Abstract
Background
Patients with severe trauma often present with critical coagulopathy, resulting in impaired hemostasis, massive hemorrhage, and a poor survival prognosis. The efficacy of hemostatic resuscitation in correcting coagulopathy and restoring tissue perfusion has not been studied. We assessed a novel approach of pre-emptive administration of fibrinogen concentrate to improve critical coagulopathy in patients with severe trauma.
Methods
We retrospectively compared blood transfusion volumes and survival prognosis between three groups of patients with trauma, with an Injury Severity Score (ISS) ≥26 over three consecutive periods: group A, no administration of fibrinogen concentrate; group B, administration of 3 g of fibrinogen concentrate after evaluation of trauma severity and a plasma fibrinogen level <1.5 g/L; group C, pre-emptive administration of 3 g of fibrinogen concentrate immediately on patient arrival based on prehospital information, including high-severity injury or assessed need for massive transfusion before measurement of fibrinogen.
Results
∼56% of patients with an ISS ≥26 and transfused with red blood cell concentrates ≥10 units, had hypofibrinogenemia (fibrinogen <1.5 g/L) on arrival. Patients who received fibrinogen concentrate in group C showed significantly higher fibrinogen levels after treatment with this agent than those in group B (2.41 g/L vs 1.88 g/L; p=0.01). Although no significant difference was observed in blood transfusion volumes between the groups, the 30-day survival of patients in group C (all, and those with an ISS ≥26) was significantly better than in group A (p<0.05). The 48-hour mortality rate in patients with an ISS ≥26 was significantly lower in group C than in group A (8.6% vs 22.9%; p=0.005). Further, among patients with an ISS ≥41, the overall mortality was significantly lower in group C than in group A (20% vs 50%; p=0.02).
Conclusion
Pre-emptive administration of fibrinogen concentrate for patients with trauma with critical coagulopathy may contribute to improved survival.
Level of evidence
Level IV.
Keywords: hemorrhage, hemostasis, transfusion, coagulopathy
Background
Although considerable progress has been made in trauma resuscitation methods, traumatic hemorrhage due to severe coagulopathy remains a major cause of mortality in patients with trauma.1 Critical hypofibrinogenemia occurs early during major blood loss and causes a bleeding tendency with uncontrollable oozing at multiple injury sites. Fibrinogen, the final substrate of coagulation and the ligand of platelet glycoprotein (GP)IIb/IIIa receptors, plays a key role in clot formation. As fibrinogen is the first coagulation factor to fall below a critical value during massive bleeding and hemodilution,2 it seems plausible that it should be the first protein to be supplied to patients with trauma. A prospective observational study reported that the fibrinogen level at presentation is an independent predictor of mortality for patients with trauma.3 Early clinical data suggest that fibrinogen supplementation, as part of an algorithm for hemostatic therapy based on point-of-care-guided coagulation factor concentrates, improves outcomes for traumatic hemorrhage by improving clot strength and reducing blood loss, thereby increasing survival.4
Conventional approaches for patients with trauma with massive hemorrhage, including damage control resuscitation using blood component therapy, have been shown to result in persistent coagulopathy, bleeding, and poor outcomes.5 There is an increasing number of reports describing the limitations of fresh frozen plasma (FFP) for treating ongoing severe hypofibrinogenemia in various clinical settings, including critically injured patients with trauma.6 However, FFP is the only treatment currently available for acquired hypofibrinogenemia in Japan. Cryoprecipitate is not generally supplied by the Japanese Red Cross, and a purified fibrinogen concentrate derived from pooled human plasma (Fibrinogen HT; Japan Blood Products Organization, Tokyo, Japan) is available for use only in patients with congenital fibrinogen deficiency in Japan. Fibrinogen concentrate shows significant effects on the recovery of plasma fibrinogen levels and subsequent hemostasis in both hereditary and acquired hypofibrinogenemic states,7 including trauma-induced coagulopathy.8 9 Analysis of decades of pharmacovigilance data shows a promising safety profile for fibrinogen concentrate,10 and off-label use has occurred in aortic replacement surgery,11 massive obstetric hemorrhage, and severe trauma in Japan.
The aim of this study was to examine the effects of pre-emptive treatment with fibrinogen concentrate on the transfusion volume and prognosis for survival of patients with trauma.
