Abstract
Background: Cadmium (Cd)‐induced testicular damage in relation to spermatogenesis has not been well studied. We studied the mechanism of Cd‐induced testicular damage in a rat model of subchronic intoxication.
Methods: Male Sprague–Dawley rats were subcutaneously injected with 0.6 mg Cd/kg per day for 6 weeks. The concentration of Cd in urine, serum and testes was measured by using atomic absorption spectrophotometry. Testicular damage was evaluated by counting the spermatogonia (SG) and spermatocytes (SC) on one cut‐surface of five seminiferous tubules in stages VII or VIII of spermatogenesis every week. The location of intratesticular cadmium was determined by using oxine‐fluorescent cytochemistry.
Results: There were no differences in the testes/bodyweight ratio between the study and control groups. The concentration of Cd in the testes increased more than 100‐fold that in serum after week 2, suggesting active testicular Cd accumulation (1–3 mg/g tissue). Cadmium accumulation was detected in SG and SC. The number of SG and SC diminished significantly in the study group (week 2: SG 74%, SC 90%; week 4: SG 47%, SC 75%; week 6: SG 30%, SC 54% of the control, respectively).
Conclusions: Cadmium accumulated in SG and SC, consequently reduced the number of these cells, and disturbed the spermatogenesis in this rat model of subchronic Cd intoxication. Therefore, the number of SG decreased in this rat model of subchronic Cd intoxication. (Reprod Med Biol 2002; 1: 59–63).
Keywords: cadmium, spermatogenesis, testes
INTRODUCTION
CADMIUM (CD) is an important industrial and environmental pollutant that accumulates in many organs after acute and chronic exposure, consequently causing various diseases. 1 The testes have been reported to be one of the target organs of Cd intoxication. 2 Cadmium has also been shown to induce cancer in male reproductive organs in rats, especially in the testes and ventral lobe of the prostate. 3 Apoptotic changes were also found in the testes of rodents after exposure to a single dose of Cd in an in vivo study of cadmium‐induced toxicity. 4 However, neither the exact mechanisms nor the target cells, especially in chronic intoxication, have been clarified. We attempted to determine the target cells of Cd‐induced testicular damage in a rat model of subchronic Cd intoxication.
METHODS
Animals
MALE SPRAGUE–DAWLEY rats (Kyodo, Japan) were subcutaneously injected with 0.6 mg Cd/kg bodyweight per day (as a solution of cadmium chloride in distilled water), six times per week for 6 weeks. 5 One milliliter per kilogram of saline was injected into control rats. Four rats (three experimental, one control) were killed every week. All rats were housed in an environment maintained at 21–25°C and in a 12‐h light–dark cycle. They were fed a commercial diet (CE‐2 containing 24.9% protein; CLEA, Tokyo, Japan) and were allowed to drink tap water ad libitum. The testes/bodyweight ratio was calculated as the average weight of the testes divided by the total bodyweight × 1000.
Quantitative determination of cadmium
Half of the harvested testis and 1 mL of serum were examined to determine the cadmium concentration by using flameless atomic absorption spectrophotometry (Z‐9000; Hitachi, Tokyo, Japan). 6 All the samples were stored at −80°C until assayed.
Detection of apoptosis by using terminal deoxynucleotidyl transferase‐mediated dUTP‐biotin nick‐end labeling
DNA fragmentation was detected by using nick‐end labeling, which was performed basically according to the method of Gavrieli et al. 7
Counting of spermatogonia and spermatocytes
The testes were fixed in neutral 10% formalin and were paraffin embedded. They were then cut into sections, stained with hematoxylin and eosin. The average number of spermatogonia and pachytene spermatocytes in one seminiferous tubule was determined by counting five seminiferous tubules in stages VII and VIII of the spermatogenic process. The average numbers of these cells in all control animals were indicated as a baseline. The statistical difference of the numbers of these cells between the control group and each experimental group was evaluated by using the Student's t‐test.
