Abstract
The cortical-striatal brain circuitry is heavily implicated in drug-use. As such, the present study investigated the functional role of cortical-striatal circuitry in modulating alcohol self-administration. Given that a functional role for the nucleus accumbens core (AcbC) in modulating alcohol-reinforced responding has been established, we sought to test the role of cortical brain regions with afferent projections to the AcbC: the medial prefrontal cortex (mPFC) and the insular cortex (IC). Long-Evans rats were trained to self-administer alcohol (15% alcohol (v/v)+2% sucrose (w/v)) during 30 min sessions. To test the functional role of the mPFC or IC, we utilized a chemogenetic technique (hM4Di-Designer Receptors Exclusively Activated by Designer Drugs) to silence neuronal activity prior to an alcohol self-administration session. Additionally, we chemogenetically silenced mPFC➔AcbC or IC➔AcbC projections, to investigate the role of cortical-striatal circuitry in modulating alcohol self-administration. Chemogenetically silencing the mPFC decreased alcohol self-administration, while silencing the IC increased alcohol self-administration, an effect absent in mCherry-Controls. Interestingly, silencing mPFC➔AcbC projections had no effect on alcohol self-administration. In contrast, silencing IC➔AcbC projections decreased alcohol self-administration, in a reinforcer-specific manner as there was no effect in rats trained to self-administer sucrose (0.8%, w/v). Additionally, no change in self-administration was observed in the mCherry-Controls. Together these data demonstrate the complex role of the cortical-striatal circuitry while implicating a role for the insula-striatal circuit in modulating ongoing alcohol self-administration.
Keywords: alcohol, insular cortex, medial prefrontal cortex, nucleus accumbens core, reinforcement, self-administration
1. INTRODUCTION
Cortical-striatal brain circuitry has been heavily implicated in “top-down” control of attentional and inhibitory behavioral processes, particularly in relation to drug-use (Kalivas, 2008; Kim et al., 2017). To this end, the present work investigates the functional role of two cortical regions, the medial prefrontal cortex (prelimbic; mPFC) and the insular cortex (anterior; IC), and specifically the outgoing projections to the nucleus accumbens core (AcbC). The mPFC is necessary for several aspects of executive “top-down” control including action selection, behavioral inhibition, complex motor planning, and decision-making (Dalley et al., 2004). Additionally, the mPFC plays an important role in modulating numerous drug-related behaviors, as the majority of preclinical studies implicate mPFC activity in driving seeking of various drugs of abuse including alcohol self-administration (Faccidomo et al., 2016; Lei et al., 2016; Moorman et al., 2015; Tapocik et al., 2014). The second focus of the present study, the IC, is proposed to integrate internal and external stimuli into interoceptive states to drive motivated behavior (Craig, 2009; Paulus and Stewart, 2014), which is highly relevant to drug-use (Naqvi and Bechara, 2010; Paulus and Stewart, 2014). Moreover, preclinical studies demonstrate that pharmacological inhibition of the IC decreased alcohol self-administration (Pushparaj and Le Foll, 2015; albeit caudal IC), as well as other addiction-related behaviors (Droutman et al., 2015).
Both the mPFC and the IC send glutamatergic projections to the AcbC (Ding et al., 2001; Jaramillo et al., 2016; Seif et al., 2013; Wright and Groenewegen, 1996), a region within the ventral striatum implicated in modulating instrumental learning and motivated decision-making (Everitt et al., 1999; Salamone and Correa, 2012; Salamone et al., 2016). Furthermore, the AcbC is implicated in modulating motivational value to stimuli associated with reward (Meredith et al., 2008), such that incoming cortical information is integrated within the AcbC and results in a behavioral output. Thus, not surprisingly the AcbC has been shown to modulate aspects of drug-related behavior (Koob and Volkow, 2010). Moreover, lesions or pharmacological inactivation of AcbC have been shown to block the self-administration and reinstatement of drug-seeking of numerous drugs of abuse (Everitt and Robbins, 2005; Koob and Volkow, 2010). In regards to alcohol, the AcbC has been proposed to play a central role in modulating alcohol-seeking (Chaudhri et al., 2008; Chaudhri et al., 2010; Hodge and Cox, 1998) and the discriminative stimulus effects of alcohol (Besheer et al., 2003; Besheer et al., 2009; Hodge and Alken, 1996), likely through modulation via glutamatergic projections (Hwa et al., 2017). Furthermore, optogenetic silencing of mPFC or IC to AcbC projections (i.e., mPFC➔AcbC or IC➔AcbC) decrease shock-resistant alcohol self-administration, but not under non-shock conditions (Seif et al., 2013), thus implicating the mPFC➔AcbC and IC➔AcbC in modulating behavior dependent on a goal-directed internal-state following extensive alcohol history.
The goal of the present work was to test the functional role of the mPFC, IC, and the efferent projections to the AcbC in modulating maintenance of ongoing operant alcohol self-administration. As such, male Long Evans rats were trained to self-administer alcohol and a chemogenetic strategy (i.e., hM4Di Designer Receptors Exclusively Activated by Designer Drugs [DREADDs]) was implemented to silence the mPFC, IC, mPFC➔AcbC, and IC➔AcbC projections. Based on the existing literature regarding the mPFC and the IC, we hypothesized that chemogenetic silencing of these regions and the projections to the AcbC, would decrease alcohol-reinforced behavior. Given the distinct roles of the IC and mPFC in modulating behavior, the present study is important for understanding the cortical-striatal circuitry modulating the maintenance of alcohol self-administration.
2. MATERIALS AND METHODS
2.1. Animals
Male Long Evans rats (Harlan Sprague–Dawley, Indianapolis, IN) were double housed, in ventilated cages. For Experiments 2–3, rats were initially double housed and then individually housed following cannulae implantation surgery. Water and food were available ad libitum in the home cage. The colony room was maintained on a 12-h light/dark cycle, with lights on at 07:00. All experiments were conducted during the light cycle. Animals were under continuous care and monitoring by veterinary staff from the Division of Laboratory Animal Medicine (DLAM) at UNC-Chapel Hill. All procedures were conducted in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines.
2.2. Viral Vectors and Stereotaxic Coordinates
hM4D-DREADDs (AAV8-hSyn-DIO-hM4Di-mCherry; UNC Vector Core, NC [lot #4980D:Experiment 1.1 and 1.2, and lot#4980H:Experiment 2.1 and 2.2] or Addgene, MA [lot#v4331:Experiment 3.2]) or mCherry-Controls (AAV8-hSyn-DIO-mCherry; UNC Vector Core, NC [lot#4981CD:Experiment 1.1, 2.2 and 3.1]) previously described by (Krashes et al., 2011; Roth, 2016) were combined with Cre recombinase (AAV8-CMV-Cre-GFP; Vector Biolabs, PA or Addgene, MA) in a ratio of 7:3 (v/v) and bilaterally infused into the mPFC (2 μl/side; AP +3.2, ML ±0.6, DV −3.0 from skull) or IC (2 μl/side; AP +3.2, ML ±4.0, DV −6.0 from skull). This injection volume was selected based on a previous rat study that infused hM4D DREADDs into the mPFC (Kerstetter et al., 2016; 2μl/side), IC (albeit at a volume of 3 μl/side; Mizoguchi et al., 2015), and on previous studies in our lab (Jaramillo et al., 2017). This volume was necessary to ensure effective DREADD expression and is likely related to our approach in which two AAV viruses (i.e., DREADD+Cre) need to be co-administered (Smith et al., 2016). Stereotaxic coordinates were based on Paxinos and Watson (2007). The coordinates used for the IC are based on previous work (Cosme et al., 2015; Kesner and Gilbert, 2007; Pelloux et al., 2013) and our work showing projections from the IC to the AcbC (Jaramillo et al., 2017; Jaramillo et al., 2016); however, it is important to consider that there is likely anatomical overlap with the orbitofrontal cortex (Schoenbaum et al., 2006).
