Abstract
Aims: It has been shown that supplementation of patients’ sera that contains sperm‐immobilizing antibodies results in failure of fertilization and embryo development in vitro. The present study was carried out to investigate if exposing retrieved eggs to a high number of sperm‐immobilizing antibodies in the follicular fluid (FF) in vivo affected subsequent fertilization and embryo development in vitro, even if they were washed with an antibody‐free culture medium.
Methods: Patients’ sera and their FF were collected in 15 in vitro fertilization‐embryo transfer (IVF‐ET) or intracytoplasmic sperm injection‐embryo transfer (ICSI‐ET) treatment cycles from 11 infertile women with sperm‐immobilizing antibodies in their sera. Quantitative sperm‐immobilizing antibody titers (SI50 titers) in the sera and FF were evaluated. The fertilization rate, good‐quality embryo rate and implantation rate by IVF‐ET were compared between infertile patients having higher (10≤) SI50 titers and lower (<10) SI50 titers in their FF.
Results: There was a significant correlation in the SI50 titers between the patients’ sera and their FF (P < 0.0001). After thoroughly washing the collected eggs in culture medium without the patient's serum before IVF, there was no difference in the fertilization rate in the patients with high (10≤) and low (<10) SI50 titers in their FF (P = 0.62). However, the good‐quality embryo rate in the patients with a high SI50 titer was significantly lower than patients with a low antibody titer (P < 0.05). There was no significant difference in the implantation rate between the two groups (P = 0.33).
Conclusions: Similar amounts of sperm‐immobilizing antibodies existed in the patients’ FF and in their sera. ICSI did not seem to be necessary in patients having the antibodies if their sera were not supplemented in the culture media. Even with careful manipulation of eggs, it might be suggested that the harmful effects of sperm‐immobilizing antibodies on embryo development cannot be completely avoided, especially in patients with high SI50 titers in the FF. (Reprod Med Biol 2006; 5: 137–143)
Keywords: antisperm antibody, embryo development, female infertility, follicular fluid, in vitro fertilization, sperm‐immobilizing antibody
INTRODUCTION
IMMUNOLOGICAL CAUSES OF infertility, such as antisperm antibodies in both males and females, are among the most frustrating pathologies for conventional treatments with timed intercourse or intrauterine insemination (IUI). The incidence of infertile women having sperm‐immobilizing antibodies, detected by the sperm immobilization test (SIT), 1 in their sera has been shown to be approximately 3% when it is tested for in female patients first visiting infertility outpatient clinics. 2
The presence of antibodies against sperm, especially sperm‐immobilizing antibodies, in the sera of infertile women has been shown to inhibit sperm migration in the female genital tract, including cervical mucus 3 and the fallopian tubes. 4 The antibodies result in poor postcoital testing or refractory response to the treatments with IUI. However, some infertile women who have sperm‐immobilizing antibodies in their sera establish pregnancy without the use of in vitro fertilization‐embryo transfer (IVF‐ET) treatment when the antibody titers are relatively low at the time of conception. 5 , 6 Kobayashi et al. found that the antibody titers evaluated by a quantitative SIT (SI50; the 50% sperm immobilization units), as described by Isojima et al., 7 correlated to possible conception by IUI. 6 They concluded that infertile women with SI50 titers lower than 10 could conceive by IUI, whereas those with antibody titers higher than 10 could not. Thereafter, the cut‐off value of 10 for SI50 titers was used when a clinical decision for treatment was made.
Such antibodies can also exert inhibitory effects on various stages of sperm–egg interaction 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 and subsequent embryo development in vitro. 14 , 15 To avoid low fertilization rates and poor embryo quality in vitro, it is recommended that the manipulation of gametes and embryos from patients having sperm‐immobilizing antibodies should be carried out more carefully than usual. Thorough washing of eggs collected in the culture medium to prohibit contamination with the patient's serum and follicular fluid (FF) should be carried out in order to obtain a better IVF result. 6 , 14 , 16 However, it has not yet been clarified whether high titers of sperm‐immobilizing antibody in FF affect fertilization and embryo development in vitro, even when the eggs are treated as mentioned above. If fertilization is inhibited by the existence of a higher number of sperm‐immobilizing antibodies, intracytoplasmic sperm injection (ICSI) should be theoretically carried out.
