Abstract
Objective
High dietary sodium intake is a risk factor for cardiovascular events and death. Recently, a J-shaped correlation between sodium intake and adverse outcomes has been shown. The evidence on the association between sodium intake and cardiovascular outcomes in the elderly is scant. The objective of this study was to evaluate the correlation between sodium intake and cardiovascular events and mortality in an elderly population, taking into account frailty status.
Design
Cohort study of community dwelling older people enrolled in the InCHIANTI (Invecchiare in Chianti - Aging in the Chianti) study from 1998 to 2000 and followed-up for 9 years.
Setting
Two communities in Tuscany, Italy.
Participants
A total of 920 participants 65 years of age and older, with 24-hour urinary sodium excretion data.
Measurements
Nine-year mortality and incident cardiovascular events were analyzed using Cox and nonlinear log-binomial models, stratified by frailty status. Sensitivity analysis in participants without hypertension and cardiovascular diseases was performed.
Results
Mean age of the population was 74.5 years (standard deviation 6.99); 55.4% were women. There was a bi-modal association between sodium excretion and mortality, with risk increasing only below sodium excretion of 6.25 g/d [hazard ratio (HR) 1.29, 95% confidence interval (CI) 1.20–1.38], confirmed in the adjusted model (HR 1.12, 95% CI 1.04–1.22). These results were confirmed in participants without cardiovascular diseases. After stratification for frailty phenotype, the association was stronger in frail participants (adjusted HR 1.23, 95% CI 1.02–1.50 vs HR 1.11, 95% CI 1.01–1.22 in robust participants). There was no association between 24-hour sodium excretion and 9-year incidence of cardiovascular diseases (adjusted risk ratio 0.96, 95% CI 0.90–1.02).
Conclusions
Reduced sodium excretion is associated with increased mortality in a sample of community-dwelling older people, especially among the frail participants. High levels of sodium excretion are not associated with adverse outcomes in this population; therefore, sodium restriction might not be beneficial in older people.
Keywords: Aged, frail elderly, mortality, cardiovascular diseases, sodium, dietary
Excess dietary sodium intake is considered an important cause of hypertension,1 and very recently an urinary sodium excretion >3.7 g/d has been associated with subclinical cardiovascular disease.2 Several studies have shown a reduced risk of cardiovascular events in people with lower sodium intake,3 and reducing sodium intake is recommended for cardiovascular prevention.4 There is evidence, however, that a J-shaped or U-shaped relationship may exist between sodium intake and adverse outcomes, with risk increasing when sodium excretion is below ~3 g/d or above ~5 g/d.5,6 The increased risk associated may be related to increased activity of renin-angiotensin-aldosterone system and sympathetic nerve activation.7
To our knowledge, only 1 study evaluated this association in an elderly population, finding no relationship between sodium intake and adverse outcomes.8 In this study, sodium intake was evaluated using a food frequency questionnaire rather than 24-hour urinary sodium excretion that is considered the most accurate method to estimate sodium intake.9 Thus, the evidence on this issue in the elderly is still not clear. In this population, comorbidities, poly-pharmacotherapy, and frailty may act as an effect modifier, in the same way as it happens for hypertension, which does not seem to be associated with mortality in people with reduced gait speed.10 The objective of our study was to evaluate the correlation between sodium intake (estimated using sodium urinary excretion), mortality, and cardiovascular events in an elderly population. The association was analyzed separately in people with and without frailty.
Methods
Data Source and Study Design
We analyzed data from the longitudinal InCHIANTI (Invecchiare in Chianti - Aging in the Chianti) study.11 The baseline study was supported by the Italian Ministry of Health and also partly supported by the US National Institute on Aging. After obtaining informed consent, participants were randomly selected from the populations of 2 town areas; data collection started in September 1998 and was completed in March 2000. The eligible participants were interviewed at home by trained study researchers. The interview was followed by a physical examination at the study clinic as well as laboratory analysis. Follow-up visits were scheduled at 3, 6, and 9 years. The Italian National Institute of Research and Care on Aging Ethical Committee approved the study protocol.