Patients and methods
More than 500 patients with blunt-end trauma, principally from traffic accidents and falls, are admitted to the Center of Emergency and Critical Care Medicine, Saitama Medical Center, every year. Trauma severity is measured with the Injury Severity Score (ISS) scale, which ranges from 0 to 75.
In this single-center retrospective cohort study, we retrospectively analyzed transfusion volumes and survival outcomes of patients with trauma over three 12-month periods. During these periods, fibrinogen levels were measured by the Clauss method in the central laboratory of our hospital. Patients with trauma received crystalloid and blood transfusion products, including FFP, according to the transfusion protocol in our emergency medical center and 1–2 g of tranexamic acid was administered, as appropriate. Our modes of resuscitation remained basically unchanged over the 3-year period. The off-label use of fibrinogen concentrate for patients with trauma began in August 2012 after approval by the Institutional Review Board of our hospital. This study also complied with the Declaration of Helsinki.
For the purpose of this study, patients were divided into three groups spanning three consecutive time periods for analysis: Group A, no administration of fibrinogen concentrate (n=476; August 2011 to July 2012); Group B, administration of 3 g of fibrinogen concentrate (Fibrinogen HT; Tokyo, Japan) after evaluation of trauma severity when plasma fibrinogen levels <1.5 g/L (n=560; April 2013 to March 2014); and Group C, pre-emptive administration of 3 g of fibrinogen concentrate on arrival at our hospital, based on prehospital information, including high-severity injury and features of hemorrhagic shock, or the assessed need for massive transfusion, before measurement of fibrinogen levels (n=566; April 2014 to March 2015). In order to determine whether pre-emptive treatment with fibrinogen concentrate contributes to better outcomes for patients with trauma, we compared fibrinogen levels, blood transfusion volumes, and the prognosis for survival between these three groups.
Differences in patient characteristics and in the plasma fibrinogen levels before and after administration of fibrinogen concentrate between groups B and C were evaluated using the unpaired t-test. The 30-day survival curves were analyzed in all patients and in patients with an ISS ≥26 (excluding patients with cardiopulmonary arrest) in each group by Gehan-Breslow-Wilcoxon tests and the Kaplan-Meier method. A univariate analysis with Pearson's χ2 test was used to assess any differences between groups in the mortality within 48 hours of hospital arrival. Statistical significance was set at p<0.05.
Results
The characteristics of the patients (n=1602) in the three groups are shown in table 1. Of the patients with trauma managed at our hospital, 50–57% had an ISS between 0 and 15; 23–27% had an ISS between 16 and 25; 16–18% had an ISS between 26 and 40; and 4–6% had an ISS >40. There were no significant differences in age; ISS; the number of patients who received more than 10 units of red blood cell concentrate (RBC) transfusion; and the transfusion volumes of RBC, FFP, or platelet concentrate (PC) between the three groups (table 1).
Table 1.
Patient characteristics in each group
| Group A | Group B | Group C | Significance | |
|---|---|---|---|---|
| Patients (n) | 476 | 560 | 566 | |
| Age (years) | 48±23.7 | 49±24 | 49±23.7 | N.S. |
| ISS | 16.8±12.5 | 15.3±11.5 | 16.8±11.8 | N.S. |
| ISS 0–15 (n) | 248 | 319 | 282 | |
| ISS 16–25 (n) | 123 | 127 | 155 | |
| ISS 26–40 (n) | 76 | 92 | 101 | |
| ISS 41– (n) | 29 | 22 | 27 | |
| Patients with RBC transfusion (n) | 104 | 115 | 114 | N.S. |
| Patients with RBC ≥10 U transfusion (n) | 44 | 44 | 59 | N.S. |
| Transfusion units of RBC (n) | 11.0±11.1 | 10.5±10.5 | 13.2±13.9 | N.S. |
| Transfusion units of FFP (n) | 13.5±11.3 | 12.0±10.2 | 15.7±15.4 | N.S. |
| Transfusion units of PC (n) | 24.6±12.3 | 22.4±7.9 | 24.7±11.9 | N.S. |
| Patients treated with FC (n) | 0 | 30 | 35 | N.S. |
The data are presented as the number (n) or the mean±SD.
