-2 gene/protein expression of ischemic myocardium in patients undergoing cardiac surgery [10, 11]. Consistent with our previous study, we also found upregulation of COX-2 protein expression in the ischemic heart in patients after cardiac surgery. Furthermore, we found that diabetes, especially uncontrolled diabetes, induced more COX-2 protein expression at baseline and after CP/CPB than did nondiabetes. In addition to the Western blot experiments, immunohistochemical staining was also performed to detect alteration in the myocardial and vascular localization of COX-1 and COX-2. We found that COX-2 was more expressed in the UDM myocardium and vessels than in ND or CDM. The CP/CPB altered COX-2 localization in diabetic and nondiabetic myocardium and coronary arterioles, but this effect was more pronounced in the UDM myocardium and coronary vessels. These novel findings suggest that diabetes combined with CP/CPB significantly upregulates COX-2 protein expression and localization. They also suggest that upregulated COX-2 plays an important role in the altered coronary arteriolar reactivity in diabetes patients after CP/CPB. However, because the majority of patients had coronary artery disease, aspirin had been routinely used for some time before surgery. Aspirin irreversibly inhibits COX-1 and modifies the enzymatic activity of COX-2, which may have a potential effect on the results of the study.
Molecular mechanisms responsible for the upregulation of COX-2 with diabetes and CP/CPB are not fully understood. Oxidative stress and systemic inflammation with diabetes and CP/CPB may be the major causes of COX-2 upregulation [3, 7, 17–23]. Indeed, current results show that protein oxidation was also enhanced in the setting of diabetes and CP/CPB. In addition, NOX-1, NOX-2, and NOX-4 were significantly upregulated with diabetes and CP/CPB. Recent work indicates that reactive oxygen species induces COX-2 overexpression in human endothelial cells exposed to high glucose as well as in monocytes from diabetes patients [24, 25]. Therefore, the increases in oxidative stress and NOX may account for the COX-2 upregulation in the setting of diabetes and CP/CPB (Fig 7) [26]. Overexpression of COX-2 in diabetes and CP/CPB can be a double-edged sword: COX-2 overexpression may contribute to CP/CPB-induced vasoconstriction through release of thromboxane A2 [10, 11], or conversely, COX-2 upregulation could also be a compensatory mechanism to the impairment of other endothelial pathways (eg, nitric oxide and endothelium-derived hyperpolarizing factor pathway) in diabetes patients during cardiac surgery [7, 9, 13]. In the diabetic and post-CP/CPB vessels, nitric oxide- and endothelium-derived hyperpolarizing factor-dependent vasodilation may be blunted [7, 9–11, 13]. Therefore, COX-2 overexpression may represent the main vasodilatory mechanism through the release of prostacyclin I-2. This notion can be supported by our previous studies indicating that CP/CPB failed to inhibit prostacyclin synthase gene/protein expression [10, 11]. Therefore, it is very important to modulate COX-2 pharmacologically in the context of cardiac surgery.
Fig 7.
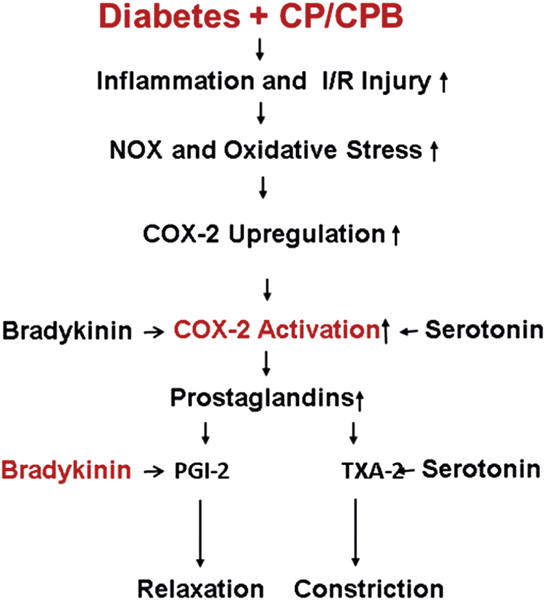
Proposed mechanisms responsible for diabetes mellitus and cardioplegia arrest and cardiopulmonary bypass (CP/CPB) upregulation of cyclooxygenase (COX)-2. (I/R = ischemia/reperfusion; NOX = nicotinamide adenine dinucleotide phosphate oxidase; PGI = prostacyclin; TXA = thromboxane.)
In conclusion, diabetes is associated with upregulation in COX-2 expression and distribution in human myocardium and coronary vasculature early after CP/ CPB and cardiac surgery. The increases in NOX expression and oxidative stress in diabetes may account for the upregulation of COX-2. These alterations may contribute to bradykinin-induced coronary arteriolar relaxation in patients with diabetes who are undergoing CP/CPB and cardiac surgery.
Acknowledgments
The authors wish to thank all nurses, physician assistants, and perfusionists at Rhode Island Hospital for collecting the patient consent forms, tissue samples, and data of patient characteristics. This research project was supported by the National Heart, Lung, and Blood Institute HL-46716 (F.W.S) and 1RO1HL127072 (J.F.), and supported in part by NIGMS/NIH grant 1P20GM103652 (pilot project [J.F.]), AHA-Grant-in-Aid-15GRNT25710105 (J.F.), Undergraduate Research and Teaching Award of Brown University (K.A.), and Summer Research—Early Identification Program of Leadership Alliance at Brown University (H.M.).
Abbreviations and Acronyms
- CDM
controlled diabetes
- COX
cyclooxygenase
- CP
cardioplegic arrest
- CPB
cardiopulmonary bypass
- ND
nondiabetes
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- PBS
phosphate-buffered saline
- UDM
uncontrolled diabetes
Footnotes
Presented at the Scientific Sessions of the American Heart Association, Orlando, FL, Nov 7–11, 2015.