Sample Selection
From 1170 participants with available 24-hour urinary sodium excretion data, we excluded participants younger than 65 years (N = 250). Follow-up data at 9 years were available for 920 participants. For the analysis on incident cardiovascular events, we selected participants without history of cardiovascular disease at baseline (N = 514).
Definition of Exposure and Outcome
Sodium intake was estimated using the 24-hour sodium urinary excretion. On the day of the study visit, participants were provided with a plastic bottle containing 1 g of boric acid as preservative, and instructed to collect all the urine produced in the following 24 hours, making the maximum effort to avoid dispersing urine during the collection period.
We considered mortality for all causes and incident cardiovascular events (angina pectoris, myocardial infarction, heart failure, and stroke) as outcome measures. Cardiovascular events were ascertained using a questionnaire and reviewing clinical documentation at the follow-up visits. Data on mortality were collected from mortality registers.
Analytic Approach
We reported the characteristics of the study sample using descriptive statistics (mean and standard deviation for continuous variables, proportion for categorical variables), according to quartiles of 24-hour urinary sodium excretion. We took into account associated diseases (eg, hypertension, diabetes), cigarette smoke (pack-years), blood pressure, total cholesterol, and estimated creatinine clearance [chronic kidney disease epidemiology collaboration (CKD-EPI)]. Total caloric intake, macronutrients and micronutrients intake, and alcohol intake were evaluated using the European Prospective Investigation into Cancer and Nutrition Questionnaire; a food frequency intake questionnaire validated in an Italian elderly population.12,13 Finally, we took into account years of education and physical activity.
Mortality risk across quartiles of sodium excretion was calculated using the Kaplan-Meier method. We used Cox regressions to evaluate the relationship between sodium excretion and mortality. Both linear and polynomial (restricted cubic splines) models were fitted; the goodness of fit of these models was evaluated using the log-likelihood test; and the best fitting unadjusted model was then adjusted for potential confounders, selected on the base of the clinical significance, prior knowledge, and results of the univariate analysis. To explore the different role of demographic and clinical variables, we first adjusted for age and sex, and then for the other potential confounders [education, CKD-EPI, pack/y, hypertension, diabetes, body mass index (BMI), caloric intake/body weight, and antihypertensive drugs and diuretics]. Finally, we repeated the analysis in participants with and without frailty, defined according to the Fried frail phenotype as the presence of 3 or more of the following criteria: unintentional weight loss (10 lbs in past year), self-reported exhaustion, reduced grip strength, slow walking speed, and low physical activity.14 Because cardiovascular diseases (angina pectoris, myocardial infarction, heart failure, and stroke) and hypertension may significantly influence the relationship between sodium excretion and mortality, we planned a sensitivity analysis that excluded participants with these conditions at baseline (N = 312).
The relationship between sodium excretion and incident cardiovascular disease was analyzed in the subgroup of participants without prevalent cardiovascular disease (angina pectoris, myocardial infarction, heart failure, and stroke), ascertained by investigating the clinical history and documentation (N = 514). This relationship was evaluated by fitting linear log-binomial regression models to directly estimate relative risks. To allow for a nonlinear relationship between exposure and outcome, we compared the goodness-of-fit of quadratic log-binomial and linear models using the Akaike information criteria (AIC). All analyses were performed using R v 3.3.3 (R Foundation for Statistical Computing, Wien, Austria).