2.3. Microinjection Procedures for Viral Vectors and Drug Infusions
Site-specific microinjections were delivered by a microinfusion pump (Harvard Apparatus, MA) through 1.0 μl Hamilton syringes (Hamilton Robotic, NV) connected to 33-gauge injectors (Plastics One, VA) as described in (Besheer et al., 2014; Jaramillo et al., 2017; Jaramillo et al., 2016). For Experiment 1–3, anesthetized rats received bilateral microinjection of viral constructs into the mPFC or IC at a 0.2 μl/min flow rate across 10-min. Additionally, the injector was left in place for 10 min following the end of the 10 min infusion, as these are important strategies to further limit the spread of the viral injection (Smith et al., 2016). CNO microinjections were delivered in Experiment 2–3 through injectors extending 2 mm below the previously implanted (aimed to terminate 2 mm above the AcbC; AP +1.7, ML +1.5, DV −6.8 from skull), 26-gauge guide (Plastics One, VA) at a volume of 0.5 μl/side across 1 min. The injectors remained in place for an additional 2-min after the infusion to allow for diffusion.
2.4. Behavioral Training Procedures
2.4.1. Self-Administration Training
Rats were trained using the same two lever (i.e., active lever and inactive lever) chambers configured for self-administration and training procedures previously described in (Besheer et al., 2015; Randall et al., 2015). Self-administration sessions (30 min) took place 5 days/week (M−F) with active lever responses on a fixed ratio 2 (FR2) schedule of reinforcement such that every second response on the lever resulted in delivery of alcohol (0.1 ml) into a liquid receptacle. Responses on the inactive lever were recorded, but produced no programmed consequences. Locomotor activity was measured during the self-administration sessions by infrared photobeams that divided the behavioral chamber into 4 parallel zones. A sucrose fading procedure was used in which alcohol was gradually added to a 10% (w/v) sucrose solution. The exact order of exposure was as follows: 10% sucrose (w/v)/2% (v/v) alcohol (10S/2A), 10S/5A, 10S/10A, 5S/10A, 5S/15A, 2S/15A. There were one or two sessions at each concentration. Following sucrose fading, sweetened alcohol (2S/15A) was the reinforcer for the remainder of the study. Based on our previous findings using similar self-administration procedures, we typically observe moderate daily alcohol intake ranging from 0.5 to 0.8 g/kg (Besheer et al., 2013; Randall et al., 2015). Sucrose self-administration trained rats did not receive alcohol and were faded to 0.8% (w/v) sucrose. The exact order of sucrose fading was as follows: 10S, 5S, 2S, 1S, 0.5S, 0.3S, 0.8S, with one or two sessions at each concentration. The final sucrose concentration was 0.8% (w/v) because this concentration resulted in comparable lever responding as compared to the alcohol self-administration groups.
2.4.2. Self-administration Testing
For all experiments, viral vector surgeries (followed by a week of recovery) occurred immediately prior to or during acquisition of self-administration training. For Experiment 1, rats had approximately 2 months of alcohol self-administration training prior to testing. For Experiments 2–3, after 1–2 months of training following the viral vector surgery, animals received cannulae implantation surgery (followed by a week of recovery). Testing was only conducted following stable self-administration behavior, (i.e., defined as no change greater than 15% in the total number of responses during the session prior to testing). For each experiment, a repeated measures design was used such that each rat received each dose in a randomized order, with at least two training sessions between testing days.
2.5. Experimental Procedures
2.5.1. Experiment 1: Examination of the functional role of mPFC and IC on the maintenance of alcohol self-administration
2.5.1.1. mPFC-silencing
Rats trained to self-administer alcohol received bilateral infusions of hM4D-DREADDs (n=12) or mCherry-Controls (n=12) in the mPFC. To determine a functional role of the mPFC in modulating maintenance of alcohol self-administration, rats received CNO (0, 3 mg/kg, intraperitoneal [IP]), 45 min prior to a self-administration session.
2.5.1.2. IC-silencing
Rats trained to self-administer alcohol received bilateral infusions of hM4D-DREADDs (n=12) or mCherry-Controls (n=10) in the IC. To determine a functional role of the IC in modulating the maintenance of alcohol self-administration, rats received CNO (0, 3 mg/kg, IP), 45 min prior to a self-administration session.
2.5.2. Experiment 2: Examination of the functional role of IC➔AcbC and mPFC➔AcbC on the maintenance of alcohol self-administration
2.5.2.1. mPFC➔AcbC silencing
Self-administration trained rats were infused with hM4D-DREADDs (n=10) in the mPFC and implanted with bilateral AcbC cannulae. To determine a role for the mPFC➔AcbC projections, rats received intra-AcbC infusion of CNO (0, 3 μM/side) 5 min prior to a self-administration session.
2.5.2.2. IC➔AcbC silencing
Self-administration trained rats were infused with hM4D-DREADDs (n=10) in the IC and implanted with bilateral AcbC cannulae. To determine a role for the IC➔AcbC projections, rats received intra-AcbC infusion of CNO (0, 3 μM/side) 5 min prior to a self-administration session.
2.5.3. Experiment 3: Examination of the functional role of IC➔AcbC on self-administration, under control conditions
2.5.3.1. mCherry-Controls
To follow-up on findings from Experiment 2 implicating the IC➔AcbC circuit in alcohol self-administration, a mCherry-Control group (n=12) was run to examine potential nonspecific viral vector and CNO effects. Rats received intra-AcbC infusion of CNO (0, 3 μM/side) 5 min prior to a self-administration session.
2.5.3.2. Sucrose-Controls
To determine whether the reductions in alcohol self-administration following silencing of the IC➔AcbC (Experiment 2) were specific to the alcohol reinforcer, a group of rats was trained to self-administer sucrose, infused with hM4D-DREADDs in the IC, and implanted with bilateral AcbC cannulae (n=11). Rats received intra-AcbC infusion of CNO (0, 3 μM/side) 5 min prior to a sucrose self-administration session.
2.6. Tissue Preparation for Viral Vector and Cannulae Confirmation
Tissue collection, immunofluorescent and Nissl staining were similar as previously described in (Besheer et al., 2014; Jaramillo et al., 2016). The brain regions examined were the mPFC (AP: +4.2 to +3.2 mm; Experiment 1–2) or the IC (AP: +2.8 to +1.9 mm; Experiment 1–3), and the AcbC (AP: +2.3 to +1.3; Experiment 2–3) according to (Paxinos and Watson, 2007). Free-floating coronal sections (40 μm) were incubated in rabbit anti-DSRed (1:2,500; Clontech, CA) for 24 h at 4 °C. Sections were then incubated at RT in fluorescent conjugated secondary antibody (goat anti-rabbit 594; Life Technologies, MA). hM4D-mCherry or mCherry-Control expression was confirmed by immunofluorescence (individual expression represented as 20% opacity [Fig. 1a–b, 2a–b, 3a, 4a, 5a and 5f]) with a Nikon 80i Upright microscope (Nikon Instruments, NY) or Zeiss AxioZoom V16 (Carl Zeiss Inc., NY). For Experiment 2–3 cannulae placements were confirmed by Nissl staining and potential damage to the ventricles was also examined (injector placements represented by circles in Fig. 3b, 4b, 5b and 5g). Only rats with accurate viral injections and cannulae placements, and no ventricle damage, were included in the analyses and data presentation.
Figure 1. Chemogenetic silencing of mPFC decreases alcohol self-administration.