References
- 1.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 2.Thourani VH, Weintraub WS, Stein B, et al. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045–52. doi: 10.1016/s0003-4975(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 3.Ruel M, Khan TA, Voisine P, Bianchi C, Sellke FW. Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg. 2004;26:1002–14. doi: 10.1016/j.ejcts.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 4.Emani S, Ramlawi B, Sodha NR, Li J, Bianchi C, Sellke FW. Increased vascular permeability after cardiopulmonary bypass in patients with diabetes is associated with increased expression of vascular growth factor and hepatocyte growth factor. J Thorac Cardiovasc Surg. 2009;138:185–91. doi: 10.1016/j.jtcvs.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voisine P, Ruel M, Khan TA, et al. Differences in gene expression profiles of diabetic and non-diabetic patients undergoing cardiopulmonary bypass and cardioplegic arrest. Circulation. 2004;110:II280–6. doi: 10.1161/01.CIR.0000138974.18839.02. [DOI] [PubMed] [Google Scholar]
- 6.Feng J, Liu Y, Sabe AA, et al. Differential impairment of adherens-junction expression/phosphorylation after cardioplegia in diabetic versus non-diabetic patients. Eur J Cardiothoracic Surg. 2015 Jun 11; doi: 10.1093/ejcts/ezv202. E-Pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng J, Liu YH, Chu LM, et al. Changes in microvascular reactivity after cardiopulmonary bypass in patients with poorly controlled versus controlled diabetes. Circulation. 2012;126:S73–80. doi: 10.1161/CIRCULATIONAHA.111.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng J, Liu YH, Khabbaz K, et al. Decreased contractile response to endothelin-1 of peripheral microvasculature from diabetic patients. Surgery. 2011;149:247–52. doi: 10.1016/j.surg.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng J, Chu LM, Dobrilovic N, Liu YH, Singh AK, Sellke FW. Decreased coronary microvascular reactivity after cardioplegic arrest in patients with uncontrolled diabetes mellitus. Surgery. 2012;152:282–9. doi: 10.1016/j.surg.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metais C, Li JY, Li J, Simons M, Sellke FW. Serotonin-induced coronary contraction increases after blood cardioplegia-reperfusion: role of COX-2 expression. Circulation. 1999;100:II328–34. doi: 10.1161/01.cir.100.suppl_2.ii-328. [DOI] [PubMed] [Google Scholar]
- 11.Metais C, Bianchi C, Li JY, Li J, Simons M, Sellke FW. Serotonin-induced human coronary microvascular contraction during acute myocardial ischemia is blocked by COX-2 inhibition. Basic Res Cardiol. 2001;96:59–67. doi: 10.1007/s003950170078. [DOI] [PubMed] [Google Scholar]
- 12.Szerafin T, Erdei N, Fülöp T, et al. Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circ Res. 2006;99:e12–7. doi: 10.1161/01.RES.0000241051.83067.62. [DOI] [PubMed] [Google Scholar]
- 13.Liu YH, Xie A, Singh AK, et al. Inactivation of endothelial SKCa/IKCa contributes to coronary arteriolar dysfunction in diabetic patients. J Am Heart Assoc. 2015;4:e002062. doi: 10.1161/JAHA.115.002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng J, Chu LM, Robich M, et al. Endothelin-1 induced contraction and signaling in the human skeletal muscle microcirculation. Circulation. 2010;122:S150–5. doi: 10.1161/CIRCULATIONAHA.109.928226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng J, Liu YH, Chu LM, et al. Thromboxane induced contractile response of human coronary arterioles is diminished post-cardioplegic arrest. Ann Thorac Surg. 2011;92:829–36. doi: 10.1016/j.athoracsur.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng J, Liu YH, Khabbaz KR, et al. Endothelin-1 induced contractile responses of human coronary arterioles through endothelin-A receptors and PKC-α signaling pathways. Surgery. 2010;147:798–804. doi: 10.1016/j.surg.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramlawi B, Feng J, Mieno S, et al. Indices of apoptosis activation after blood cardioplegia and cardiopulmonary bypass. Circulation. 2006;114:I257–63. doi: 10.1161/CIRCULATIONAHA.105.000828. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez A, Contreras C, Martinez P, et al. Enhanced cyclooxygenase 2-mediated vasorelaxation in coronary arteries from insulin-resistant obese Zucker rats. Atherosclerosis. 2010;213:392–9. doi: 10.1016/j.atherosclerosis.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Bagi Z, Erdei N, Toth A, et al. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol. 2005;25:1610–6. doi: 10.1161/01.ATV.0000172688.26838.9f. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z, Su W, Allen S, et al. COX-2 upregulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res. 2005;67:723–35. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Cosentino F, Eto M, De Paolis P, et al. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–23. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- 22.Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–62. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 23.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paneni F, Costantino S, Battista R, et al. Adverse epigenetic signatures by histone methyltransferase Set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ Cardiovasc Genet. 2015;8:150–8. doi: 10.1161/CIRCGENETICS.114.000671. [DOI] [PubMed] [Google Scholar]
- 25.Schröder K, Zhang M, Benkhoff S, et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–25. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 26.Feng J, Scott MD, Monica L, Sellke FW, Ferran CJ, Abid R. Endothelium-dependent coronary vasodilatation requires NADPH oxidase-derived ROS. Arterioscler Thromb Vasc Biol. 2010;30:1703–10. doi: 10.1161/ATVBAHA.110.209726. [DOI] [PMC free article] [PubMed] [Google Scholar]


