Abstract
Benzalkonium chloride (BAC) and cetyl pyridinium chloride (CPC) are two of the most common household antiseptics, but show weaker efficacy against Gram-negative bacteria as well as against methicillin-resistant Staphylococcus aureus (MRSA) strains, relative to other S. aureus strains. We prepared 28 novel quaternary ammonium compounds (QACs) that represent a hybrid of these two structures, using 1- to 2-step synthetic sequences. The biscationic (bisQAC) species prepared show uniformly potent activity against six bacterial strains tested, with nine novel antiseptics displaying single-digit micromolar activity across the board. Effects of unequal chain lengths of two installed side chains had less impact than the overall number of side chain carbon atoms present, which was optimal at 22–25 carbons. This is further indication that simple refinements to multiQAC architectures can show improvement over current household antiseptics.
Keywords: antiseptics, benzalkonium chloride, cetyl pyridinium chloride, methicillin-resistant Staphylococcus aureus (MRSA), quaternary ammonium compounds
Household antiseptics represent a first line of defense against pathogenic bacteria. The home is full of common and well-known antiseptics, presented in an enormous variety of commercial products, including wipes, sprays, and concentrated solutions.[1] Many such antiseptics are cationic amphiphiles, deriving charge from quaternary ammonium groups, and bearing nonpolar long alkyl chains. Their positive charge and amphiphilic nature facilitates the attraction to, and disruption of bacterial cell membranes, respectively. Quaternary ammonium-based antiseptics have been commonplace for roughly a century,[2,3] and are only increasing in their application.
Household QACs are exemplified by benzalkonium chloride, developed in the 1930s,[4] which features a quaternary ammonium group bearing two methyl groups, a benzyl group, as well as a variable long-chained alkyl group, generally from 8–18 carbons long. While modest changes to this parent structure have been noted over time, including modification to add an ethyl group onto the aromatic moiety, as well as replacing the benzyl group with a decyl group (resulting in DDAC), it remains a workhorse of household antiseptic use.[1]
While BAC is present in select personal care applications, such as oral formulations for use against canker sores, even more common in personal hygiene products such as mouthwashes is cetyl pyridinium chloride (CPC), which uses a hexadecyl chain to quaternize a pyridine nitrogen. One can infer the relative lack of toxicity of these compounds, as few cases have been reported of overexposure to these structures, perhaps owing to their rapid clearance from the body.[1,5]
While these structures enjoy a privileged position in the household, their ability to eradicate bacteria is not without shortcoming. Both compounds show a diminished activity against Gram-negative bacteria, more noticeably for BAC; this is overcome by the use of relatively high concentrations of BAC in household formulations. Of greater concern is the diminished activity that each presents against methicillin-resistant Staphylococcus aureus (MRSA) strains, as compared to methicillin-susceptible S. aureus (MSSA) strains. Our research group has investigated the diminished activity of such mono-QACs against MRSA strains, endeavoring to uncover structural reasons for this phenomenon, or structure-resistance relationships.[6] In any case, it is becoming clear that MRSA strains showing resistance to QACs are rapidly increasing in prevalence in clinical settings;[7] we can no longer be complacent with the activity of BAC and CPC to eradicate pathogens in the household (Figure 1).
Figure 1.
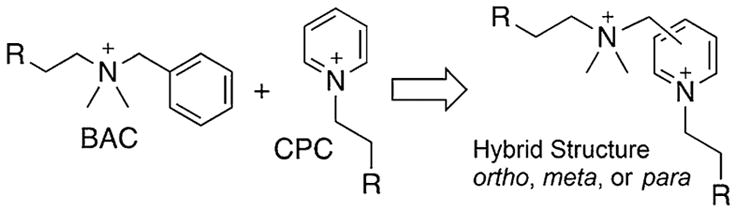
Conceptual development of hybrid bisQACs.