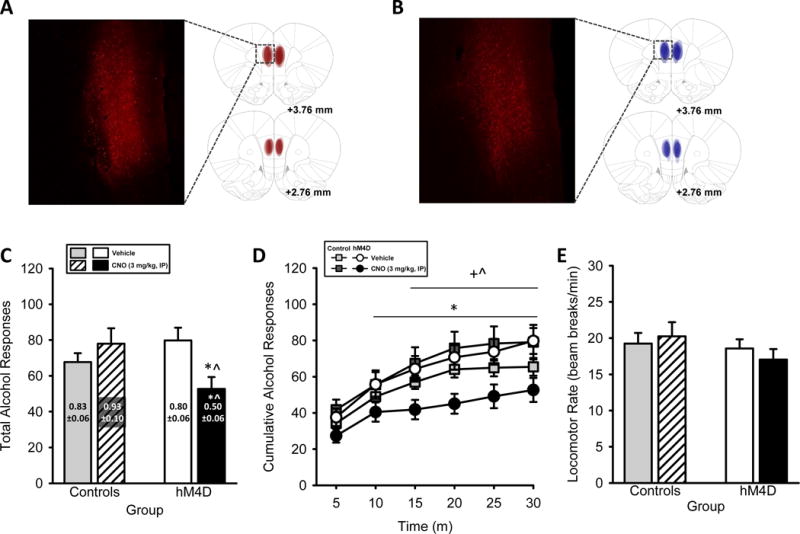
(A) Representative intra-mPFC hM4D-mCherry expression and (B) intra-mPFC mCherry-Control expression (2X, 1 mm scale bar) with schematic demonstrating individual bilateral expression in rats trained to self-administer alcohol. (C) Total session alcohol responses show decreased responding in the hM4D group, with decreased alcohol intake (g/kg, text on bars) following mPFC silencing. (D) The pattern of alcohol-reinforced responses across the self-administration session demonstrate decreased responses in the hM4D group after silencing the mPFC by CNO, beginning 10 min into the session (relative to vehicle), 15 min into the session relative to both m-Cherry control groups, and remaining decreased for the remainder of the session. (E) Locomotor rates were unaffected. *Significant difference from hM4D-vehicle, ^Significant difference from Control-CNO, +Significant difference from Control-vehicle (Tukey). Values on graphs represent mean ± S.E.M. (n=10–12/group; p≤0.05).
Figure 2. Chemogenetic silencing of IC increases alcohol intake in rats trained to self-administer alcohol.
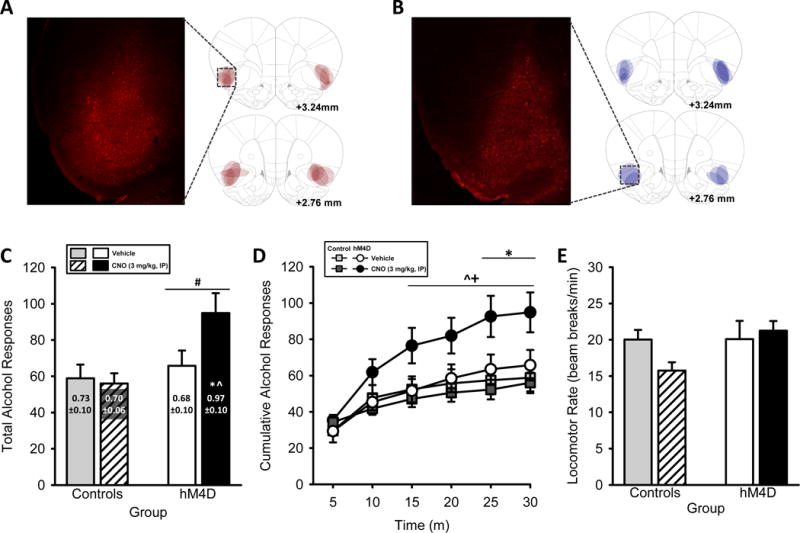
(A) Representative intra-IC hM4D-mCherry expression and (B) intra-IC mCherry-Control expression (2X, 1 mm scale bar) with schematic demonstrating individual bilateral expression in rats trained to self-administer alcohol. (C) Total session alcohol responses show increased responding in the hM4D group, with increased alcohol intake (g/kg, text on bars) following IC silencing. (D) The pattern of alcohol-reinforced responses across the self-administration session demonstrate potentiated responses in the hM4D group after silencing the IC by CNO, beginning 15 min into the session and remaining elevated for the remainder of the session. (E) Locomotor rates were unaffected. #Significant main effect of group (two-way RM ANOVA). #Significant main effect of group, *Significant difference from hM4D-vehicle, ^Significant difference from Control-CNO, +Significant difference from Control-vehicle (Tukey). Values on graphs represent mean ± S.E.M. (n=7–8/group; p≤0.05).
Figure 3. Chemogenetic silencing of mPFC➔AcbC projections does not affect alcohol self-administration.
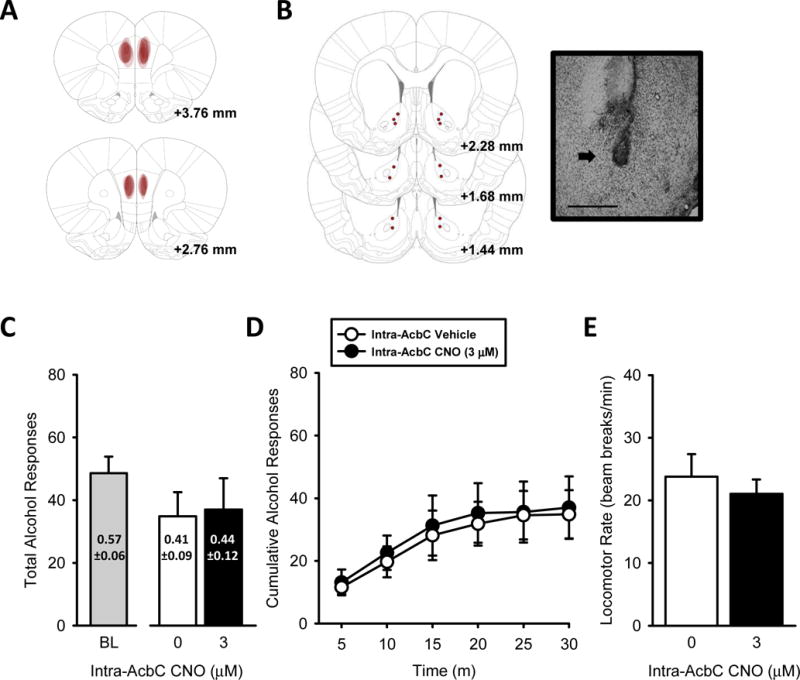
(A) Representative intra-mPFC hM4D-mCherry expression and (B) bilateral AcbC injector tip placements (depicted as red circles) from individual rats trained to self-administer alcohol, with corresponding photomicrograph (4X, 200 μm scale bar) inset showing an injector tip (arrow). (C) Total session alcohol responses and alcohol intake (g/kg, text on bars) were unchanged following mPFC➔AcbC silencing. (D) The pattern of alcohol-reinforced responses across the self-administration session was not affected by silencing mPFC➔AcbC projections with CNO. (E) Locomotor rates were unaffected. Baseline self-administration performance shown to the left of x-axis break. Values on graphs represent mean ± S.E.M. (n=7).
Figure 4. Chemogenetic silencing of IC➔AcbC projections decreases alcohol intake in rats trained to self-administer alcohol.
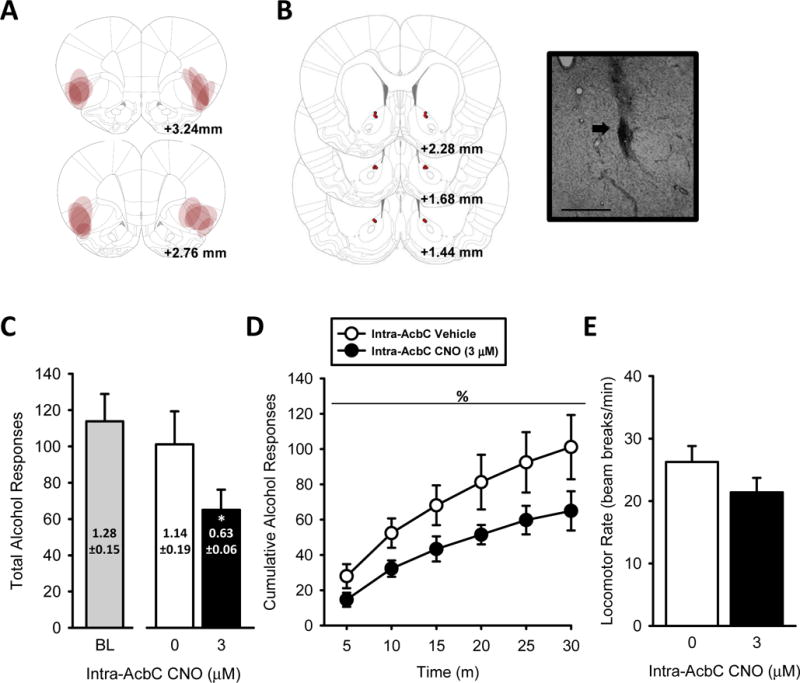
(A) Representative intra-IC hM4D-mCherry expression and (B) bilateral AcbC injector tip placements (depicted as red circles) from individual rats trained to self-administer alcohol, with corresponding photomicrograph (4X, 200 μm scale bar) inset showing an injector tip (arrow). (C) Total session alcohol responses show a trend (p=0.068) for increased responding, with increased alcohol intake (g/kg, text on bars) following IC➔AcbC silencing. (D) The pattern of alcohol-reinforced responses across the self-administration session demonstrate potentiated responses after silencing the IC➔AcbC by intra-AcbC CNO. (E) Locomotor rates were unaffected. Baseline self-administration performance shown to the left of x-axis break. *Significant difference from vehicle (paired t-test). %Significant main effect of intra-AcbC CNO (two-way RM ANOVA). Values on graphs represent mean ± S.E.M. (n=8; p≤0.05).