FC, fibrinogen concentrate; FFP, fresh frozen plasma; ISS, Injury severity score; N.S., not significant at a level of p ≥0.05; PC, platelet concentrate; RBC, red blood cell concentrate.
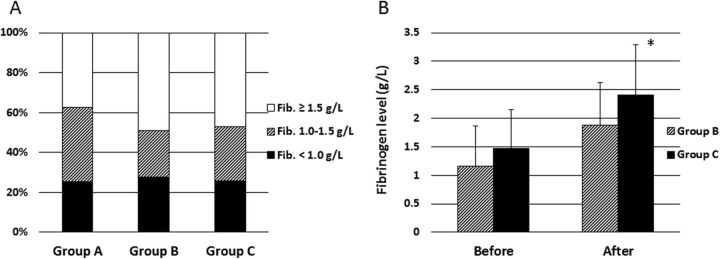
Figure 1A shows the fibrinogen levels of patients with trauma with an ISS ≥26 and transfused with RBC ≥10 units, on arrival at our hospital for groups A to C. Approximately 56% of patients showed hypofibrinogenemia (defined as a fibrinogen level 1.0–1.5 g/L) and 26% had critical hypofibrinogenemia (defined as a fibrinogen level <1.0 g/L). We observed no significant differences between the groups in terms of the number of patients with hypofibrinogenemia or critical hypofibrinogenemia. In addition, no significant differences in fibrinogen levels measured before administration of fibrinogen concentrate were detected between groups B (n=30) and C (n=35) (figure 1B). However, patients who received fibrinogen concentrate in group C showed significantly higher fibrinogen levels after treatment with this agent than those in group B (2.41 g/L vs 1.88 g/L; p=0.01; figure 1B).
Figure 1.
The proportion of patients with hypofibrinogenemia. (A) The percentage of each category of fibrinogen level on arrival in trauma patients with ISS ≥26 and transfused with red blood cell concentrates (RBC) ≥10 units (group A, n=56; group B, n=60; and group C, n=64). Fibrinogen concentration in plasma: black portions, <1.0 g/L; hatched portions, 1.0–1.5 g/L; white portions, >1.5 g/L. (B) The fibrinogen levels in patients before and after administration of fibrinogen concentrate in group B (hatched columns; n=30) and C (black columns; n=35). The data are presented as the mean and SD. Differences between groups B and C were evaluated using the unpaired t-test (*p=0.01). ISS, Injury Severity Score.
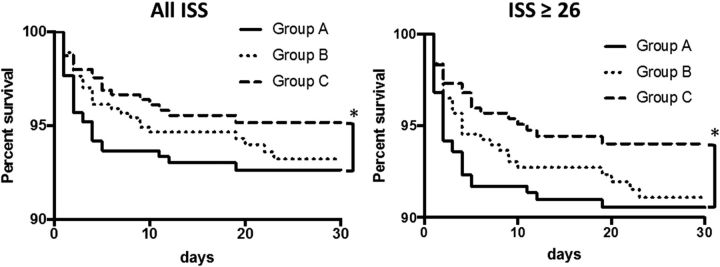
We analyzed the 30-day survival curves in all patients and in patients with an ISS ≥26, excluding patients with cardiopulmonary arrest, in all three groups. A significantly better prognosis for survival in both analyses was observed in group C (n=536 in total and n=101 with an ISS ≥26) compared with group A (n=441 in total and n=75 with an ISS ≥26) (p<0.05; figure 2).
Figure 2.
The 30-day survival curves were analyzed in all patients (left panel) and in patients with an ISS ≥26 (right panel), excluding patients with cardiopulmonary arrest, in all three groups using Gehan-Breslow-Wilcoxon tests and the Kaplan-Meier method. Significant differences (*p<0.05) were detected in both analyses between group A (n=441 in total and 75 with an ISS ≥26) and group C (n=536 in total and 101 with an ISS ≥26). ISS, Injury Severity Score.