Staining of cadmium using 8‐hydroxyquinoline
Frozen samples were sliced with a microtome to obtain 8 µm‐thick slices, and were mounted on a glass slide. They were rinsed with absolute ethanol for approximately 30 s, and then immersed in 8‐hydroxyquinoline‐saturated ethanol for 30 min. Thereafter, they were immersed in a solution of 0.2 g 8‐hydroxyquinoline, 2 mg glacial acetic acid, and 120 mL distilled water adjusted to pH 8.5 for 30 min. 8 The slides were examined under a fluorescence microscope (BHS‐RF‐A; Olympus, Tokyo, Japan) and photographed with Ektachrome 1000 film (Kodak, Rochester, NY, USA) under a 1000× magnification.
RESULTS
Testes/bodyweight ratio
THE CONTROL GROUP gained 20.5 g per week during the study period, on average. Cadmium‐injected rats gained only 2.8 g per week during the same period. The testes/bodyweight ratios of these groups were not statistically different all throughout the study period (control group 4.1 ± 0.20, experimental group 3.8 ± 0.28; average ± SE).
Serum and testicular cadmium concentration
The serum concentration of Cd peaked at week 3. The testicular concentration of Cd increased hundreds of times compared with that of serum (Table 1).
Table 1.
Concentration of cadmium (Cd; average ± SE) in serum and testes
| Week | n | Serum (ng/mL) | Testes (ng/g wet tissue) |
|---|---|---|---|
| Control | 6 | 2.8 ± 0.6 | 3.7 ± 0.6 |
| 1 | 3 | 8.1 ± 1.2 | 378.2 ± 87.3 |
| 2 | 3 | 7.2 ± 0.2 | 717.0 ± 143.3 |
| 3 | 3 | 19.5 ± 0.5 | 3910.0 ± 1720.0 |
| 4 | 3 | 17.5 ± 3.5 | 2025.0 ± 1085.0 |
| 5 | 3 | 16.5 ± 6.4 | 1815.0 ± 485.0 |
| 6 | 3 | 31.5 ± 10.5 | 4520.0 ± 2310.0 |
Cadmium accumulation in the testes was prominent because the testicular concentration of Cd was almost 100‐fold more than that in serum.
Histological findings and number of spermatogonia and spermatocytes
Histological examination revealed marked hypospermatogenesis and a decrease of spermatogenic cells in the experimental group (Fig. 1). The average number of spermatogonia in one seminiferous tubule was: 43.6 at baseline, 32.4 at week 2 (P > 0.05), 20.8 at week 4 (P < 0.01) and 13.0 at week 6 (P < 0.01). The average number of pachytene spermatocytes was: 52.0 at baseline, 47.2 at week 2 (P > 0.05), 39.4 at week 4 (P < 0.05), and 28.4 at week 6 (P < 0.01). Apoptotic cells were detected in all specimens by using terminal deoxynucleotidyl transferase‐mediated dUTP‐biotin nick‐end labeling (TUNEL). However, the number of apoptotic cells did not change significantly during the study period in either group.
Figure 1.
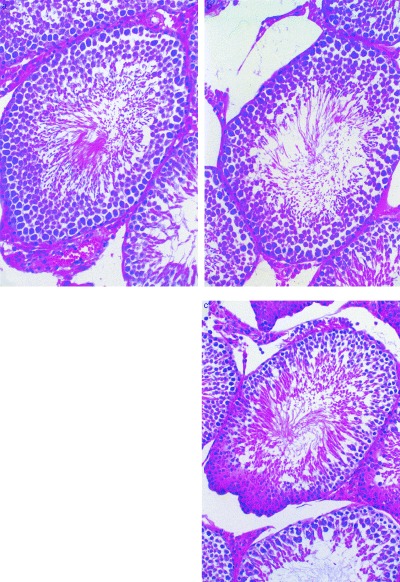
Histological findings of the testes (hematoxylin & eosin stain). Control week 3 (a; reduced from ×66), experimental group week 3 (b; reduced from ×50), experimental group week 5 (c; reduced from ×80). The number of spermatogonia decreased in (b; 37) and (c; 27) compared with (a; 49). The number of pachytene spermatocytes decreased in (c; 30) compared to (a; 56) and (b; 58). Meanwhile, the number of Sertoli cells did not change in all specimens (approximately 35).