Figure 5. Intra-AcbC CNO treatment does not affect alcohol self-administration in controls or sucrose self-administration following chemogenetic silencing of IC➔AcbC projections.
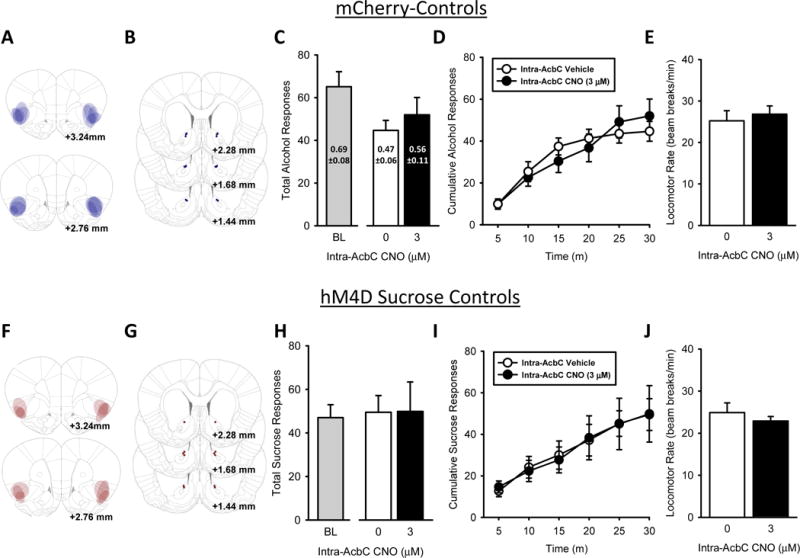
(A) Representative intra-IC mCherry-Control expression and (B) bilateral AcbC injector tip placements (depicted as blue circles) from individual rats trained to self-administer alcohol. (C) Total session alcohol responses, alcohol intake (g/kg, text on bars) show no effect of intra-AcbC CNO. (D) The pattern of alcohol-reinforced responses across the self-administration session (E) and locomotor rate were unaffected following intra-AcbC CNO. (F) Representative intra-IC hM4D-mCherry expression and (G) bilateral AcbC injector tip placements (depicted as red circles) from individual rats trained to self-administer sucrose. (H) Total session sucrose responses, sucrose intake (ml/kg, text on bars) show no effect of intra-AcbC CNO. (I) The pattern of alcohol-reinforced responses across the self-administration session (J) and locomotor rates were unaffected following intra-AcbC CNO. Baseline self-administration performance shown to the left of x-axis break. Values on graphs represent mean ± S.E.M. (n=6–8/group; p≤0.05).
2.7. Drugs
Alcohol [95 percent (w/v); Pharmco-AAPER, Shelbyville, KY, USA] was diluted in distilled water to 15 percent (v/v). For systemic administration CNO, injected at a volume of 1 ml/kg (NIDA Drug Supply Program), was dissolved in 1% dimethyl sulfoxide in water (v/v), or in aCSF for intracranial administration. The CNO doses were chosen based on pilot studies and previous work from our lab (Jaramillo et al., 2017) and others (Krashes et al., 2011; Roth, 2016; Stachniak et al., 2014).
2.8. Data Analysis
Alcohol intake (g/kg) was approximated based on body weight and number of reinforcements delivered. For Experiment 1 two-way analysis of variance (ANOVA) with DREADD condition as a between-subject factor and CNO dose as a within-subject factor, was used to analyze total alcohol lever responses, total inactive lever responses, alcohol intake, and locomotor rate. Three-way ANOVA with DREADD group as a between-subject factor, CNO treatment and time as within-subject factors, was used to analyze cumulative alcohol responses. For Experiments 2–3 paired t-tests were used to analyze total lever responses and locomotor rate. Two-way repeated measures ANOVA were used to analyze cumulative lever responses with CNO treatment and time as within-subject factors. Tukey post hoc analyses were used to explore significant main effects and interactions. Data are represented as means ± S.E.M. and significance was declared at p≤0.05.
3. RESULTS
3.1. Experiment 1: Examination of the functional role of mPFC and IC on maintenance of alcohol self-administration
3.1.1. mPFC-silencing
Two rats in the Control group had inefficient vector infusions (i.e., no mCherry expression) likely due to a clogged injector at the time of vector infusion. These rats are not included in any analyses or figures and the data presented in Figure 1 are based on hM4D-DREADD (n=12) and mCherry-Control (n=10) groups, with similar baseline self-administration performance (i.e., two sessions prior to initiation of testing): hM4D group – alcohol-reinforced responses: 74.2±6.0 alcohol reinforced responses, alcohol intake: 0.87±0.06 g/kg; mCherry-control group – alcohol-reinforced responses: 70.1±5.1, alcohol intake: 0.89 ± 0.05 g/kg. Representative hM4D-mCherry and mCherry-Control expression is represented in Figures 1A and 1B, respectively. Total session alcohol responses and total alcohol intake (g/kg; text on bars) are shown in Figure 1C. The two-way ANOVA on total alcohol responses showed a significant CNO by group interaction (F[1,20]=11.16, p<0.01), with a significant reduction in alcohol responses in the hM4D group following CNO relative to vehicle (p≤0.05) and relative to the Control group (p≤0.05). Furthermore, there was a significant CNO by group interaction on alcohol intake (F[1,20]=7.11, p<0.05), whereby CNO decreased intake in the hM4D group relative to vehicle (p≤0.05) and relative to the Control group (p≤0.05). There was no significant main effect of CNO or group. In examining the pattern of alcohol lever responses during the session (Figure 1D), the three-way ANOVA showed a significant main effect of time (F[5,190]= 94.26, p<0.05), and a CNO by group interaction (F[1,38] = 2.85, p<0.05). There was a significant three-way interaction (time X CNO X group) (F[5,190]=3.24, p<0.01), with attenuated alcohol responses across time in the hM4D group following CNO treatment beginning 10 min into the session and remaining for the remainder of the session relative to vehicle. Furthermore, CNO decreased responding in hM4D group relative to Control group (following both vehicle and CNO conditions) beginning at 15 min until the remainder of the session. These findings suggest that mPFC projections are at least partially involved in motivating alcohol self-administration as silencing these projections decreased alcohol responding. There were no effects of CNO on inactive lever responses (Table 1) or locomotor rate (Figure 1E) in either group, indicating that changes in self-administration were likely not due to locomotor suppression.
Table 1.