Our laboratories have endeavored to develop inexpensive and easily prepared alternatives to commercial antiseptics, with an eye on multicationic quaternary ammonium compounds (multiQACs).[8] Along with many other research groups,[9] we have observed superior antiseptic activity for such species, notably against MRSA strains as well as biofilms,[8j] which are noticeably recalcitrant against standard antiseptics. Improved bioactivity may result from superior attraction to the bacterial cell surface, enhanced membrane disruption, diminished susceptibility to bacterial resistance mechanisms, or a combination thereof. Herein, we report our progress to prepare bisQACs that represent a hybrid structure of BAC and CPC, to see if we could not only improve upon the bioactivity of these two, but also breathe new life into a set of structures that would not show diminished activity toward Gram-negative species or MRSA strains.
Our synthetic campaign toward such hybrid bisQACs aimed to take advantage of the three possible modes of superimposition of a “BAC residue” upon the pyridine ring of the “CPC residue”; we envisioned possible linkage at the 2, 3, or 4 positions of the pyridine ring. An inexpensive starting material was identified in 2-dimethylaminomethyl pyridine (Figure 2, top), and it showed the ability to alkylate once, strictly at the tertiary alkyl amine, in excellent yield. We dubbed the resultant “hybrid” QAC as o-Hy-16,0, indicating the pseudo-ortho nature of the substitution on the ring, as well as the attachment of one 16-carbon chain, but no second alkylation at the pyridine nitrogen. Disappointingly, a variety of conditions failed to subsequently alkylate at said pyridine nitrogen, likely owing to steric hindrance. Subsequently, we prepared and investigated the known and somewhat volatile 4-diethylaminomethyl pyridine[10] (Figure 2, middle), which again was limited to monoalkylation, this time at the pyridine nitrogen. In an attempt to extend the linker length separating the alkyl amine from the pyridine moiety and perhaps diminish steric encumbrance, we investigated the alkylation of 4-(2-diethylaminoethyl) pyridine, which simply led to decomposition under a variety of synthetic conditions.
Figure 2.
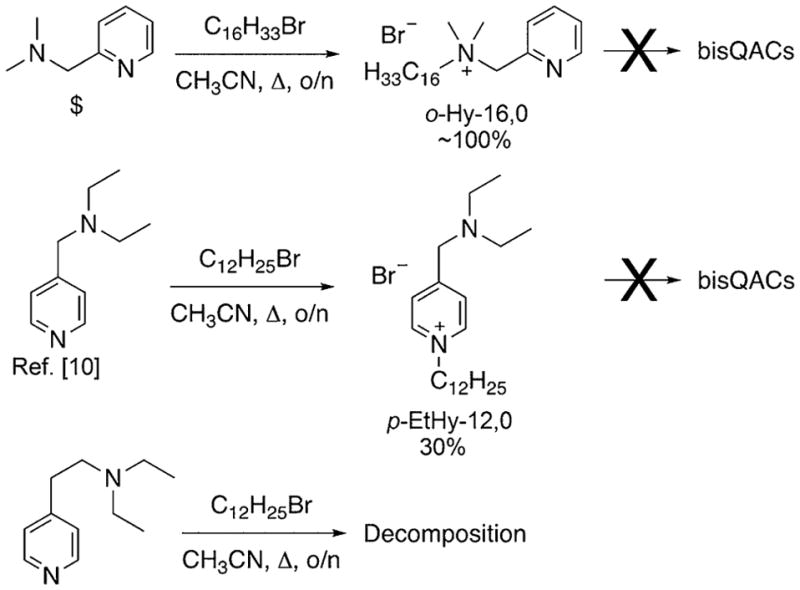
Failed attempts to prepare “hybrid” bisQAC structures.
Sensing that the placement of the tertiary alkyl amine at the ortho position led to excessive steric hindrance, as did the use of ethyl groups on the nitrogen, we identified an alternative launch point in the commercially available 3-dimethylamino-methyl pyridine (Scheme 1). We were delighted to find that bi-salkylation was successful and high-yielding for this bis-amine starting material, providing a set of “hybrid” bisQACs we dubbed m-Hy-n,n, wherein n represented the number of carbons on the attached alkyl chains. Bis-alkylation yields ranged from 54–89 %; pure products were obtained after triturations and completely characterized (see Supporting Information).
Scheme 1.