The present study was carried out to investigate if exposing the retrieved eggs to a high number of sperm‐immobilizing antibodies in the follicular fluid (FF) in vivo affected subsequent fertilization and embryo development in vitro, even if they were washed with the antibody‐free culture medium.
MATERIALS AND METHODS
Patients’ sera
A TOTAL OF 1020 infertile women between May 1999 and December 2004 at the Department of Obstetrics and Gynecology, School of Medicine, Jichi Medical University, Japan, were tested for sperm‐immobilizing antibodies in their sera as described below. Twenty‐seven of 1020 infertile women had sperm‐immobilizing antibodies in their sera, giving the positive rate of 2.6%. So far, 11 of them proceeded to IVF‐ET or ICSI‐ET treatment after the unsuccessful treatment cycles by intrauterine insemination. The average age was 37.7 years (range 26–44 years). The treatment by ICSI was selected for two infertile women with sperm‐immobilizing antibodies because of their husband's severe asthenozoospermia.
Patients’ sera were collected in 11 IVF‐ET or four ICSI‐ET treatment cycles from 11 infertile women having sperm‐immobilizing antibodies in their sera, with informed consent. All sera were heat‐treated at 56°C for 30 min to inactivate them and were kept frozen at −20°C until use.
Collection of follicular fluid and procedure of IVF‐ET and ICSI‐ET
A standardized ovarian stimulation was carried out, as previously described. 17 , 18 The patients were stimulated using a gonadotropin releasing hormone (GnRH) agonist (Nafarelin acetate, Yamanouchi Pharmaceutical, Tokyo, Japan) started in the midluteal phase (suppression protocol) followed by gonadotropins. After the onset of withdrawal bleeding, ovarian stimulation was initiated by injections of follicle stimulating hormone (FSH; Fertinom P, Serono, Tokyo, Japan) for 3 days followed by human menopausal gonadotropins (Humegon, Nippon Organon K.K., Osaka, Japan) for >6 days. Ovarian stimulation was monitored by the measurement of serum E2 concentration and by ultrasonographic assessment of the follicle diameter. Human chorionic gonadotropin (hCG) (HCG, Mochida, Tokyo, Japan) was injected when at least one of the leading follicles reached 17 mm in diameter. Oocyte retrieval was carried out through transvaginal ultrasonography‐guided aspiration 36 h after the hCG administration. Retrieved oocytes were examined under a microscope and washed with culture media supplemented with human serum albumin (HSA) at least three times before pre‐incubation. Before insemination with the husband's swim‐up sperm, they were washed again with new culture media four times.
The FF from the leading follicle was collected in 11 IVF‐ET or four ICSI‐ET treatment cycles from 11 infertile women having sperm‐immobilizing antibodies in their sera, with informed consent. All FF were immediately centrifuged for 5 min at 600 × g and the supernatants were heat‐treated at 56°C for 30 min for inactivation. They were kept frozen at −20°C until use.
On the second or third day after IVF or ICSI, the morphological assessment of embryos was carried out under an inverted microscope using the Veeck's classification 19 before transfer. Grade 1 embryos with regular blastomeres and no cytoplasmic fragments, and grade 2 embryos with regular blastomeres and minor cytoplasmic fragments were considered good‐quality embryos. Grade 3, 4 and 5 embryos were considered poor‐quality embryos. A maximum of three embryos of good quality were transferred. In some selected cases, elective transfer of two good‐quality embryos was carried out to reduce high‐order multiple pregnancies. 18 , 20 Clinical pregnancy was diagnosed when the gestational sac was detected under transvaginal ultrasonography.
Sperm immobilization test and quantitative sperm immobilization test
A SIT was carried out as previously described by Isojima et al. 1 In the present study, 10 µL of a patient's inactivated serum or FF, 2 µL of guinea pig serum (C’H50 = 200) and 1 µL of human sperm suspension (40 × 106/mL) were mixed on a Terasaki microplate and incubated at 32°C for 3 h. As a control, the same amount of heat‐inactivated guinea pig serum was used instead of the active complement. The sperm immobilization value (SIV) was calculated by dividing the sperm motility of the control serum or FF by that of the test serum or FF. A SIV of two or more was considered positive.