Results
The mean age of the sample was 74.5 years [standard deviation (SD) 6.99]; 55.4% were women. Cut-off for sodium excretion quartiles were 3.70 g/d, 5.44 g/d, and 7.04 g/d. Participants in the first (ie, lower) quartile of sodium excretion were older (mean age 77.4, SD 7.6 vs 72.5, SD 6 in the fourth quartile, P <.001), more frequently women (68% vs 38% in the fourth quartile, P < .001), and more sedentary (74% vs 52% in the fourth quartile, P < .001). They also had lower estimated glomerular filtration rate and BMI, and lower prevalence of diabetes, and higher blood systolic blood pressure and prevalence of dementia. Participants in the fourth quartile of sodium excretion had higher intake of energy (2093.5 kcal/d compared 1784.2 kcal/d, P < .001), micronutrients, including sodium, potassium, and vitamins, and also had higher intake of alcohol (12.3 g/d in the first quartile vs 18 g/d in the fourth, P = .001) (Table 1).
Table 1.
General Characteristics of the Population Distributed by Quartiles of 24-Hour Sodium Excretion
| I Quartile (n = 230) | II Quartile (n = 230) | III Quartile (n = 230) | IV Quartile (n = 230) | P | |
|---|---|---|---|---|---|
| Age, mean (SD), years | 77.4 (7.6) | 74.9 (6.9) | 73.2 (6.4) | 72.5 (6) | <.001 |
| Female sex, No. (%) | 156 (68) | 136 (60) | 125 (55) | 88 (38) | <.001 |
| Education, mean (SD), years | 5.3 (3.6) | 4.9 (2.9) | 5.5 (3.4) | 5.6 (3) | .094 |
| Sedentary, No. (%) | 170 (74) | 149 (65) | 132 (58) | 120 (52) | <.001 |
| BMI, mean (SD), kg/m2 | 26.6 (4) | 27.4 (4.4) | 27.5 (3.9) | 28.1 (3.6) | .002 |
| CKD-EPI, mean (SD), mL/min/1.73 m2 | 68.4 (15.6) | 71.1 (14.2) | 72.5 (12.8) | 72.5 (13) | .005 |
| Total cholesterol, mean (SD), mg/dL | 215.6 (36.8) | 217.5 (40.5) | 218.4 (40) | 218.8 (38.1) | .819 |
| Systolic blood pressure, mean (SD), mmHg | 155.6 (20.4) | 148.9 (17.8) | 148.6 (18.6) | 149.5 (20.3) | <.001 |
| Diastolic blood pressure, mean (SD), mmHg | 85.6 (8.2) | 83 (7.8) | 83.9 (9) | 83.7 (8.6) | .01 |
| Smoke, mean (SD), pack/year | 11.2 (21) | 10.4 (19.5) | 13.6 (21.8) | 16 (22.3) | .021 |
| Hypertension, No. (%) | 145 (63) | 149 (65) | 133 (58) | 141 (61) | .462 |
| Diabetes, No. (%) | 26 (11) | 27 (12) | 21 (9) | 42 (18) | .021 |
| Peripheral Arteriopathy, No. (%) | 36 (16) | 29 (13) | 21 (9) | 23 (10) | .128 |
| COPD, No. (%) | 19 (8) | 17 (7) | 14 (6) | 21 (9) | .652 |
| Metabolic syndrome, No. (%) | 48 (21) | 59 (26) | 53 (23) | 57 (25) | .635 |
| Dementia, No. (%) | 18 (8) | 12 (5) | 4 (2) | 4 (2) | .002 |
COPD, chronic obstructive pulmonary disease.