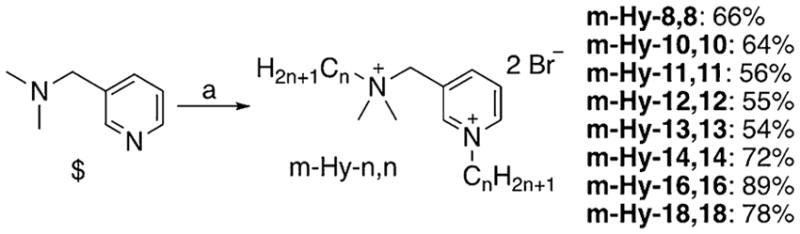
Synthesis of m-Hy-n,n bisQAC structures: a) CnH2n +1Br (2.5 equiv), CH3CN, Δ, 24 h.
To broaden the structures available in this hybrid structural class, we considered our previous insight from a bisQAC investigation where we noted that some modest levels of asymmetry in bis-QACs led to marginally improved antiseptic activity.[8k] We were unable, however, to effect a clean monoalkylation with the above conditions, even while limiting the alkyl bromide to a single equivalent. To our delight, we were able to effect a modest level of selectivity of alkylation when employing the corresponding alkyl iodide, which perhaps led to a less-soluble resultant monoQAC bearing the iodide counterion (Scheme 2); reaction proceeded at the alkylamine. Although we were not able to then bis-alkylate at the pyridine position using traditional alkyl electrophiles, we were able to use more reactive amide-substituted alkyl halide electrophiles to effect the preparation of bisQACs; we had used such electrophiles in a recent report,[8a] as they provided a level of designed instability in acidic conditions. As a result, we were able to prepare 16 more hybrid bisQACs, in variable yields that averaged 48 %. Purification of these structures took careful (and often, repeated) trituration with varying proportions of acetone/hexanes. Our naming scheme for the amide-containing side chains counts the total number of atoms in the chain, including the amide nitrogen and carbonyl carbon, for direct comparison with simple alkyl chains; the letter A is appended to the atom length to indicate the amide present.
Scheme 2.
Preparation of monoQACs and asymmetric bisQACs of the hybrid scaffold: a) CnH2n +1I (1.0 equiv), CH3CN, Δ, 24 h; b) CH3CN, Δ, 24 h.
With 28 novel mono- and bisQACs in hand, varying in both alkyl chain lengths and the nature of the substituent, we inspected both antimicrobial activity and toxicity, using red blood cell (RBC) lysis as a proxy. These assessments followed standard protocols employed by our group and others.[8,11] The complete set of MIC values against six bacteria [Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, community-acquired methicillin-resistant SA (USA300-0114), hospital-acquired methicillin-resistant SA (ATCC 33591)], along with red blood cell lysis (presented as lysis20, the highest concentration at which <20 % of RBCs are lysed), is presented in Table 1.
Table 1.
Antimicrobial activity (MIC) and red blood cell lysis data for amphiphiles; Gram-negative bacteria (E. coli and P. aeruginosa) are highlighted in red.[a]
| Series | Compound | Minimum Inhibitory Concentration (MIC) [μM] | Lysis20 [μM] | |||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | E. faecalis | E. coli | P. aeruginosa | USA300-0114 | ATCC 33591 | |||
| BAC | 8 | 8 | 32 | 63 | 32 | 16 | 63 | |
| CPC | 0.5 | 1 | 8 | 63 | 16 | 1 | 8 | |
| m-Hy-n,0 | m-Hy-10,0 | 32 | >250 | 125 | 250 | 32 | 63 | NT |
| m-Hy-12,0 | 63 | 250 | 125 | 250 | 63 | 125 | NT | |
| m-Hy-14,0 | 8 | 250 | 32 | >250 | 8 | 32 | 63 | |
| m-Hy-16,0 | 2 | 125 | 16 | 250 | 2 | 4 | 16 | |
| Hybrid BisQACs m-Hy-n,n | m-Hy-8,8 | 63 | >250 | >250 | 250 | 32 | 250 | NT |
| m-Hy-10,10 | 2 | 32 | 4 | 16 | 1 | 4 | 32 | |
| m-Hy-11,11 | 2 | 4 | 2 | 4 | 1 | 2 | 16 | |
