Abstract
Aim: The present study has been designed with the objective of determining if fatty acids bound to bovine serum albumin‐V (BSA‐V) can improve motility, viability, and increase acrosome reaction (AR) and utilization of glucose in boar spermatozoa.
Methods: Boar spermatozoa were washed, swum‐up and incubated at 37°C for 6 h in TALP medium supplemented with fatty acids bound to bovine serum albumin fraction V (BSA‐V), fatty acid free BSA (BSA‐FAF), polyvinyl alcohol + main fatty acids bound to BSA‐V (PVA + FA) and PVA. Sperm motility, viability, AR, and the incorporation and oxidation of 14C‐glucose were evaluated during 6 h of incubation.
Results: The results show that the BSA‐V was superior to BSA‐FAF and PVA in improving motility and AR. Viability was significantly increased (P < 0.05) by only BSA‐V compared with PVA. When the main fatty acids compound of BSA‐V were added to PVA, the sperm motility, viability and AR became almost the same as with BSA‐V. The rate of incorporation and oxidation of 14C‐glucose were significantly increased (P < 0.05) by BSA‐V compared with BSA‐FAF and PVA. Fatty acids bound to BSA‐V are important for improvement of sperm functions.
Conclusions: The present study postulates that fatty acids bound to BSA‐V are important to acrosome reaction and the utilization of glucose in boar spermatozoa.
Keywords: acrosome reaction, boar spermatozoa, bovine serum albumin, motility, utilization of glucose.
INTRODUCTION
CULTURE MEDIA HAVE a great impact on the results obtained from reproductive techniques such as in vitro fertilization (IVF) in domestic animals. Sperm motility, viability and acrosome reaction (AR) depend strongly on the media used during the process of in vitro manipulation. Improvements in the culture systems that are most commonly used have relied on macromolecules, such as bovine serum albumin (BSA), or complex biological fluids, such as serum in the culture medium, for the provision of components essential for development. However, these macromolecules are inconsistent in their composition and their ability to support development in vitro.
Most sperm incubation media contain BSA for supporting sperm motility, viability and AR. 1 Bovine serum albumin maintains the osmolality of media, and a variety of molecules, such as fatty acids, lipids, steroids, amino acids and growth factor bound to BSA, are released from it and stimulate sperm functions. BSA preparations can contain up to 2–3 mol of fatty acids/mol of protein, 2 which could represent the introduction of as much as 15 µg of fatty acid/mg of BSA. 3 Concentration of bovine serum albumin fraction V (BSA‐V) used in culture media usually ranges from about 1–30 mg/mL or more, which may carry a large amount of fatty acids. Spermatozoa can use these fatty acids as a source of energy. 4 , 5 BSA serves as a highly efficient fatty acids delivery system, making a distinct contribution in inducing acrosome reaction of guinea pig spermatozoa. 6 Therefore, the beneficial effects of BSA to culture medium on sperm motility, viability and AR are presumably at least partly due to those of the fatty acids.
However, it is not clear what role the fatty acids bound to BSA play in inducing AR of boar spermatozoa. Thus, the present study has been undertaken to investigate the effect of the fatty acids bound to BSA on boar sperm motility, viability, AR and utilization of glucose.
MATERIALS AND METHODS
Chemicals
RADIOACTIVE GLUCOSE WAS purchased from American Radiolabeled Chemicals Inc (St Louis, MO, USA). Fatty acids bound to BSA (BSA‐V), fatty acid free BSA (BSA‐FAF) and polyvinyl alcohol (PVA) came from Sigma Chemical Company (St Louis, MO, USA). The purity of BSA was verified by sodium dodecyl sulfate‐agarose gel electrophoresis (SDS‐AGE). The purity of BSA‐V was ≥98% (SDS‐AGE) and BSA‐FAF was ≥99% (SDS‐AGE). All other chemicals were analytical grade and purchased from Nacalai Tesque (Kyoto, Japan).