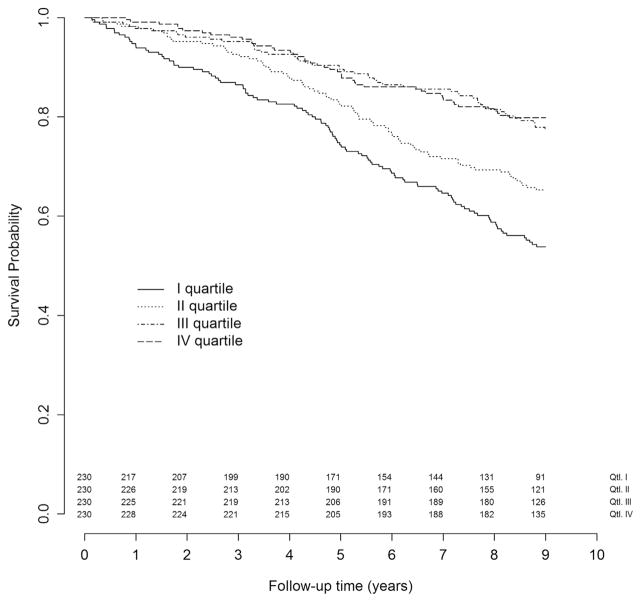
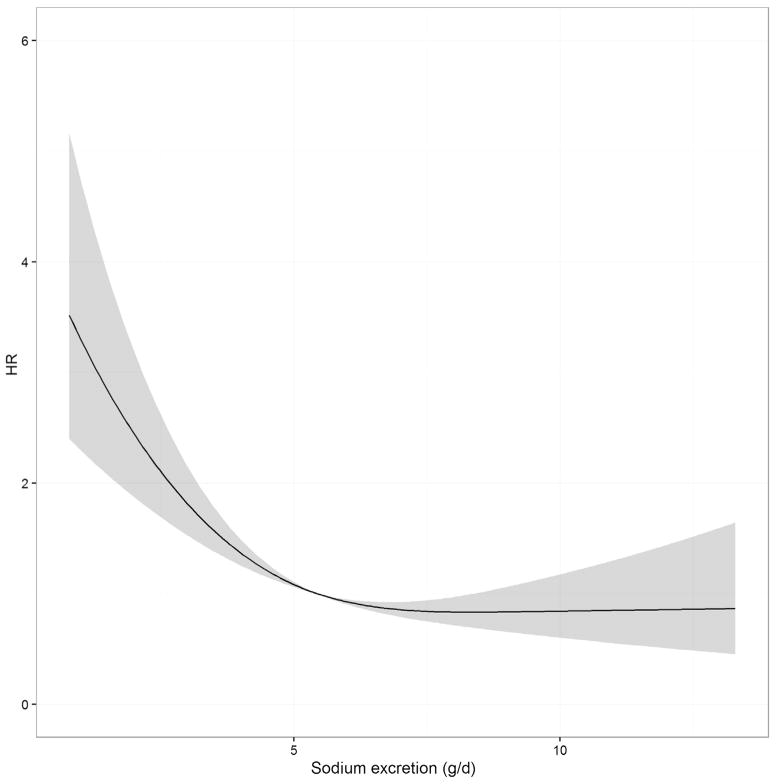
The median follow-up time was 9 years, with a cumulative follow-up time of 7009 years. Over this time, 281 participants died, with an incidence rate of 4.01/100 person-year [95% confidence interval (CI) 3.57–4.49] and a cumulative risk of 31% (95% CI 28%–34%). There was no difference in the mortality risk between the third and the fourth quartile (22.7% and 20.2%, respectively), while participants in the first 2 quartiles had a clearly increased risk (46.2% and 34.7%, respectively) (Figure 1). The analysis of the relationship between sodium excretion and risk of mortality using a nonlinear Cox model (restricted cubic spline with 3 knots) showed that a linear model was not appropriate to describe the data, whereas a linear spline allowing an increase in risk when sodium excretion is below 6.25 g/d seemed to provide an adequate fit (Figure 2). The likelihood ratio obtained with a linear, restricted cubic spline, or linear spline models were 36.7, 48.1, and 49.4, respectively. Accordingly, we chose to use the linear spline model with 1 knot at 6.25 g/d because it allows an easy interpretation of the coefficient that expresses the increase of the risk for 1-unit decrease of sodium excretion below 6.25 g/d.
Fig. 1.
Six-year all-cause mortality according to 24-hour sodium excretion quartiles.
Fig. 2.