| m-Hy-12,12 | 2 | 4 | 1 | 4 | 1 | 2 | 8 | |
| m-Hy-13,13 | 2 | 32 | 2 | 16 | 2 | 2 | 8 | |
| m-Hy-14,14 | 2 | 63 | 4 | 63 | 2 | 4 | 8 | |
| m-Hy-16,16 | 2 | 250 | 63 | 250 | 2 | 4 | 16 | |
| m-Hy-18,18 | 8 | >250 | 125 | >250 | 16 | 8 | 63 | |
| Asymmetric Hybrid BisQACS m-Hy-n,nA | m-Hy-10,11A | 2 | 8 | 2 | 8 | 1 | 2 | 32 |
| m-Hy-10,12A | 2 | 16 | 4 | 8 | 2 | 4 | 32 | |
| m-Hy-10,13A | 2 | 4 | 2 | 4 | 2 | 2 | 16 | |
| m-Hy-12,11A | 2 | 4 | 2 | 4 | 2 | 2 | 16 | |
| m-Hy-12,12A | 2 | 4 | 2 | 8 | 2 | 2 | 8 | |
| m-Hy-12,13A | 4 | 4 | 2 | 4 | 2 | 2 | 16 | |
| m-Hy-10,15A | 2 | 8 | 2 | 8 | 2 | 4 | 16 | |
| m-Hy-14,11A | 2 | 8 | 2 | 16 | 4 | 2 | 16 | |
| m-Hy-14,12A | 2 | 16 | 2 | 32 | 2 | 4 | 16 | |
| m-Hy-14,13A | 4 | 32 | 4 | 63 | 4 | 4 | 8 | |
| m-Hy-16,11A | 4 | 16 | 4 | 32 | 2 | 4 | 8 | |
| m-Hy-12,15A | 4 | 8 | 4 | 8 | 4 | 4 | 16 | |
| m-Hy-16,12A | 4 | 63 | 4 | 63 | 4 | 4 | 16 | |
| m-Hy-14,15A | 8 | 63 | 8 | 125 | 8 | 8 | 16 | |
| m-Hy-16,13A | 4 | 63 | 16 | 125 | 8 | 4 | 16 | |
| m-Hy-16,15A | 16 | >250 | 63 | >250 | 16 | 16 | 32 | |
NT =not tested. All MIC and Lysis20 data were acquired through compilation of the highest value of three independent trials; all trials were within one dilution.
Inspection of the bioactivity profile of the 28 novel hybrid antiseptics, in comparison with the commercial standards which inspired them, indicates some clear trends. The mono-cationic hybrid compounds showed uniformly modest bioactivity, roughly similar to the activity of BAC. CPC, however, showed strong bioactivity against Gram-positive strains, yet had both diminished activity against Gram-negative bacteria (e.g., MIC of 63 μM vs. P. aeruginosa) and community acquired MRSA (32-fold decrease in activity relative to MSSA), indicative of resistance pathways.[3,12] The amide side chain seemed slightly less effective than the alkyl counterpart, as evidenced by a comparison of m-Hy-12,12 to m-Hy-12,12A, though this might be outweighed by the desire for environmental degradation capabilities. Resistance was generally not observed in MRSA against the bisQAC structures, with the exception of a fourfold increase observed for the smallest bisQAC, m-Hy-8,8. This increased MIC against MRSA has been observed for similarly small multiQACs,[8b] but we cannot yet offer a hypothesis for what resistance mechanisms are in operation in this case.
The bisQACs displaying the strongest bioactivity (e.g., m-Hy-11,11, m-Hy-12,12) showed a remarkably consistent MIC level (1–4 μM) against the bacteria tested, with uniform activity against both Gram-positive and Gram-negative bacteria, as well as across the three S. aureus strains tested. In fact, nine different bisQACs showed single-digit MICs against all six strains tested, though BAC and CPC did not function at this level. It should be noted that longer chained bisQACs showed a diminished effect against select bacterial species, most notably E. faecalis and P. aeruginosa. While we often see diminished water solubility for our longest-chained compounds, the disparity of the activity across the board was intriguing; m-Hy-18,18 was both potent against three Staphylococcus strains (MIC =8–16 μM) and essentially inactive against E. faecalis and the two Gram-negative strains (MIC =125 to >250 μM).