All sera with sperm‐immobilizing antibodies were tested to determine the antibody titers by a quantitative SIT (SI50) as described by Isojima et al. 7
The fertilization rate (FR), good‐quality embryo rate and implantation rate by IVF‐ET were compared between infertile patients having high and low SI50 titers in their FF according to the classification by Kobayashi et al. 6
Statistical analysis
Statistical analysis of the data was carried out by the Pearson's product‐moment correlation coefficient and Fisher's χ2‐test using spss 13.0J for Windows (SPSS, Chicago, IL). P < 0.05 represented a significant difference.
RESULTS
Comparison of SI50 titers between the serum and the follicular fluid
SIX PATIENTS HAD higher (10≤) SI50 titers, whereas nine patients had lower (<10) SI50 titers in their sera. SI50 titers between the serum and the FF were compared in 15 treatment cycles from 11 immunologically infertile women. There was a significant correlation in the SI50 titers between the patients’ sera and their FF (P < 0.0001) (Fig. 1).
Figure 1.
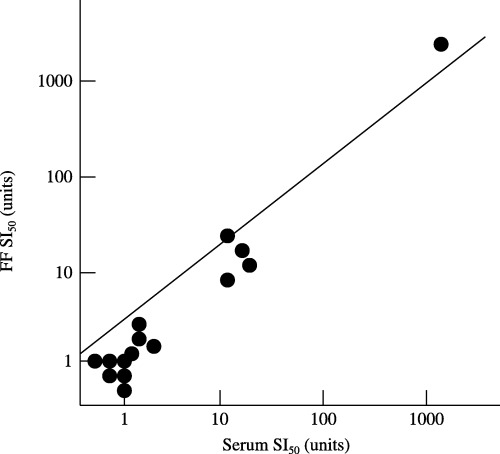
Comparison of quantitative sperm‐immobilizing antibodies titers (SI50) between serum and follicular fluid (FF). SI50 titers between serum and FF were compared in 15 treatment cycles and SI50 titers in patients’ FF were similar to those in their sera. There was a significant correlation in the SI50 titers between the patients’ sera and their FF (P < 0.0001).
Comparison of the fertilization rate by IVF in infertile patients having high and low SI50 titers in their follicular fluid
The results of 11 IVF‐ET treatment cycles from the 10 infertile patients are analyzed. Therefore, the results of four ICSI‐ET treatment cycles, including FR, embryo quality, and the implantation rate, were excluded. They were divided into two groups according to the levels of SI50 titers in the patient's FF, and the FR was compared between the two groups. In total, 52 of the 65 mature oocytes inseminated were fertilized and the FR was 80.0%. In the four IVF treatment cycles with high (10≤) SI50 titers, 36 mature oocytes were inseminated and 28 were fertilized, giving a FR of 77.8%. In the seven treatment cycles with low (<10) SI50 titers, 29 mature oocytes were inseminated and 24 were fertilized, giving a FR of 82.8%. There was no difference in FR in patients having high (10≤) or low (<10) SI50 titers in their FF (P = 0.62) (Fig. 2).
Figure 2.
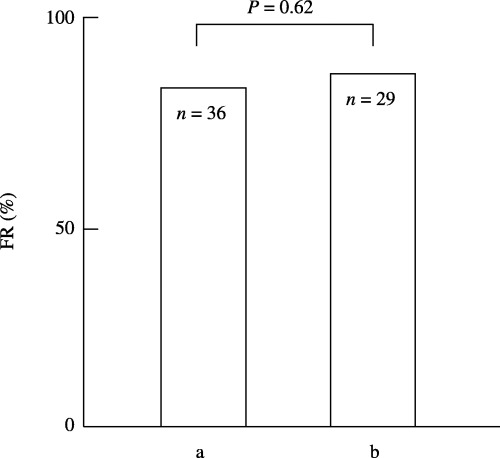
Comparison of the fertilization rate (FR) in infertile patients who have (a) high (10≤) and (b) low (<10) SI50 titers in their follicular fluid (FF). In total, 52 of the 65 mature oocytes inseminated were fertilized and the FR was 80.0%. (a) In the four IVF treatment cycles with high (10≤) SI50 titers in FF, 36 mature oocytes were inseminated and 28 fertilized, giving a FR of 77.8%. (b) In the seven treatment cycles with low (<10) SI50 titers in FF, 29 mature oocytes were inseminated and 24 fertilized, giving a FR of 82.8%. There was no difference in FR between the two groups (P = 0.62).