Twenty-four-hour sodium excretion and HR for all-cause death
We found an association between decreasing sodium excretion and mortality [hazard ratio (HR) 1.29; 95% CI 1.20–1.38] that was reduced but still significant after adjustment for age and sex only (HR 1.15; 95% CI 1.07–1.24), and after further adjustment for education, estimated GFR, systolic blood pressure, smoking history, hypertension, diabetes, BMI, caloric intake/body weight ratio, and antihypertensive drug and diuretics (HR 1.12; 95% CI 1.04–1.22). Compared with robust participants, frail participants were older (mean age 80.9 years, SD 6.9 vs 73.7 years, SD 6.5), more frequently women (64% vs 54%), and more frequently had a low level of physical activity (95% vs 58%), and comorbidities, such as diabetes, peripheral arteriopathy, dementia; there was no difference in total energy intake/kg of body weight (28.9 kcal/kg, SD 8.4 vs 26.6 kcal/kg, SD 7.5 in frail vs robust group). After stratification for frailty phenotype, we found that the crude association between decreasing daily sodium excretion and mortality was more evident in robust (HR 1.25, 95% CI 1.15–1.30) compared with frail participants (HR 1.15; 95% CI 1–1.33). After adjustment for potential confounders, however, among frail participants the risk associated with decreased sodium excretion was higher (HR 1.23, 95% CI 1.02–1.05) compared with robust participants (HR 1.11, 95% CI 1.01–1.22) (Table 2). In the subsample of participants without prevalent cardiovascular disease or hypertension at baseline, we observed a stronger association between sodium excretion and mortality (crude HR 1.69, 95% CI 1.18–1.56; adjusted HR 1.32, 95% CI 1.12–1.56). A stratified analysis for frailty was not possible for the small number of frail people in this subsample (N = 24).
Table 2.
Association Between Decreasing 24-Hour Sodium Excretion and Mortality
| Overall | Frail | Robust | |
|---|---|---|---|
| N | 920 | 87 | 812 |
| N events | 281 | 69 | 197 |
| Person/y | 7009 | 783 | 6430 |
| Crude model HR (95% CI) |
1.29 (1.20–1.38) | 1.15 (1–1.33) | 1.25 (1.15–1.30) |
| Adjusted model 1 HR (95% IC) |
1.15 (1.07–1.24) | 1.19 (1.02–1.39) | 1.11 (1.02–1.22) |
| Adjusted model 2 HR (95% IC) |
1.12 (1.04–1.22) | 1.23 (1.02–1.50) | 1.11 (1.01–1.22) |
Model adjusted 1: adjusted for age and sex.
Model adjusted 2: Adjusted for age, sex, education, CKD-EPI, systolic blood pressure, pack/y, hypertension, diabetes, BMI, caloric intake/body weight, and antihypertensive drugs and diuretics.
Over the 9-year follow-up, we observed 169 cardiovascular events, with an incidence rate of 3.75/100 person-year (95% CI 3.23–4.33). Fitting log-binomial models of 24-hour sodium excretion vs incident cardiovascular disease, we found no differences in the goodness of fit comparing a linear (AIC = 651.3) and a quadratic (AIC = 653.2) model, suggesting a linear relationship. There was a weak inverse association between 24-hour sodium excretion and 9-year incidence of cardiovascular diseases (RR 0.95; 95% CI 0.90–1), which was not confirmed after adjustment of the model for possible confounders (age, sex, education, CKD-EPI, systolic blood pressure, pack/y, hypertension, diabetes, BMI, caloric intake/body weight, and antihypertensive drugs and diuretics), with a RR of 0.96 (95% CI 0.90–1.02).