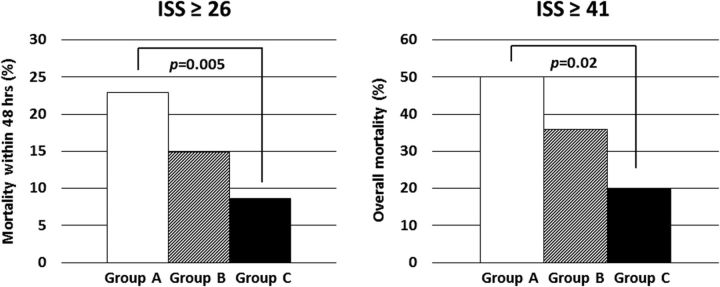
In patients with trauma with an ISS ≥26, the 48-hour mortality rate was significantly lower in group C (n=128; 8.6%) than in group A (n=105; 22.9%, p=0.005), as shown in the left panel in figure 3. The cohort analyzed in this figure was different from that in figure 2; in figure 2 patients with cardiopulmonary arrest were excluded. Moreover, the mortality rate of patients with an ISS ≥41 was also significantly lower in group C (n=25; 20%) than in group A (n=30; 50%) (p=0.02; right panel in figure 3). Of the patients with an ISS ≥41 who survived, 42% in group B and 45% in group C received fibrinogen concentrate (data not shown). No thromboembolic events caused by treatment with fibrinogen concentrate were observed in this study (data not shown).
Figure 3.
Mortality of trauma patients with high severity. Left panel: The mortality within 48 hours of arrival at the hospital for patients with an ISS ≥26 in groups A to C (n=105 for group A; n=114 for group B; n=128 for group C). A significant difference was detected between group A and C (p=0.005) after evaluation by Pearson's χ2 test. Right panel: The overall mortality of patients with an ISS ≥41 in groups A to C (n=30 for group A; n=22 for group B; n=25 for group C). A significant difference was detected between group A and C (p=0.02) after evaluation by Pearson's χ2 test. White columns, group A; hatched columns, group B; black columns, group C. ISS, Injury Severity Score.
Discussion
The lethal triad of coagulopathy, acidosis, and hypothermia starts early after traumatic injury and is associated with increased mortality.12 Trauma-induced coagulopathy is primarily diagnosed as hypofibrinogenemia, which is accelerated by acidosis and hypothermia.13 14 As demonstrated in figure 1, ∼56% of the patients with trauma with an ISS ≥26 had hypofibrinogenemia, and a further 25% had critical hypofibrinogenemia. The average initial fibrinogen level of the patients on arrival in groups B and C who were treated with fibrinogen concentrate, was also <1.5 g/L (figure 1B). Critical hypofibrinogenemia leads to uncontrollable microvascular bleeding, including oozing at multiple injury sites, resulting in massive hemorrhage. This pathology results from the dilutional coagulopathy caused by fluid supplementation to maintain blood pressure, and also from fibrinogenolysis due to hyperfibrinolysis induced by tissue-type plasminogen activator released from injured endothelial cells.15
Importantly, the elevation of fibrinogen levels observed in patients after treatment with fibrinogen concentrate in group C was significantly higher than in group B (figure 1B), suggesting that pre-emptive administration of fibrinogen concentrate can efficiently increase the fibrinogen level over the threshold for hemostasis. Rapid elevation of the fibrinogen level over 2.0 g/L makes complete hemostasis at injury sites possible, contributing to better survival outcomes for patients with trauma. Thus, fibrinogen is a key molecule in trauma-induced coagulopathy to be targeted for supplementation in transfusion therapy.2 13 16
As shown in figures 2 and 3, the prognosis for survival tended to improve with the use of fibrinogen concentrate (see and compare group A and B) even though the difference was not statistically significant. In group B, fibrinogen concentrate was given after measurement of patient's fibrinogen level. It usually takes longer than half an hour to receive laboratory confirmation of plasma fibrinogen levels, which is a critical period for patients with severe trauma. Presumably then, the administration of concentrated fibrinogen started around 2 hours after the onset of trauma in group B. In addition, the activation and consumption of fibrinogen progresses quickly during the first hour after trauma. Thus, some patients in group B may have lost a critical window for survival by waiting for the fibrinogen results. Evaluation of coagulopathy via rotational thromboelastometry has been recently published and could reduce the time needed for fibrinogen assessment and supplementation.8 17 Our study used immediate administration of fibrinogen concentrate on patient arrival to treat critical coagulopathy based on prehospital information, including high-severity injury and features of hemorrhagic shock, and an assessed need for massive transfusion. In patients with trauma with hypofibrinogenemia, early treatment with fibrinogen concentrate may control active bleeding and oozing at multiple injury sites.