Comparison of the good‐quality embryo rate in infertile patients having high and low SI50 titers in their follicular fluid
The good‐quality embryo rate was compared between the two groups described above. In total, 36 of the 52 fertilized eggs developed to good‐quality embryos and the good‐quality embryo rate was 69.2%. In the four IVF treatment cycles with high (10≤) SI50 titers, 16 of the 28 fertilized eggs developed to good‐quality embryos, giving a good‐quality embryo rate of 57.1%. In the seven treatment cycles with low (<10) SI50 titers, 20 of the 24 fertilized eggs developed to good‐quality embryos, giving a good‐quality embryo rate of 83.3%. There was a significant difference between the two groups (P < 0.05) (Fig. 3).
Figure 3.
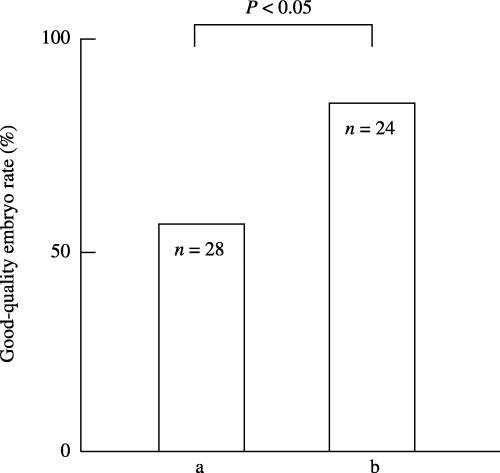
Comparison of the good‐quality embryo rate in infertile patients who have (a) high (10≤) and (b) low (<10) SI50 titers in their follicular fluid (FF). In total, 36 of the 52 fertilized eggs developed to good‐quality embryos and the good‐quality embryo rate was 69.2%. (a) In the four IVF treatment cycles with high (10≤) SI50 titers, 16 of the 28 fertilized eggs developed to good‐quality embryos, giving a good‐quality embryo rate of 57.1%. (b) In the seven treatment cycles with low (<10) SI50 titers, 20 of the 24 fertilized eggs developed to good‐quality embryos, giving a good‐quality embryo rate of 83.3%. There was a significant difference between the two groups (P < 0.05).
Comparison of the implantation rate in infertile patients having high and low SI50 titers in their follicular fluid
Five of the ten patients with sperm‐immobilizing antibodies conceived by IVF‐ET, giving a pregnancy rate per patient of 50.0%. The implantation rates between the two groups according to the levels of SI50 titers were compared. In total, 6 of the 27 embryos transferred implanted and the implantation rate was 22.2%. In the four IVF treatment cycles with high (10≤) SI50 titers, nine embryos were transferred and three implanted, giving an implantation rate of 33.3%. In the seven treatment cycles with low (<10) SI50 titers, 18 embryos were transferred and three implanted, giving an implantation rate of 16.7%. There was no significant difference in implantation rate between the two groups (P = 0.33) (Fig. 4).
Figure 4.
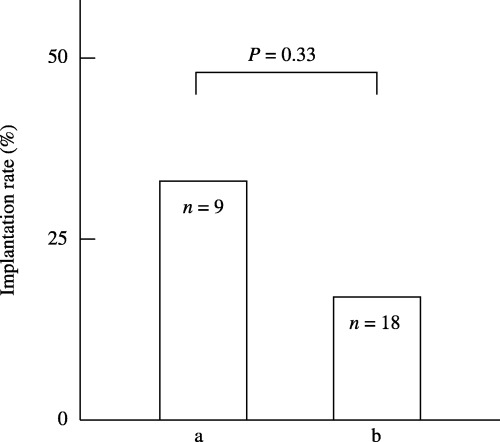
Comparison of the implantation rate in infertile patients who have (a) high (10≤) and (b) low (<10) SI50 titers in their follicular fluid (FF). In total, 6 of the 27 embryos transferred implanted and the implantation rate was 22.2%. (a) In the four IVF treatment cycles with high (10≤) SI50 titers, nine embryos were transferred and three implanted, giving an implantation rate of 33.3%. (b) In the seven treatment cycles with low (<10) SI50 titers, 18 embryos were transferred and three implanted, giving an implantation rate of 16.7%. There was no significant difference in implantation rate between the two groups (P = 0.33).