Discussion
We found that risk for mortality does not increase for sodium excretion of 6.25 g/d or more, and a linear increase in risk was evident for sodium excretion below 6.25 g/d. After stratification for frailty phenotype, this association was somewhat stronger in frail participants. We did not find an association between sodium excretion and cardiovascular events in a sample of older community dwelling people. To our knowledge, only 1 other study investigated this issue focusing on older people.8 However, in that study, sodium intake was assessed by a food questionnaire, which is not the gold standard,9 and people with difficulties with walking, stair climbing, or activities of daily living, or with cognitive impairment, which are at higher risk of negative outcomes, were excluded. The nonlinear association between sodium excretion and mortality found in our sample is in line with the evidence obtained in younger people, in which different studies found an association between mortality and low sodium excretion, whereas an association with high sodium excretion was observed only in the hypertensive subgroup.6,15 These findings were confirmed by a meta-analysis showing a 9% reduction of the risk of all-cause mortality in the usual vs low sodium intake group.16 In contrast with other studies,5,17 we did not find an association between higher sodium excretion and mortality.16 The discrepancy between these findings and our results may be due the difference in the sample characteristics, as the mean age in our study was considerably higher and also other selection criteria (eg, baseline cardiovascular risk) differed.
To our knowledge, this is the first study comparing the effects of sodium intake in frail and robust people. Frailty is associated with reduced physical activity. In the elderly, frailty is associated with a reduced activation of the sympathetic nervous system but also of renin-angiotensin-aldosterone system, although for the latter, evidence is more contrasting18; therefore, frail persons may have an overactivation of these systems, which in turn may lead to increased risk for cardiovascular events and mortality.19,20 This activation of sympathetic nervous system and renin-angiotensin-aldosterone system may be exacerbated by low sodium intake,7,21 explaining the stronger association with mortality compared with robust participants. Another mechanism underlying the stronger association between low sodium intake and mortality highlighted in frail people may be related to the lower caloric intake that we found to be associated with low sodium excretion. Frailty and malnutrition are closely related,22,23 and frail people may have a reduced energy reserve that would make them more susceptible to low caloric intake, with consequent high risk of macronutrients and micronutrients deficits. These nutrients have a primary role in cellular metabolism but also in several biological processes,24 and their deficits are associated with higher risk of mortality, especially in the elderly.25
The strength of this study is that it is the first study that evaluates the prognostic role of sodium intake in the elderly using the 24-hour sodium excretion, the gold standard to estimate sodium intake. This is also the first study evaluating the role of frailty as an effect modifier of the relationship between sodium intake and clinical outcomes. Furthermore, our study represents a relative large sample of the real life community-dwelling elderly population, providing precious information on these persons that are generally excluded in large clinical trials. A limitation of this study is the lack of information of sodium excretion at follow-up. Furthermore, we did not exclude people affected by hypertension in the study of the relationship between sodium excretion and cardiovascular diseases and also those affected by cardiovascular diseases in the study of the relationship with mortality, with the risk of introducing a bias related to different dietary patterns due to medical prescriptions. However, we corrected our models for cardiovascular disease, hypertension, and systolic blood pressure. Furthermore, the analysis performed on nonhypertensive participants confirmed our results in the total sample, despite a loss of statistical significance. Finally, our results may be partially related to the reverse causation. Elderly persons may have a reduction of total caloric intake and, thus, sodium intake, in their last years of life. Therefore, the association between reduction of sodium intake and mortality may be related to a physiological reduction of sodium intake in the last years of life of these persons. However, the long follow-up time in frail persons may reduce this effect.