Two cohort studies reported lower mortality rates for patients receiving high doses of fibrinogen during traumatic hemorrhage.18 19 A randomized controlled trial (RCT) of fibrinogen concentrate is now ongoing20 and a small RCT of cryoprecipitate, another source of concentrated fibrinogen, showed that early fibrinogen supplementation was feasible in patients with trauma.21 While hemostatic resuscitation offers advantages over previous strategies, it does not correct coagulopathy during the acute phase of trauma hemorrhage without a high total fibrinogen load.22 We observed better survival rates for patients with trauma with an ISS ≥26 who received pre-emptive treatment with fibrinogen concentrate (figures 2 and 3), despite no significant reduction in transfusion volumes administered (table 1). Notably, a better survival rate (ie, up to 80% survival) was achieved by pre-emptive administration of this agent in patients with trauma with an ISS ≥41, who generally show an extremely low survival rate (figure 3). A limitation of this retrospective study is that we compared the cohort over the course of 3 years. Over such a period, the treatment of patients with trauma may have varied. However, in our institution, the management of patients with trauma, for example, damage control resuscitation, transfusion protocols, use of tranexamic acid, and surgical procedures, remained basically unchanged over the 3-year period. Primary hemostasis, accomplished by pre-emptive administration of fibrinogen concentrate, enables surgeons to perform early mobilization of patients for imaging diagnosis to detect bleeding sites, leading to definitive surgical fixation and hemostasis. Taken together, this time saving, aggressive supplementation with fibrinogen may contribute to improved outcomes and may prevent death due to massive hemorrhage, especially during the acute phase of trauma.
Acknowledgments
The authors thank Y Suzuki and H Maeda for management of the clinical data on patient transfusions. We would like to thank Editage for English language editing (http://www.editage.jp).
Footnotes
Contributors: KY analyzed the data and wrote the manuscript. AY and MM collected and analyzed the data. MA collected the data. KI and MS designed the study and analyzed the data. SS critically reviewed the manuscript. All authors read and approved the final manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Institutional Review Board of Saitama Medical Center.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg 2007;31:1507–11. doi:10.1007/s00268-007-9087-2 [DOI] [PubMed] [Google Scholar]
- 2.Schöchl H, Schlimp CJ, Maegele M. Tranexamic acid, fibrinogen concentrate, and prothrombin complex concentrate: data to support prehospital use? Shock 2014;41(Suppl 1):44–6. doi:10.1097/SHK.0000000000000093 [DOI] [PubMed] [Google Scholar]
- 3.Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost 2012;10:1342–51. doi:10.1111/j.1538-7836.2012.04752.x [DOI] [PubMed] [Google Scholar]
- 4.Schöchl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, Kozek-Langenecker S, Solomon C. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care 2010;14:R55 doi:10.1186/cc8948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan S, Davenport R, Raza I, Glasgow S, De'Ath HD, Johansson PI, Curry N, Stanworth S, Gaarder C, Brohi K. Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma hemorrhage. Intensive Care Med 2015;41:239–47. doi:10.1007/s00134-014-3584-1 [DOI] [PubMed] [Google Scholar]
- 6.Scalea TM, Bochicchio KM, Lumpkins K, Hess JR, Dutton R, Pyle A, Bochicchio GV. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg 2008;248:578–84. doi:10.1097/SLA.0b013e31818990ed [DOI] [PubMed] [Google Scholar]
- 7.Fenger-Eriksen C, Lindberg-Larsen M, Christensen A, Ingerslev J, Sørensen B. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth 2008;101:769–73. doi:10.1093/bja/aen270 [DOI] [PubMed] [Google Scholar]