DISCUSSION
TREATMENT WITH IVF‐ET has been used to overcome the immunological causes of female infertility by antisperm antibodies in vivo, and satisfactory results under suitable culture conditions for gametes and embryos have been obtained. 6 , 16 , 21 , 22 It has been shown that treatment of infertile women who have sperm‐immobilizing antibodies in their sera by the IVF‐ET procedure can achieve a high pregnancy rate, 6 , 16 , 22 because IVF‐ET treatments that do not use the patient's serum in the culture medium overcome the inhibitory effects of the antibodies on sperm penetration in the female genital tract and the fertilization process. The authors have found in another recent study, that if the culture medium contains the patient's serum which has sperm‐immobilizing antibodies, the sperm‐immobilizing antibodies themselves exert inhibitory effects on fertilization and subsequent embryo development in vitro. 14 In the present study, the morphological assessment of embryos followed the Veeck's classification, 19 which has been widely accepted for clinical use. The principle of the classification is the observation of regularity in blastomeres and the severity in cytoplasmic fragmentation. It has been shown that the high quality embryos assessed using this classification have higher potentiality in implantation. Therefore, it has been applied to reduce the number of embryos transferred to avoid high‐order multiple pregnancies. 18 , 20
A significantly lower fertilization rate and a significantly higher incidence of embryo fragmentation were observed in embryos cultured in the medium supplemented with sera from infertile women which contained sperm‐immobilizing antibodies. These inhibitory effects by antihuman sperm‐immobilizing antibodies on fertilization and embryo development to blastocyst in vitro have also been observed in mice. 15 It was shown in rats that the sperm‐specific antigens were transferred to the fertilized eggs and were located on the cell membrane in the various developmental stages from one cell to blastocyst. 23 Antibodies against rat sperm impaired the in vitro development of fertilized eggs in the presence of a complement.
In humans, it has been shown that a similar amount of sperm‐immobilizing antibodies can exist in a patient's FF 24 and peritoneal fluid, 4 as in their sera. Careful manipulation of eggs, such as thorough washing in culture medium before IVF, has been recommended to avoid possible harmful effects of the antibodies attached to the cumulus cells on fertilization and embryo development, especially in patients with high SI50 titers in the FF. So far, it has not yet been clarified if high titers of sperm‐immobilizing antibody affect the results of IVF‐ET, even when the eggs are washed thoroughly.
In the present study, levels of sperm‐immobilizing antibodies found in the FF were similar to those in the patients’ sera, and there was a significant correlation in the SI50 titers between the FF and the patients’ sera (Fig. 1). Previous studies have also shown that concentrations of sperm‐immobilizing antibodies in the FF and peritoneal fluid were consistent with that in the transudation from sera. 4 , 24 The present study supports their conclusions. The relationship between the antibody titer and the results of the IVF‐ET treatment were compared. Regarding the fertilization rate, there was no difference in patients having high (10≤) and low (<10) SI50 titers in their FF after thoroughly washing the collected eggs in culture medium before IVF (Fig. 2). Furthermore, the fertilization rates (77.8% and 82.8%) obtained for infertile women with high and low sperm‐immobilizing antibodies in the present study were the same as those (80.5%) obtained for 110 infertile women without the antibodies nor male factors in our previous report. 25 These findings indicate that ICSI does not seem to be necessary in patients who have the antibodies if their sera are not supplemented in the culture media.