Conclusions
Reduced sodium excretion is associated with increased mortality in a sample of older community-dwelling people, especially among frail and those without hypertension or history cardiovascular disease. High levels of sodium excretion are not associated with adverse outcomes in this population; therefore, a sodium restriction might not be appropriated for older people, especially in those without cardiovascular disease.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Stamler J. The INTERSALT Study: Background, methods, findings, and implications. Am J Clin Nutr. 1997;65:626S–642S. doi: 10.1093/ajcn/65.2.626S. [DOI] [PubMed] [Google Scholar]
- 2.Selvaraj S, Djoussé L, Aguilar FG, et al. Association of estimated sodium intake with adverse cardiac structure and function: From the HyperGEN Study. J Am Coll Cardiol. 2017;70:715–724. doi: 10.1016/j.jacc.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strazzullo P, D’Elia L, Kandala N-B, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–2238. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 7.Graudal NA, Galløe AM, Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: A meta-analysis. JAMA. 1998;279:1383–1391. doi: 10.1001/jama.279.17.1383. [DOI] [PubMed] [Google Scholar]
- 8.Kalogeropoulos AP, Georgiopoulou VV, Murphy RA, et al. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: The Health, Aging, and Body Composition Study. JAMA Intern Med. 2015;175:410–419. doi: 10.1001/jamainternmed.2014.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean RM. Measuring population sodium intake: A review of methods. Nutrients. 2014;6:4651–4662. doi: 10.3390/nu6114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: The impact of frailty. Arch Intern Med. 2012;172:1162–1168. doi: 10.1001/archinternmed.2012.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 12.Bartali B, Turrini A, Salvini S, et al. Dietary intake estimated using different methods in two Italian older populations. Arch Gerontol Geriatr. 2004;38:51–60. doi: 10.1016/s0167-4943(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 13.Pisani P, Faggiano F, Krogh V, et al. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26:S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 15.Mente A, O’Donnell M, Rangarajan S, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: A pooled analysis of data from four studies. Lancet Lond Engl. 2016;388:465–475. doi: 10.1016/S0140-6736(16)30467-6. [DOI] [PubMed] [Google Scholar]
- 16.Graudal N, Jürgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: A meta-analysis. Am J Hypertens. 2014;27:1129–1137. doi: 10.1093/ajh/hpu028. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q. Sodium and potassium intake and mortality among US adults: Prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171:1183. doi: 10.1001/archinternmed.2011.257. [DOI] [PubMed] [Google Scholar]
- 18.Femminella GD, de Lucia C, Iacotucci P, et al. Neuro-hormonal effects of physical activity in the elderly. Front Physiol. 2013;4:378. doi: 10.3389/fphys.2013.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma S, Gupta M, Holmes DT, et al. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur Heart J. 2011;32:2135–2142. doi: 10.1093/eurheartj/ehr066. [DOI] [PubMed] [Google Scholar]
- 20.Benedict CR, Shelton B, Johnstone DE, et al. Prognostic significance of plasma norepinephrine in patients with asymptomatic left ventricular dysfunction. SOLVD Investigators. Circulation. 1996;94:690–697. doi: 10.1161/01.cir.94.4.690. [DOI] [PubMed] [Google Scholar]
- 21.Brunner HR, Laragh JH, Baer L, et al. Essential hypertension: Renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286:441–449. doi: 10.1056/NEJM197203022860901. [DOI] [PubMed] [Google Scholar]
- 22.Yannakoulia M, Ntanasi E, Anastasiou CA, Scarmeas N. Frailty and nutrition: From epidemiological and clinical evidence to potential mechanisms. Metabolism. 2017;68:64–76. doi: 10.1016/j.metabol.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Bonnefoy M, Berrut G, Lesourd B, et al. Frailty and nutrition: Searching for evidence. J Nutr Health Aging. 2015;19:250–257. doi: 10.1007/s12603-014-0568-3. [DOI] [PubMed] [Google Scholar]
- 24.Soukoulis V, Dihu JB, Sole M, et al. Micronutrient deficiencies an unmet need in heart failure. J Am Coll Cardiol. 2009;54:1660–1673. doi: 10.1016/j.jacc.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 25.McCullough PA, Fallahzadeh MK, Hegazi RM. Nutritional deficiencies and sarcopenia in heart failure: A therapeutic opportunity to reduce hospitalization and death. Rev Cardiovasc Med. 2016;17:S30–S39. doi: 10.3909/ricm17S1S004. [DOI] [PubMed] [Google Scholar]