- 8.Schöchl H, Forster L, Woidke R, Solomon C, Voelckel W. Use of rotation thromboelastometry (ROTEM) to achieve successful treatment of polytrauma with fibrinogen concentrate and prothrombin complex concentrate. Anaesthesia 2010;65:199–203. doi:10.1111/j.1365-2044.2009.06188.x [DOI] [PubMed] [Google Scholar]
- 9.Stephens CT, Gumbert S, Holcomb JB. Trauma-associated bleeding: management of massive transfusion. Curr Opin Anaesthesiol 2016;29:250–5. doi:10.1097/ACO.0000000000000306 [DOI] [PubMed] [Google Scholar]
- 10.Solomon C, Gröner A, Ye J, Pendrak I. Safety of fibrinogen concentrate: analysis of more than 27 years of pharmacovigilance data. Thromb Haemost 2015;113:759–71. doi:10.1160/TH14-06-0514 [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto K, Usui A, Takamatsu J. Fibrinogen concentrate administration attributes to significant reductions of blood loss and transfusion requirements in thoracic aneurysm repair. J Cardiothorac Surg 2014;9:90 doi:10.1186/1749-8090-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma 1997;42:857–61. doi:10.1097/00005373-199705000-00016 [DOI] [PubMed] [Google Scholar]
- 13.Fries D, Mortini W. Role of fibrinogen in trauma-induced coagulopathy. Br J Anaesth 2010;105:116–21. doi:10.1093/bja/aeq161 [DOI] [PubMed] [Google Scholar]
- 14.Meyer MA, Ostrowski SR, Sørensen AM, Meyer AS, Holcomb JB, Wade CE, Johansson PI, Stensballe J. Fibrinogen in trauma, an evaluation of thrombelastography and rotational thromboelastometry fibrinogen assays. J Surg Res 2015;194:581–90. doi:10.1016/j.jss.2014.11.021 [DOI] [PubMed] [Google Scholar]
- 15.Theusinger OM, Wanner GA, Emmert MY, Billeter A, Eismon J, Seifert B, Simmen HP, Spahn DR, Baulig W. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM) is associated with higher mortality in patients with severe trauma. Anesth Analg 2011;113:1003–12. doi:10.1213/ANE.0b013e31822e183f [DOI] [PubMed] [Google Scholar]
- 16.Schöchl H, Cotton B, Inaba K, Nienaber U, Fischer H, Voelckel W, Solomon C. FIBTEM provides early prediction of massive transfusion in trauma. Crit Care 2011;15:R265 doi:10.1186/cc10539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theusinger OM, Baulig W, Seifert B, Müller SM, Mariotti S, Spahn DR. Changes in coagulation in standard laboratory tests and ROTEM in trauma patients between on-scene and arrival in the emergency department. Anesth Analg 2015;120:627–35. doi:10.1213/ANE.0000000000000561 [DOI] [PubMed] [Google Scholar]
- 18.Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, Hess JR, Dubick MA, Simon CD, Beekley AC, et al. . The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma 2008;64:S79–85. doi:10.1097/TA.0b013e318160a57b [DOI] [PubMed] [Google Scholar]
- 19.Dente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Patel S, Shah A, Vercruysse GA, Feliciano DV, Rozycki GS, et al. . Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma 2009;66:1616–24. doi:10.1097/TA.0b013e3181a59ad5 [DOI] [PubMed] [Google Scholar]
- 20.Steinmetz J, Sørensen AM, Henriksen HH, Lange T, Larsen CF, Johansson PI, Stensballe J. Pilot randomized trial of fibrinogen in trauma haemorrhage (PRooF-iTH): study protocol for a randomized controlled trial. Trials 2016;17:327 doi:10.1186/s13063-016-1439-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curry N, Rourke C, Davenport R, Beer S, Pankhurst L, Deary A, Thomas H, Llewelyn C, Green L, Doughty H, et al. . Early cryoprecipitate for major haemorrhage in trauma: a randomised controlled feasibility trial. Br J Anaesth 2015;115:76–83. doi:10.1093/bja/aev134 [DOI] [PubMed] [Google Scholar]
- 22.Khan S, Brohi K, Chana M, Raza I, Stanworth S, Gaarder C, Davenport R, International Trauma Research Network (INTRN). Hemostatic resuscitation is neither hemostatic nor resuscitative in trauma hemorrhage. J Trauma Acute Care Surg 2014;76:561–7; discussion 567–8 doi:10.1097/TA.0000000000000146 [DOI] [PubMed] [Google Scholar]