We have previously shown that poor‐quality embryos with fragmentation were obtained when the embryos were cultured in a medium containing the patient's serum with sperm‐immobilizing antibodies. 14 In the present study, the embryo culture medium was supplemented with HSA. However, the good‐quality embryo rate (57.1%) in patients with a high SI50 titer was significantly lower than that (83.3%) with a low antibody titer (Fig. 3). Recently, we reported that the good‐quality embryo rates in the day 2 and day 3 elective transfer of two good quality embryos were 72.0% and 79.5%, respectively. 20 These data indicate that infertile women with a low SI50 titer show the same good‐quality embryo rate after IVF as patients without sperm‐immobilizing antibodies, but those with a high SI50 titer do not. In our previous experiments using mice, it was observed that embryo development in a medium without sperm‐immobilizing antibodies was inhibited after fertilization using sperm treated with sperm‐immobilizing antibodies. 15 Therefore, it might be suggested that the harmful effects by sperm‐immobilizing antibodies on embryo development cannot be completely avoided, especially in patients with high SI50 titers in the FF, even when careful manipulation of eggs and embryos, such as thoroughly washing in the culture medium, is performed. However, further analysis is necessary to confirm the harmful effects of sperm‐immobilizing antibodies on embryo development in infertile women with higher SI50 titers.
There was no significant difference in the implantation rate between the two groups (Fig. 4). This result might indicate that embryos with good quality after exposure to a high level of sperm‐immobilizing antibodies in the patient's FF can be expected to develop in vivo.
Although we have shown that chemical pregnancy rates tended to be higher in patients with higher sperm immobilizing antibody titers, 26 no chemical pregnancy or abortion was found in the present study. Further studies are required to clarify the mechanisms of sperm‐immobilizing antibodies for inhibiting embryo development in vitro.
REFERENCES
- 1. Isojima S, Li TS, Ashitaka Y. Immunologic analysis of sperm immobilizing factor found in sera of women with unexplained sterility. Am J Obstet Gynecol 1968; 101: 677–683. [Google Scholar]
- 2. Isojima S. Recent advances in reproductive immunology. Asia-Oceania J Obstet Gynaecol 1983; 9: 15–26. [DOI] [PubMed] [Google Scholar]
- 3. Koyama K, Ikuma K, Kubota K, Isojima S. Effects of antisperm antibodies on sperm migration through cervical mucus. Excerpta Med Int Congr Series 1979; 512: 705–708. [Google Scholar]
- 4. Shibahara H, Shigeta M, Toji H, Koyama K. Sperm immobilizing antibodies interfere with sperm migration from the uterine cavity through the fallopian tubes. Am J Reprod Immunol 1995; 34: 120–124. [DOI] [PubMed] [Google Scholar]
- 5. Koyama K, Kubota K, Ikuma K, Shigeta M, Isojima S. Application of the quantitative sperm immobilization test for follow‐up study of sperm‐immobilizing antibody in the sera of sterile women. Int J Fertil 1988; 33: 201–206. [PubMed] [Google Scholar]
- 6. Kobayashi S, Bessho T, Shigeta M, Koyama K, Isojima S. Correlation between quantitative antibody titers of sperm immobilizing antibodies and pregnancy rates by treatments. Fertil Steril 1990; 54: 1107–1113. [DOI] [PubMed] [Google Scholar]
- 7. Isojima S, Koyama K. Quantitative estimation of sperm immobilizing antibody in the sera of women with sterility of unknown etiology: the 50 % sperm immobilization unit (SI50). Excerpta Med Int Cong Series 1974; 370: 10–15. [Google Scholar]
- 8. Alexander NJ. Antibodies to human spermatozoa impede sperm penetration of cervical mucus or hamster eggs. Fertil Steril 1984; 41: 433–439. [PubMed] [Google Scholar]
- 9. Kamada M, Daitoh T, Hasebe H, Irahara M, Yamano S, Mori T. Blocking of human fertilization in vitro by sera with sperm‐immobilizing antibodies. Am J Obstet Gynecol 1985; 153: 328–331. [DOI] [PubMed] [Google Scholar]