Total inactive lever responses following CNO-induced silencing
| Vehicle | CNO | |
|---|---|---|
|
|
||
| Experiment 1 | ||
| mPFC silencing | ||
| hM4D | 1.9±0.7 | 2.4±0.7 |
| mCherry-Controls | 1.8±0.9 | 3.7±2.3 |
| IC silencing | ||
| hM4D | 1.0±0.4 | 2.1±0.7 |
| mCherry-Controls | 0.7±0.1 | 0.7±0.1 |
| Experiment 2 | ||
| mPFC➔AcbC silencing | 0.4±0.2 | 0.1±0.1 |
| IC➔AcbC silencing | 1.1±0.5 | 0.6±0.3 |
| Experiment 3 | ||
| IC➔AcbC silencing | ||
| mCherry-Controls | 2.0±1.1 | 1.1±0.6 |
| Sucrose-Controls | 0.2±0.2 | 0.3±0.2 |
Data represent mean ± standard error of the mean (p≤0.05)
3.1.2. IC- silencing
Four rats had inefficient vector infusions (i.e., only unilateral expression; 3 in hM4D and 1 in Control group), and 3 rats had no mCherry expression (1 in hM4D and 2 in Control group), likely due to a clogged injector at the time of vector infusion. These rats are not included in any analyses or figures and the data presented in Figure 2 are based on hM4D-DREADD (n=8) and mCherry-Control (n=7) groups, with similar baseline self-administration performance (i.e., two sessions prior to initiation of testing): hM4D group – alcohol-reinforced responses: 77.8±7.6 ; alcohol intake: 0.8±0.1 g/kg; mCherry-Control group – alcohol-reinforced responses: 74.9±6.2; alcohol intake: 1.0±0.1 g/kg. Representative hM4D-mCherry and mCherry-Control expression is represented in Figures 2A and 2B, respectively. Total session alcohol responses and total alcohol intake (g/kg; text on bars) are shown in Figure 2C. The two-way ANOVA on total alcohol responses showed a main effect of group [F(1,13)=6.70, p≤0.02; Fig. 2C], with increased alcohol responses in the hM4D group. There was no significant main effect of CNO treatment or interaction. The two-way ANOVA of total alcohol intake demonstrated a significant CNO by group interaction [F(1,13)=5.10, p≤0.04; Fig. 2C text on bars], with increased alcohol intake following CNO in the hM4D group relative to vehicle (p≤0.05) and relative to the Control group (p≤0.05), showing that silencing the IC potentiated alcohol intake. There was no significant main effect of CNO or group. Figure 2D shows the pattern of alcohol-reinforced responses across the self-administration session. The three-way ANOVA of alcohol responses showed a significant main effect of time [F(5,65)=5.22, p<0.001; Fig. 2D], a significant main effect of CNO treatment [F(1,13)=58.58, p<0.001], and a CNO by time interaction [F(5,65)=7.88, p<0.001]. There was a significant main effect of group [F(1,13)=5.06, p≤0.04], a significant group by CNO interaction [F(1,13)=6.67, p≤0.02], and a trend for a group by time interaction [F(5,65)=3.62, p≤0.06]. There was a significant three-way interaction (time X CNO X group) [F(5,65)=2.81, p≤0.02], with elevated alcohol responses across time in the hM4D group following CNO treatment beginning 15 min into the session and remaining elevated for the remainder of the session relative to Control group (following vehicle and CNO conditions) and elevated relative to the hM4D under vehicle condition at 25 and 30 min (p<0.05). These data show that silencing the IC outgoing projections results in an increase in alcohol self-administration. Inactive lever responses (Table 1) and locomotor rate (Fig. 2E) were unchanged by CNO.
3.2. Experiment 2: Examination of the functional role of IC➔AcbC and mPFC➔AcbC on maintenance of alcohol self-administration
3.2.1. mPFC➔AcbC silencing
Three rats had inefficient vector infusions (i.e., no hM4D-mCherry expression), likely due to a clogged injector at the time of vector infusion. These rats are not included in any analyses or figures and the data presented in Figure 3 represent hM4D-DREADD (n=7). Representative hM4D-mCherry expression and AcbC injector tip placements are represented in Figure 3A and 3B, respectively. Total session alcohol responses and total alcohol intake (g/kg; text on bars) are shown in Figure 3C. Baseline self-administration performance is shown to the left of the x-axis break (Fig. 3C) as a visual reference (i.e., not included in overall analyses). Intra-AcbC CNO treatment did not affect total alcohol responses, alcohol intake, pattern of alcohol responding, inactive lever responses, or locomotor rate. These findings suggest that mPFC projections to AcbC do not directly modulate alcohol self-administration.
3.2.2. IC➔AcbC silencing
Two rats had inefficient vector infusions (i.e., no hM4D-mCherry expression), likely due to a clogged injector at the time of vector infusion. These rats are not included in any analyses or figures and the data presented in Figure 3 are based on hM4D-DREADD (n=8). Representative hM4D-mCherry expression and AcbC injector tip placements are represented in Figure 4A and 4B, respectively. Total session alcohol responses and total alcohol intake (g/kg; text on bars) are shown in Figure 4C. Baseline self-administration performance is shown to the left of the x-axis break (Fig. 4C) as a visual reference (i.e., not included in overall analyses). Intra-AcbC CNO did not affect total alcohol responses, albeit a trend was noted (p=0.068). However, total alcohol intake (g/kg) was significantly attenuated following CNO treatment [t(7)=2.67, p=0.03; Fig. 4C text on bars], indicating that silencing IC➔AcbC projections decreased alcohol intake. Examination of the pattern of alcohol-reinforced responses across the self-administration session (Figure 4D), with a two-way ANOVA, showed a significant main effect of time [F(5,35)=35.53, p≤0.001] and a significant main effect of CNO [F(1,7)=7.82, p≤0.03; Fig. 4D], with decreased alcohol responses following intra-AcbC CNO treatment. These results show that silencing the IC➔AcbC projections resulted in a reduction in alcohol self-administration. There was no significant time by CNO interaction. Inactive lever responses (Table 1) and locomotor rate (Fig. 4F) were unchanged by CNO.
3.3. Experiment 3: Examination of the functional role of IC➔AcbC on self-administration, under control conditions
3.3.1. mCherry-Controls
To follow up the significant findings showing IC➔AcbC silencing decreased alcohol self-administration, an additional group of rats trained to self-administer alcohol served as mCherry-Controls and were infused with mCherry in the IC and implanted with bilateral cannulae in the AcbC. Four rats had inefficient vector infusions (i.e., no mCherry expression), likely due to a clogged injector at the time of vector infusion. These rats are not included in any analyses or figures and the data presented in Figure 5A – 5E are based on mCherry-Controls (n=8). Representative mCherry-Control expression and AcbC injector tip placements are represented in Figure 5A and 5B, respectively. Baseline self-administration performance is shown to the left of the x-axis break (Fig. 5C) as a visual reference (i.e., not included in overall analyses). Total session alcohol responses and total alcohol intake (g/kg; text on bars) are shown in Figure 5C and demonstrate no significant effect of intra-AcbC CNO treatment. Figure 5d shows the pattern of alcohol-reinforced responses across the self-administration session. The two-way ANOVA of alcohol responses showed a main effect of time [F(5,35)=54.09, p≤0.001; Fig. 5D]. There was no main effect of intra-AcbC CNO treatment or interaction, suggesting no off-target effects of CNO in the non-DREADD expressing group. Inactive lever responses (Table 1) and locomotor rate (Fig. 5E) were unchanged by intra-AcbC CNO.
3.3.2. Sucrose-Controls
To examine if the reduction of alcohol self-administration following IC➔AcbC silencing was reinforcer-specific, a group of rats trained to self-administer sucrose were infused with hM4D-mCherry in the IC and implanted with cannulae in the AcbC. Three rats had inefficient vector infusions on one side (i.e., unilateral expression), and 2 rats had no hM4D-mCherry expression. These rats are not included in any analyses or figures and the data presented in Figure 5F – 5J are based on hM4D-mCherry (n=6). Representative hM4D-mCherry expression and AcbC injector tip placements are represented in Figure 5F and 5G, respectively. Baseline self-administration performance is shown to the left of the x-axis break (Fig. 5H) as a visual reference (i.e., not included in overall analyses). Total session sucrose responses are shown in Figure 5h and show no significant effect of intra-AcbC CNO treatment. Figure 5I shows the pattern of sucrose-reinforced responses across the self-administration session. The two-way ANOVA of sucrose responses showed a significant main effect of time [F(5,25)=14.97, p≤0.001; Fig. 5I]. There was no main effect of intra-AcbC CNO treatment or interaction, indicating that silencing the IC➔AcbC does not decrease sucrose self-administration. Inactive lever responses (Table 1) and locomotor rate (Fig. 5J) were unchanged by intra-AcbC CNO.