Localization of cadmium by 8‐hydroxyquinoline staining
Oxine‐fluorescence staining revealed an accumulation of Cd in both the spermatogonia and spermatocytes (Fig. 2). No apparent Cd accumulation was detected in Leydig cells or in Sertoli cells.
Figure 2.
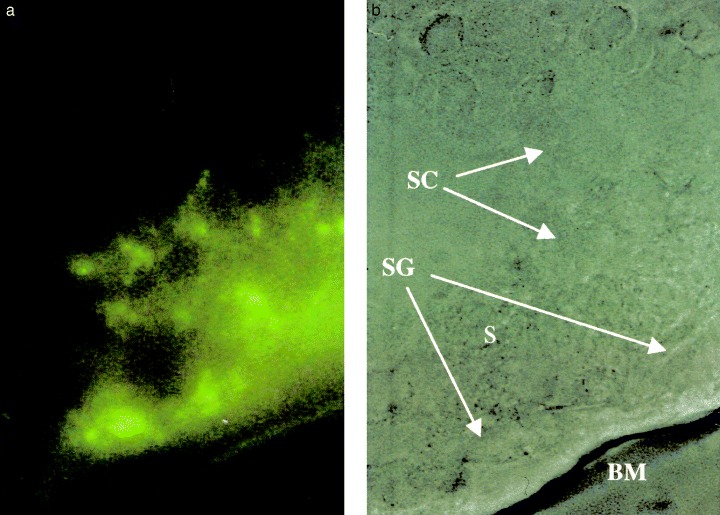
(a) Oxine‐fluorescent cytochemistry of seminiferous tubules from rats in the experimental group at week 5 (left, reduced from ×1000). The same view without fluorescent light (right; BM, basement membrane; S, Sertoli cell; SC, spermatocyte; SG, spermatogonium). Cadmium was identified mainly at the cytoplasm of spermatogonia, and weakly at spermatocytes.
DISCUSSION
MAMMALIAN TESTES ARE known to be susceptible to Cd both after acute and chronic exposure. 2 As for acute Cd exposure, Cd was reported to cause hemorrhage in the capillaries of the testes, followed by degeneration and necrosis of the seminiferous tubules; these appearances resembled testicular infarction. 9 The testes produce metallothionein 1 and 2, which actively incorporate zinc during spermatogenesis. 10 Metallothionein also actively binds to Cd. Therefore, in the case of chronic Cd exposure, Cd accumulates in the testes as demonstrated in the present study.
Histological examination revealed that no hemorrhage, necrotic change or testicular atrophy occurred under the present experimental conditions. The testes/bodyweight ratio remained constant during Cd exposure. However, spermatogenesis was apparently disturbed. Oxine‐fluorescent cytochemistry revealed that Cd accumulated in spermatogonia (SG) and spermatocytes (SC), and that the number of SG decreased in relation to the duration of Cd exposure, followed by the decrease of the number of SC. In rats, spermatogenesis is reported to take 8.4 weeks. Histologically, the seminiferous tubules are divided into 14 stages according to the spermatogenetic appearance. The process of stage alteration, from stages I to XIV, takes 310.8 h. Stages VII and VIII are said to be suitable for the assessment of toxic effects, because the final events of spermatogenesis are seen in these stages. 11 Two cyclic alterations from stages I to XIV are required for the maturation of SG to pachytene SC; 3.7 weeks. The decrease in the number of SG in week 2 compared with the control was almost the same as that of pachytene SC observed in week 6. The decrease in the number of SG was supposed to be caused by necrosis not by apoptosis, because the number of apoptotic cells were not changed by Cd exposure.