Regarding the option of asymmetry that we designed into this set of compounds, we saw little differentiation, for example, in comparing m-Hy-12,15A to m-Hy-16,11A; only a fourfold difference was observed against P. aeruginosa. What we return to as a guiding principle is the total number of carbons on the side chains of our multiQACs, which favor 22–25 carbons as optimal substitution. m-Hy-12,12 might represent the best compound in this set, regarding ease of preparation, cost, and uniform activity against a panel of bacteria.
Overall, this work has successfully developed a series of bis-QACs that are structural hybrids of two of the most employed antiseptics in the modern household: BAC and CPC. The hybrids developed herein, exemplified by the easily prepared m-Hy-11,11 and m-Hy-12,12, display strong antimicrobial activity against a variety of bacteria, overcoming some inconsistencies of the current state-of-the-art. We will continue to develop novel and simple structures for potential use in the household and beyond.
Supplementary Material
Acknowledgments
This work was funded by the National Institute of General Medical Sciences (GM119426 to W.M.W.) and Villanova University.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting information (complete experimental details and compound characterization) and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/cmdc.201700597.
References
- 1.Walker EB, Paulson D. Quaternary Ammonium Compounds. Marcel Dekker; New York: 2002. [Google Scholar]
- 2.a) Jacobs WA. J Exp Med. 1916;23:563–568. doi: 10.1084/jem.23.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jacobs WA. J Exp Med. 1916;23:569–576. doi: 10.1084/jem.23.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Jacobs WA. J Exp Med. 1916;23:577–599. doi: 10.1084/jem.23.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennings MC, Minbiole KPC, Wuest WM. ACS Infect Dis. 2015;1:288–303. doi: 10.1021/acsinfecdis.5b00047. [DOI] [PubMed] [Google Scholar]
- 4.Domagk G. Dtsch Med Wiss. 1935;61:829–832. [Google Scholar]
- 5.a) Stookey GK, Beiswanger B, Mau M, Isaacs RL, Witt JJ, Gibb R. Am J Dent. 2005;18:24A–28A. [PubMed] [Google Scholar]; b) Lin GHY, Voss KA, Davidson TJ. Food Chem Toxicol. 1991;29:851–854. doi: 10.1016/0278-6915(91)90113-l. [DOI] [PubMed] [Google Scholar]; c) Deutschle T, Porkert U, Reiter R, Keck T, Riechelmann H. Toxicol In Vitro. 2006;20:1472–1477. doi: 10.1016/j.tiv.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Forman ME, Fletcher MH, Jennings MC, Duggan SM, Minbiole KPC, Wuest WM. ChemMedChem. 2016;11:958–962. doi: 10.1002/cmdc.201600095. [DOI] [PubMed] [Google Scholar]