4. DISCUSSION
The present findings demonstrate that global silencing of the IC and mPFC (i.e., systemic CNO administration) and efferent projections to the AcbC differentially affected ongoing alcohol self-administration. First, silencing the mPFC decreased, while silencing the mPFC➔AcbC projections did not alter alcohol self-administration. Second, silencing the IC increased, while silencing IC➔AcbC projections decreased alcohol self-administration. Lastly, silencing IC➔AcbC projections did not affect sucrose self-administration, suggesting alcohol-reinforcer specificity. Together these findings demonstrate an important role for these cortical regions and their projections to the AcbC in modulating ongoing alcohol self-administration, and emphasize circuit-specificity in regulating the maintenance of ongoing alcohol self-administration.
4.1. Silencing the mPFC and mPFC➔AcbC projections
There is an extensive literature demonstrating a role for the mPFC in reinforcement learning and drug self-administration (Tzschentke, 2000; Van den Oever et al., 2010). Moreover, numerous studies have demonstrated the effects of lesions or inactivation of mPFC on drug-seeking and -intake (Di Pietro et al., 2006; LaLumiere and Kalivas, 2008; McFarland and Kalivas, 2001; Rocha and Kalivas, 2010; Seif et al., 2013). Consistent with these studies, we show that chemogenetic silencing of mPFC (i.e., following systemic CNO) decreased alcohol reinforced-responding. Due to the nature of this strategy (i.e., silencing the region as a whole with systemic CNO), this effect is likely the result of affecting both incoming and outgoing projections. For example, incoming dopaminergic afferents from ventral tegmental area that regulate glutamatergic pyramidal cells in mPFC are potentially affected which could have effects on alcohol intake. Indeed, it has been shown previously that differentially modulating dopamine D2 and D3 receptor signaling in mPFC decreases alcohol self-administration (Hodge et al., 1996; Samson and Chappell, 2003). Moreover, outgoing projections to nucleus accumbens would also be affected by silencing these cells as lesion/inhibition studies have demonstrated decreased glutamate release in AcbC (McFarland et al., 2004; McFarland et al., 2003). Together, these findings demonstrate that mPFC plays an important role in modulating behavioral output in response to drugs and drug-related cues.
In addition, although not explored in the present study, an important consideration when studying factors that can impact drinking is the influence of the pharmacological and interoceptive effects of alcohol. That is, pre-session administration of alcohol (e.g., preload) can decrease ongoing alcohol consumption and self-administration (Randall et al., 2015; Samson et al., 2002), which is evidence that rats can titrate intake based on the preload dose (Czachowski et al., 2003; Czachowski et al., 2006). Our lab has previously shown that preload of 1 g/kg alcohol decreases alcohol self-administration (Randall et al., 2015). Interestingly, pharmacological inhibition of the mPFC, fully substitutes for the interoceptive effects of alcohol (1 g/kg) (Jaramillo et al., 2016). To this end, a possible explanation for the decrease in alcohol self-administration is that silencing the mPFC produced interoceptive effects similar to an alcohol preload resulting in decreased self-administration.
mPFC➔AcbC circuity has been implicated in various aspects of drug-related behavior and is believed to modulate drug-seeking responses (Koob and Volkow, 2010). Our present findings showed that silencing this projection did not affect alcohol self-administration. Previous studies have shown that alcohol self-administration increases extracellular dopamine in both the mPFC (Doherty et al., 2016) and the AcbC (Doyon et al., 2003). This, considered in the context of our current findings, would suggest that independent dopamine release in AcbC in response to alcohol is sufficient to maintain alcohol self-administration in the absence of input from mPFC. However, Seif et. al., 2013 have demonstrated a functional role for mPFC➔AcbC projections in modulating compulsive (i.e., shock-resistant) alcohol self-administration via adaptations in NMDA receptors. Together this suggests that while mPFC➔AcbC activity is not necessary to maintain alcohol self-administration, enhanced recruitment of mPFC➔AcbC activity is necessary for alcohol self-administration under aversive conditions. Alternatively, these findings may suggest that mPFC➔AcbC may be more important for drug-seeking behavior under non-reinforced conditions (i.e., extinction). For example, Sparta et al., (2014) showed that inhibition of this projection did not affect consummatory behavior during reinforced training sessions, but enhanced the rate of extinction. Similarly, silencing this projection also blocks cue-induced reinstatement for cocaine and heroin (LaLumiere and Kalivas, 2008; Stefanik et al., 2013). An important consideration is that alcohol self-administration in this cohort of rats was generally lower than the other groups. Consequently, it is possible that under conditions with a lower rate of behavior it is more difficult to observe a CNO-induced reduction in self-administration behavior, than under conditions with a higher rate of behavior. Therefore, it will be important to replicate this finding in a cohort of animals with higher levels of self-administration. Taken together, our findings investigating the mPFC demonstrate that the region is involved in modulating alcohol intake; however, the role of its projections is less clear and may depend on the drinking behavior and/or the behavior being tested (i.e., alcohol-seeking vs. ongoing drinking).
4.2. Silencing the IC and IC➔AcbC projections
Previous work has found that inactivation of the caudal granular IC by intra-IC infusion of a muscimol+baclofen (GABAA agonist + GABAB agonist) cocktail reduced alcohol self-administration (Pushparaj and Le Foll, 2015). Interestingly, in the present work, we find an escalation in alcohol self-administration following chemogenetic silencing of the IC. One difference between the two studies are the techniques used to inactivate the IC. The use of pharmacological inhibition (GABA agonists) likely results in complete suppression of neural activity, whereas activation of the inhibitory DREADDs results in a reduction, not elimination of neural activity (Smith et al., 2016). Furthermore, the neuronal populations affected by each procedure may differ as pharmacological and chemogenetic manipulations are limited to neurons expressing GABAergic and DREADD receptors, respectively. To this end, it is possible, that some neuronal activity still occurred following chemogenetic silencing or two distinct neuronal populations exist in the IC which differentially regulate drinking. Another difference between the two studies is the targeted area of the IC. In the present work, manipulations were aimed at the anterior IC as this region has projections to the AcbC, as we have previously confirmed (Jaramillo et al., 2016). Accordingly, these findings suggest differences in the functional importance of the anatomical subregions of the IC. Indeed, interoceptive information (e.g., nociceptive, viscerosensory) initially integrated in the caudal granular IC is relayed and further integrated before reaching the anterior IC which is considered a high-order multimodal cortical region (Allen et al., 1991; Shi and Cassell, 1998). Further, previous work in the anterior IC (same coordinates as used in this work) has shown that pharmacological inhibition, does not fully substitute for alcohol (as observed in the mPFC), but rather induces partial alcohol-like interoceptive effects (Jaramillo et al., 2016) and chemogenetic silencing increases sensitivity to low alcohol doses (Jaramillo et al., 2017). Therefore, a possible explanation for the escalation in alcohol self-administration is that the partial alcohol-like effects induced by silencing the IC may have stimulated or primed further alcohol self-administration. To this end, it is interesting that silencing the IC➔AcbC projections decreased alcohol self-administration, as silencing this projection has been shown to fully substitute for the interoceptive effects of 1 g/kg alcohol and potentiate the effects of alcohol (Jaramillo et al., 2017). Further, there is evidence for an IC➔AcbC role in the execution of choice based on incentive value, as pharmacological disconnection of IC➔AcbC projections disrupts satiety-induced decreases in instrumental responding for food (Parkes et al., 2015). Interestingly, in another study investigating IC➔AcbC projections, optogenetic inhibition of IC➔AcbC circuitry decreased shock-resistant alcohol self-administration (e.g., compulsive), but had no effect on alcohol self-administration (i.e., under non-shock conditions; Seif et al., 2013). This latter outcome is in contrast to the present findings in which we observe a reduction in alcohol self-administration. This discrepancy could be attributed to differences in alcohol training as the intermittent alcohol drinking model, which produces aversion resistant intake through synaptic changes (Seif et al., 2013), was not utilized in the present study. However, this finding along with (Parkes et al., 2015) demonstrating a role for the IC➔AcbC in outcome devaluation, implicate a specific role of the insular-striatal projections in modulating goal-directed behavior in conditions under strong interoceptive control.