Spermatogenesis is known to be influenced by the amount of sex hormones. Serum follicular stimulating hormone and testosterone were reported to decrease in postpubertal SD rats, to some extent, as a consequence of chronic Cd exposure. 12 However, our results showed that the initiation of Cd‐induced hypospermatogenesis was the decrease of SG. It was thought that this occurred not by the result of hormonal change but by the direct toxicity of Cd to SG, because Cd accumulation was apparent to SG and SC.
The mechanism of chronic Cd‐induced testicular injury was completely different from that of the acute injury. In chronic Cd‐induced injury, the target cells were SG and SC. Spermatogenesis was disturbed by the SG decrease. Nowadays, Cd is widely used in various industrial products. Even a low dose Cd pollutant may become a problem in the future, because Cd will accumulate in the male reproductive organs and disturb spermatogenesis as well as behave as a carcinogen, as reported elsewhere. 3
ACKNOWLEDGEMENTS
THE AUTHORS WISH to thank Dr Sumie Yamanaka and Dr Atsushi Takayanagi from the Department of Hygiene, Tokyo Dental College, for measuring the cadmium concentration in the samples. ACKNOWLEDGMENTSTHE AUTHORS WISH to thank Dr Sumie Yamanaka and Dr Atsushi Takayanagi from the Department of Hygiene, Tokyo Dental College, for measuring the cadmium concentration in the samples.
REFERENCES
- 1. Friberg L, Kjellstrom T, Nordberg GF. Cadmium In: Friberg L, Nordberg GF, Vouk VB,eds. Handbook on the Toxicology of Metals, Vol. 2 New York: Elsevier, 1986;. 130–184. [Google Scholar]
- 2. Samarawickrama GP. In: Webb M, ed. The Chemistry, Biochemistry and Biology of Cadmium. Amsterdam: Elsevier, 1979;. 341–408. [Google Scholar]
- 3. Waalkes MP, Rehm S, Riggs CW etal. Cadmium carcinogenesis in male Wistar rats:dose–response analysis of tumor induction in the prostate and testes and at the injection site. Cancer Res 1988; 48: 4656–4663. [PubMed] [Google Scholar]
- 4. Xu C, Johnson JE, Singh PK et al. In vivo studies of cadmium‐induced apoptosis in testicular tissue of the rat and its modulation by a chelating agent. Toxicology 1996; 197: 1–8. [DOI] [PubMed] [Google Scholar]
- 5. Tanimoto A, Hamada T, Koide O. Cell death and regeneration of renal proximal tubular cellsi n rats with subchronic cadmium intoxication. Toxicol Pathol 1993; 21: 341–352. [DOI] [PubMed] [Google Scholar]
- 6. Vesterberg O, Wrangskogh K. Determination of cadmium in urine by graphite‐furnace atomic absorption spectroscopy. Clin Chem 1978; 24: 681–685. [PubMed] [Google Scholar]
- 7. Gavrieli Y, Shrman Y, Ben‐Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992; 119: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamada T, Tanimoto A, Iwai S et al. Cytopathological changes induced by cadmium‐exposure in canine proximal tubular cells: acytochemical and ultrastructural study. Nephron 1994; 68: 104–111. [DOI] [PubMed] [Google Scholar]
- 9. Nolan CV, Shaikh ZA. The vascular endothelium as a target tissue in acute cadmium toxicity. Life Sci 1986; 39: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki JS, Kodama N, Molotkov A et al. Isolation and identification of metallothionein isoforms (MT‐1 and MT‐2) in the rat testis. Biochem J 1998; 334: 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsui H, Takahashi M. A novel quantitative morphometry of germ cells for the histopathological evaluation of rat testicular toxicity. J Toxicol Sci 1999; 24: 17–25. [DOI] [PubMed] [Google Scholar]
- 12. Lafuente A, Marquez N, Perez‐Lorenzo M et al. Pubertal and postpubertal cadmium exposure differentially affects the hypothalamic‐pituitary‐testicular axis function in the rat. Food Chem Toxicol 2000; 38: 913–923. [DOI] [PubMed] [Google Scholar]