- 10. Shibahara H, Shigeta M, Koyama K, Burkman LJ, Alexander NJ, Isojima S. Inhibition of sperm‐zona pellucida tight binding by sperm immobilizing antibodies as assessed by the hemizona assay (HZA). Acta Obstet Gynaecol Jpn 1991; 43: 237–238. [PubMed] [Google Scholar]
- 11. Bandoh R, Yamano S, Kamada M, Daitoh T, Aono T. Effect of sperm‐immobilizing antibodies on the acrosome reaction of human spermatozoa. Fertil Steril 1992; 57: 387–392. [PubMed] [Google Scholar]
- 12. Shibahara H, Burkman LJ, Isojima S, Alexander NJ. Effects of sperm‐immobilizing antibodies on sperm‐zona pellucida tight binding. Fertil Steril 1993; 60: 533–539. [PubMed] [Google Scholar]
- 13. Shibahara H, Shigeta M, Inoue M et al. Diversity of the blocking effects of antisperm antibodies on fertilization in human and mouse. Hum Reprod 1996; 11: 2595–2599. [DOI] [PubMed] [Google Scholar]
- 14. Taneichi A, Shibahara H, Hirano Y et al. Sperm immobilizing antibodies in the sera of infertile women cause low fertilization rates and poor embryo quality in vitro. Am J Reprod Immunol 2002; 47: 46–51. [DOI] [PubMed] [Google Scholar]
- 15. Taneichi A, Shibahara H, Takahashi K et al. Effects of sera from infertile women with sperm immobilizing antibodies on fertilization and embryo development in vitro in mice. Am J Reprod Immunol 2003; 49: 146–151. [DOI] [PubMed] [Google Scholar]
- 16. Sugimoto Y, Shigeta M, Ikeda Y et al. Successful application of in vitro fertilization and embryo replacement for the treatment of infertile women with sperm immobilizing antibody. Acta Obstet Gynaecol Jpn 1986; 38: 1135–1136. [PubMed] [Google Scholar]
- 17. Shibahara H, Mitsuo M, Inoue M, Hasegawa A, Shigeta M, Koyama K. Relationship between human in‐vitro fertilization and intracytoplasmic sperm injection and the zona‐free hamster egg penetration test. Hum Reprod 1998; 13: 1928–1932. [DOI] [PubMed] [Google Scholar]
- 18. Veeck LL. Oocyte assessment and biological performance. Ann NY Acad Sci 1988; 541: 259–274. [DOI] [PubMed] [Google Scholar]
- 19. Shibahara H, Suzuki T, Tanaka Y et al. Establishment and application of criteria for the elective transfer of two good‐quality embryos to reduce high‐order multiple pregnancies. Reprod Med Biol 2002; 1: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suzuki T, Shibahara H, Hirano Y, Ohno A, Takamizawa S, Suzuki M. Randomized study comparing day 2 versus day 3 elective transfer of two good‐quality embryos. Reprod Med Biol 2004; 3: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yovich J, Stranger JD, Kay D, Boettcher B. In vitro fertilization of oocytes from women with serum antisperm antibodies. Lancet 1984; 1: 369–370. [DOI] [PubMed] [Google Scholar]
- 22. Daitoh T, Kamada M, Yamano S et al. High implantation rate and consequently high pregnancy rate by in vitro fertilization‐embryo transfer treatment in infertile women with antisperm antibody. Fertil Steril 1995; 63: 87–91. [DOI] [PubMed] [Google Scholar]
- 23. Koyama K, Hasegawa A, Isojima S. Effects of antisperm antibody on the in vitro development of rat embryos. Gamete Res 1984; 10: 143–152. [Google Scholar]
- 24. Kay DJ, Boettcher B, Yovich JL, Stanger JD. Antispermatozoal antibodies in human follicular fluid. Am J Reprod Immunol Microbiol 1985; 7: 113–117. [DOI] [PubMed] [Google Scholar]
- 25. Obara H, Shibahara H, Tsunoda H et al. Prediction of unexpectedly poor fertilization and pregnancy outcome using the strict criteria for sperm morphology before and after sperm separation in IVF‐ET. Int J Androl 2001; 24: 102–108. [DOI] [PubMed] [Google Scholar]
- 26. Shibahara H, Mitsuo M, Ikeda Y, Shigeta M, Koyama K. Effects of sperm immobilizing antibodies on pregnancy outcome in infertile women treated with IVF‐ET. Am J Reprod Immunol 1996; 36: 96–100. [DOI] [PubMed] [Google Scholar]