The findings that silencing the IC➔AcbC projections decreased alcohol self-administration, but not sucrose self-administration, suggests reinforcer specificity. The lack of effect on sucrose self-administration is consistent with self-administration studies, finding no effect on food self-administration or reinstatement of food-seeking following pharmacological inactivation of the posterior IC (Forget et al., 2010) or anterior IC (Cosme et al., 2015), respectively. However, the IC is implicated in cue-triggered food approach (Kusumoto-Yoshida et al., 2015) and taste processing (Carleton et al., 2010). Moreover, to our knowledge, the functional involvement of the IC➔AcbC pathway in sucrose or food self-administration has not been determined. While we did not find a change in sucrose self-administration following silencing of IC➔AcbC projections, it may be premature to conclude that this circuit is not involved in regulating sucrose self-administration, as it is possible that this circuit may be recruited at a different sucrose concentration, reinforcement schedule, or under extinction conditions. Furthermore, it has been shown that IC➔AcbC inhibition decreases intake of alcohol adulterated with the bitter tastant quinine (Seif et al., 2013), which provides evidence for an insular-striatal role in taste under conditions of conflict. Lastly, considering that silencing the IC➔AcbC produces alcohol-like interoceptive effects (Jaramillo et al., 2017), within this framework, it is interesting to note that sucrose self-administration was unchanged in alcohol-naïve rats. This suggests that alcohol-like effects (induced by IC➔AbC silencing) do not alter ongoing sucrose self-administration and may depend on previous experience with the pharmacological effects of alcohol (i.e., alcohol experienced).
Silencing the IC and the IC➔AcbC projections produced opposite effects (increase and decrease, respectively) on self-administration, suggesting the functional role for the IC➔AcbC projections to reduce alcohol self-administration was overshadowed following general silencing of all IC outgoing projections. Together these findings suggest that suppression of neuronal activity within this IC➔AcbC circuit is, in part, sufficient to terminate ongoing alcohol self-administration, whereas it is likely that other IC-related circuits may be more prominent to drive escalations in alcohol self-administration. Accordingly, it will be important for future work to investigate other IC-related circuits that may promote escalations in alcohol self-administration (e.g., IC projections to amygdala, mPFC). Although strategies were taken to localize chemogenetic manipulations to the IC (and the mPFC), the potential for the functional contribution of neighboring regions needs to be considered. For example, given the close proximity of the piriform cortex, primary somatosensory cortex, and orbitofrontal cortex (a region often encompassed in IC studies) to the IC, it is possible that neuronal populations within these regions were recruited following systemic CNO administration.
4.3. Consideration of the mCherry controls
Inclusion of the mCherry-Control group is an important feature of the present work. Silencing the IC or mPFC did not disrupt alcohol self-administration behavior in the mCherry-Control group, indicating that the increases and decreases in self-administration, respectively, in the hM4D groups were not due to non-specific effects of systemic CNO pretreatment. These are important findings as CNO, a major metabolite of the anti-psychotic drug clozapine, can convert to clozapine and therefore have biological effects which may depend on animal, strain, and CNO dose (Chang et al., 1998; Gomez et al., 2017; Jann et al., 1994; MacLaren et al., 2016). Additionally, clozapine can serve as a discriminative stimulus in drug discrimination experiments (Goudie et al., 1998; Prus et al., 2016) and has been shown to decrease alcohol-stimulated activity (Thrasher et al., 1999). Of direct relevance to the present work are findings that a high dose of clozapine (12 mg/kg/day) decreased home cage alcohol consumption, but did not affect the maintenance of alcohol consumption in alcohol-preferring rats (Chau et al., 2013). Further, clozapine has also been shown to decrease home cage alcohol drinking in Syrian golden hamsters (Chau et al., 2010; Green et al., 2004). Thus, the inclusion of these CNO-only control groups (i.e., mCherry-Controls) and the absence of behavioral effects within the context of this study are highly relevant. Although a sucrose self-administration mCherry-Control group was not tested in the present study, the lack of a behavioral effect following intra-AcbC CNO in the sucrose self-administration trained rats suggests that CNO did not have a general effect on behavior. However, future studies testing the effects of intra-AcbC CNO on sucrose self-administration in mCherry-Controls will be important.
4.4. Conclusions
Here, we demonstrate differential roles for the mPFC and IC and their projections to the AcbC in regulating ongoing self-administration. These cortical projections are predominantly glutamatergic, which is highly relevant given the extent of glutamatergic adaptations during different phases of the development and progression of alcohol use disorder (Hwa et al., 2017). Accordingly, while rats in this self-administration model have an extensive alcohol drinking history, it will be important for future work to determine whether these circuits show differential recruitment following alcohol dependence. Further, it will be important to examine the contribution of these circuits in relapse-like behavior. In summary, the present findings demonstrate a role of cortical-striatal circuits involving the mPFC and IC and the AcbC, while showing that suppression of the insular-striatal circuit decreases alcohol-reinforced behavior.
Highlights.
mPFC silencing decreased alcohol self-administration
No effect following mPFC➔AcbC silencing
IC silencing increased alcohol self-administration
IC➔AcbC silencing decreased alcohol self-administration
Acknowledgments
This work was supported, in part, by the National Institute of Health [AA019682, AA011605, F31AA024973, F32AA024674]; the National Science Foundation [DGE-1144081], and by the Bowles Center for Alcohol Studies. The authors would like to thank the NIDA Drug Supply Program for providing the CNO.