- 7.Tezel U, Pavlostathis SG. Curr Opin Biotechnol. 2015;33:296–304. doi: 10.1016/j.copbio.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Allen RA, Jennings MC, Mitchell MA, Al-Khalifa SE, Wuest WM, Minbiole KPC. Bioorg Med Chem Lett. 2017;27:2107–2112. doi: 10.1016/j.bmcl.2017.03.077.Al-Khalifa SE, Jennings MC, Wuest WM, Minbiole KPC. ChemMedChem. 2017;12:280–283. doi: 10.1002/cmdc.201600546.Forman ME, Jennings MC, Wuest WM, Minbiole KPC. ChemMedChem. 2016;11:1401–1405. doi: 10.1002/cmdc.201600176.Minbiole KPC, Jennings MC, Ator LE, Black JW, Grenier MC, LaDow JE, Caran KL, Seifert K, Wuest WM. Tetrahedron. 2016;72:3559–3566.and references cited therein. Joyce MD, Jennings MC, Santiago CN, Fletcher MH, Wuest WM, Minbiole KPC. J Antibiot. 2016;69:344–347. doi: 10.1038/ja.2015.107.Mitchell MA, Iannetta AA, Jennings MC, Fletcher MH, Wuest WM, Minbiole KPC. ChemBioChem. 2015;16:2299–2303. doi: 10.1002/cbic.201500381.Jennings MC, Buttaro BA, Minbiole KPC, Wuest WM. ACS Infect Dis. 2015;1:304–308. doi: 10.1021/acsinfecdis.5b00032.Paniak TJ, Jennings MC, Shanahan PC, Joyce MD, Santiago CN, Wuest WM, Minbiole KPC. Bioorg Med Chem Lett. 2014;24:5824–5828. doi: 10.1016/j.bmcl.2014.10.018.Ator LE, Jennings MC, McGettigan AR, Paul JJ, Wuest WM, Minbiole KPC. Bioorg Med Chem Lett. 2014;24:3706–3709. doi: 10.1016/j.bmcl.2014.07.024.Jennings MC, Ator LE, Paniak TJ, Minbiole KPC, Wuest WM. ChemBioChem. 2014;15:2211–2215. doi: 10.1002/cbic.201402254.Black JW, Jennings MC, Azarewicz J, Paniak TJ, Grenier MC, Wuest WM, Minbiole KPC. Bioorg Med Chem Lett. 2014;24:99–102. doi: 10.1016/j.bmcl.2013.11.070.Grenier MC, Davis RW, Wilson-Henjum KL, LaDow JE, Black JW, Caran KL, Seifert K, Minbiole KPC. Bioorg Med Chem Lett. 2012;22:4055–4058. doi: 10.1016/j.bmcl.2012.04.079.
- 9.a) Imam T, Devinsky F, Lacko I, Mlynarcik D, Krasnec L. Pharmazie. 1983;38:308–310. doi: 10.1002/chin.198340149. [DOI] [PubMed] [Google Scholar]; b) Devinsky F, Lacko I, Mlynarcik D, Racansky V, Krasnec L. Tenside Deterg. 1985;22:10–15. [Google Scholar]; c) Zana R, Talmon Y. Nature. 1993;362:228–230. [Google Scholar]; d) Menger FM, Keiper JS. Angew Chem Int Ed. 2000;39:1906–1920. doi: 10.1002/1521-3773(20000602)39:11<1906::aid-anie1906>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2000;112:1980–1996. [Google Scholar]; e) El A-chouri M, Bensouda Y, Gouttaya HM, Nciri B, Perez L, Infante MR. Ten-side Surfactants Deterg. 2001;38:208–215. [Google Scholar]; f) Haldar J, Kondaiah P, Bhattacharya S. J Med Chem. 2005;48:3823–3831. doi: 10.1021/jm049106l. [DOI] [PubMed] [Google Scholar]; g) Vieira DB, Carmona-Ribeiro AM. J Antimicrob Chemother. 2006;58:760–767. doi: 10.1093/jac/dkl312. [DOI] [PubMed] [Google Scholar]; h) Brycki B. Pol J Microbiol. 2010;59:227–231. [PubMed] [Google Scholar]; i) Hoque J, Akkapeddi P, Yarlagadda V, Uppu DSSM, Kumar P, Haldar J. Langmuir. 2012;28:12225–12234. doi: 10.1021/la302303d. [DOI] [PubMed] [Google Scholar]
- 10.Heydari A, Khaksar S, Akbari J, Esfandyari M, Pourayoubi M, Tajbakhsh M. Tetrahedron Lett. 2007;48:1135–1138. [Google Scholar]
- 11.Approved Standard, 9th ed, CLSI Document M07-A9. 2. Vol. 32. Wayne, PA (USA): 2012. Methods for Dilution Antimicrobial Tests for Bacteria that Grow Aerobically. [Google Scholar]
- 12.Jennings MC, Forman ME, Duggan SM, Minbiole KPC, Wuest WM. ChemBioChem. 2017;18:1573–1577. doi: 10.1002/cbic.201700233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