Abbreviations
- AcbC
nucleus accumbens core
- CNO
clozapine-N-oxide
- DREADDs
designer receptors exclusively activated by designer drugs
- IC
insular cortex
- mPFC
medial prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- Besheer J, Cox AA, Hodge CW. Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res. 2003;27:450–456. doi: 10.1097/01.ALC.0000057036.64169.C1. [DOI] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Jaramillo AA, Frisbee S, Cannady R. Stress hormone exposure reduces mGluR5 expression in the nucleus accumbens: functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology. 2014;39:2376–2386. doi: 10.1038/npp.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Lindsay TG, Cannady R. Transient increase in alcohol self-administration following a period of chronic exposure to corticosterone. Neuropharmacology. 2013;72:139–147. doi: 10.1016/j.neuropharm.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Frisbee S, Randall PA, Jaramillo AA, Masciello M. Gabapentin potentiates sensitivity to the interoceptive effects of alcohol and increases alcohol self-administration in rats. Neuropharmacology. 2015;101:216–224. doi: 10.1016/j.neuropharm.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Salling MC, Spanos M, Stevenson RA, Hodge CW. Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci. 2009;29:9582–9591. doi: 10.1523/JNEUROSCI.2366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton A, Accolla R, Simon SA. Coding in the mammalian gustatory system. Trends Neurosci. 2010;33:326–334. doi: 10.1016/j.tins.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WH, Lin SK, Lane HY, Wei FC, Hu WH, Lam YW, Jann MW. Reversible metabolism of clozapine and clozapine N-oxide in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:723–739. doi: 10.1016/s0278-5846(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Chau DT, Gulick D, Xie H, Dawson R, Green AI. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58:351–356. doi: 10.1016/j.neuropharm.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau DT, Khokhar JY, Dawson R, Ahmed J, Xie H, Green AI. The comparative effects of clozapine versus haloperidol on initiation and maintenance of alcohol drinking in male alcohol-preferring P rat. Alcohol. 2013;47:611–618. doi: 10.1016/j.alcohol.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci. 2008;28:2288–2298. doi: 10.1111/j.1460-9568.2008.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosme CV, Gutman AL, LaLumiere RT. The Dorsal Agranular Insular Cortex Regulates the Cued Reinstatement of Cocaine-Seeking, but not Food-Seeking, Behavior in Rats. Neuropsychopharmacology. 2015;40:2425–2433. doi: 10.1038/npp.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Assessment of sucrose and ethanol reinforcement: the across-session breakpoint procedure. Physiol Behav. 2003;78:51–59. doi: 10.1016/s0031-9384(02)00963-0. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Prutzman S, DeLory MJ. Volume and dose effects of experimenter-administered ethanol preloads on ethanol seeking and self-administration. Alcohol. 2006;40:35–40. doi: 10.1016/j.alcohol.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Ding DC, Gabbott PL, Totterdell S. Differences in the laminar origin of projections from the medial prefrontal cortex to the nucleus accumbens shell and core regions in the rat. Brain Res. 2001;917:81–89. doi: 10.1016/s0006-8993(01)02912-2. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Schier CJ, Vena AA, Dilly GA, Gonzales RA. Medial Prefrontal Cortical Dopamine Responses During Operant Self-Administration of Sweetened Ethanol. Alcohol Clin Exp Res. 2016;40:1662–1670. doi: 10.1111/acer.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Droutman V, Read SJ, Bechara A. Revisiting the role of the insula in addiction. Trends Cogn Sci. 2015;19:414–420. doi: 10.1016/j.tics.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Reid GT, Agoglia AE, Ademola SA, Hodge CW. CaMKII inhibition in the prefrontal cortex specifically increases the positive reinforcing effects of sweetened alcohol in C57BL/6J mice. Behav Brain Res. 2016;298:286–290. doi: 10.1016/j.bbr.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010;68:265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie AJ, Smith JA, Taylor A, Taylor MA, Tricklebank MD. Discriminative stimulus properties of the atypical neuroleptic clozapine in rats: tests with subtype selective receptor ligands. Behav Pharmacol. 1998;9:699–710. doi: 10.1097/00008877-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Green AI, Chau DT, Keung WM, Dawson R, Mesholam RI, Schildkraut JJ. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128:9–20. doi: 10.1016/j.psychres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Alken AS. Discriminative stimulus function of ethanol: role of GABAA receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1996;20:1221–1228. doi: 10.1111/j.1530-0277.1996.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1996;20:1631–1638. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139:95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- Hwa L, Besheer J, Kash T. Glutamate plasticity woven through the progression to alcohol use disorder: a multi-circuit perspective. F1000Res. 2017;6:298. doi: 10.12688/f1000research.9609.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann MW, Lam YW, Chang WH. Rapid formation of clozapine in guinea-pigs and man following clozapine-N-oxide administration. Arch Int Pharmacodyn Ther. 1994;328:243–250. [PubMed] [Google Scholar]
- Jaramillo AA, Agan VE, Makhijani VH, Pedroza S, McElligott ZA, Besheer J. Functional role for suppression of the insular-striatal circuit in modulating interoceptive effects of alcohol. Addict Biol. 2017 doi: 10.1111/adb.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Frisbee S, Besheer J. Modulation of sensitivity to alcohol by cortical and thalamic brain regions. Eur J Neurosci. 2016;44:2569–2580. doi: 10.1111/ejn.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Wunsch AM, Nakata KG, Donckels E, Neumaier JF, Ferguson SM. Corticostriatal Afferents Modulate Responsiveness to Psychostimulant Drugs and Drug-Associated Stimuli. Neuropsychopharmacology. 2016;41:1128–1137. doi: 10.1038/npp.2015.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE. The role of the agranular insular cortex in anticipation of reward contrast. Neurobiol Learn Mem. 2007;88:82–86. doi: 10.1016/j.nlm.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Yun K, Kim JH. Alterations in Striatal Circuits Underlying Addiction-Like Behaviors. Mol Cells. 2017;40:379–385. doi: 10.14348/molcells.2017.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumoto-Yoshida I, Liu H, Chen BT, Fontanini A, Bonci A. Central role for the insular cortex in mediating conditioned responses to anticipatory cues. Proc Natl Acad Sci U S A. 2015;112:1190–1195. doi: 10.1073/pnas.1416573112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu JH, Mototake A, Hu B, Hopf FW. Nucleus Accumbens Shell and mPFC but Not Insula Orexin-1 Receptors Promote Excessive Alcohol Drinking. Front Neurosci. 2016;10:400. doi: 10.3389/fnins.2016.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD. Clozapine N-Oxide Administration Produces Behavioral Effects in Long-Evans Rats: Implications for Designing DREADD Experiments. eNeuro. 2016:3. doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi H, Katahira K, Inutsuka A, Fukumoto K, Nakamura A, Wang T, Nagai T, Sato J, Sawada M, Ohira H, Yamanaka A, Yamada K. Insular neural system controls decision-making in healthy and methamphetamine-treated rats. Proc Natl Acad Sci U S A. 2015;112:E3930–3939. doi: 10.1073/pnas.1418014112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2015;1628:130–146. doi: 10.1016/j.brainres.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes SL, Bradfield LA, Balleine BW. Interaction of insular cortex and ventral striatum mediates the effect of incentive memory on choice between goal-directed actions. J Neurosci. 2015;35:6464–6471. doi: 10.1523/JNEUROSCI.4153-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76(Pt B):342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press/Elsevier; Amsterdam; Boston: 2007. [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ. Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur J Neurosci. 2013;38:3018–3026. doi: 10.1111/ejn.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prus AJ, Wise LE, Pehrson AL, Philibin SD, Bang-Andersen B, Arnt J, Porter JH. Discriminative stimulus properties of 1.25mg/kg clozapine in rats: Mediation by serotonin 5-HT2 and dopamine D4 receptors. Brain Res. 2016;1648:298–305. doi: 10.1016/j.brainres.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Pushparaj A, Le Foll B. Involvement of the caudal granular insular cortex in alcohol self-administration in rats. Behav Brain Res. 2015;293:203–207. doi: 10.1016/j.bbr.2015.07.044. [DOI] [PubMed] [Google Scholar]
- Randall PA, Jaramillo AA, Frisbee S, Besheer J. The role of varenicline on alcohol-primed self-administration and seeking behavior in rats. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Pardo M, Yohn SE, Lopez-Cruz L, SanMiguel N, Correa M. Mesolimbic Dopamine and the Regulation of Motivated Behavior. Curr Top Behav Neurosci. 2016;27:231–257. doi: 10.1007/7854_2015_383. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A. Dopaminergic involvement in medial prefrontal cortex and core of the nucleus accumbens in the regulation of ethanol self-administration: a dual-site microinjection study in the rat. Physiol Behav. 2003;79:581–590. doi: 10.1016/s0031-9384(03)00126-4. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A, Legg B. Effects of self-administered alcohol or sucrose preloads on subsequent consumption in the rat. J Stud Alcohol. 2002;63:107–113. [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399:440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV. DREADDS: Use and application in behavioral neuroscience. Behav Neurosci. 2016;130:137–155. doi: 10.1037/bne0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Hovelso N, Mason AO, Kantak PA, Ung RL, Decot HK, Stuber GD. Activation of prefrontal cortical parvalbumin interneurons facilitates extinction of reward-seeking behavior. J Neurosci. 2014;34:3699–3705. doi: 10.1523/JNEUROSCI.0235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus–>midbrain pathway for feeding behavior. Neuron. 2014;82:797–808. doi: 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, LaLumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, Barbier E, Flanigan M, Solomon M, Pincus A, Pilling A, Sun H, Schank JR, King C, Heilig M. microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci. 2014;34:4581–4588. doi: 10.1523/JNEUROSCI.0445-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher MJ, Freeman PA, Risinger FO. Clozapine’s effects on ethanol’s motivational properties. Alcohol Clin Exp Res. 1999;23:1377–1385. [PubMed] [Google Scholar]
- Tzschentke TM. The medial prefrontal cortex as a part of the brain reward system. Amino Acids. 2000;19:211–219. doi: 10.1007/s007260070051. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35:276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ. Patterns of overlap and segregation between insular cortical, intermediodorsal thalamic and basal amygdaloid afferents in the nucleus accumbens of the rat. Neuroscience. 1996;73:359–373. doi: 10.1016/0306-4522(95)00592-7. [DOI] [PubMed] [Google Scholar]