Culture medium
Boar spermatozoa were prepared in a basic TALP medium. 7 According to the experimental design, the basic medium was supplemented with BSA‐V and BSA‐FAF as 0.3 g/100 mL and PVA as 0.1 g/100 mL concentration. To find out the reason for the excellent performance of BSA‐V, fatty acids of BSA‐V were mixed together then added with PVA and compared with BSA‐V for sperm functions. Fatty acids mixture (FA) was prepared on the basis of the fatty acids binding to BSA 8 and the percentage of fatty acids (myristic 0.030 mg, palmitic 0.066 mg, palmitoleic 0.028 mg, stearic 0.069 mg, oleic 0.111 mg, linoleic 0.025 mg, arachidonic 0.156 mg, docosahexaenoic 0.051 mg and lignoceric 0.096 mg/100 mL) present in BSA‐V. 9 A total of 0.64 mg fatty acids mixture was added per 100 mL medium.
Semen collection and preparation
Semen from Duroc boars aged between 2 and 3 years was collected by the gloved‐hand technique at Nagano Animal Industry Experiment Station, Nagano, Japan. The semen was diluted according to the method of Johnson et al., 10 with the Modena extender giving a sperm concentration of 1 × 108/mL at room temperature. The vial of semen was carried to the laboratory within 30 min using a cork box to keep it at 21°C temperature. The upper most 10 mL of the semen was removed from the vial and incubated for 10 min in a water bath at 37°C temperature for anabiosis. Then, 3 ml of the anabiosed sperm sample was washed twice with TALP medium by centrifugation at 900 × g for 5 min. The sperm pellet was suspended in TALP medium and pre‐incubated at 37°C, 95% humidified air and 5% CO2 for 1 h to swim‐up. A 50 µL of sperm aliquots obtained from swim‐up separation was resuspended to the previously prepared drops with the specified medium to give a final concentration of spermatozoa 5 × 106/mL. It was then incubated at 37°C, 95% humidified air and 5% CO2 for 0–6 h to evaluate the sperm motility. To evaluate the acrosome status, swim‐up spermatozoa were resuspended separately at a concentration of 20 × 106/mL and incubated at 37°C for 0–6 h in the same conditions.
Progressive motility
After completion of the specified incubation periods, three subsamples of each sample were placed on warm glass slides for motility and movement quality evaluations. Slides were warmed at 37°C by using a stage warmer set (MPF‐10‐SZX, Kitazato supply, Tokyo, Japan). The slides were then examined under a light microscope (×400). Sperm motility was assessed by determining the percentage of spermatozoa that showed the following four categories of movement: (a) rapid progressive motility; (b) slow or sluggish progressive motility; (c) non‐progressive motility; and (d) immotility. It was then expressed as a progressive motility percentage. The percentage of spermatozoa with progressive motility was determined subjectively by scoring 400 individual spermatozoa in each sample.
Acrosome reaction and viability
To evaluate the AR and viability, spermatozoa were stained by applying the triple staining technique. 11 Namely; dead spermatozoa were stained with trypan blue at the post‐acrosomal region, whereas live spermatozoa were not stained with this dye but were stained with Bismark brown. The acrosome was stained by Rose Bengal. The triple stained slides were examined by light microscopy under oil immersion (×1000). Viability and acrosome reaction of the spermatozoa were evaluated from randomly selected fields of the triple stained slides until 400 spermatozoa had been examined. Therefore, based on the staining patterns, the spermatozoa might be categorized according to the following characteristics: (a) dead and acrosome unreacted (Fig. 1a); (b) live and acrosome unreacted (Fig. 1b); (c) dead and acrosome reacted (Fig. 1c); and (d) live and acrosome reacted or lost (Fig. 1d).
Figure 1.
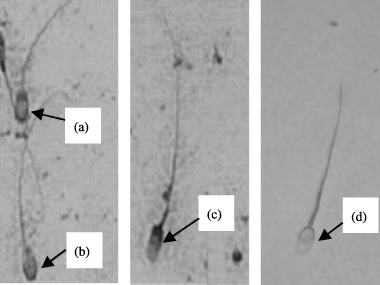
Triple stained boar spermatozoa with different acrosome status. (a) Dead spermatozoa which had not undergone acrosome reaction (AR). (b) Live spermatozoa which had not undergone AR. (c) Dead spermatozoa which had undergone AR. (d) Live spermatozoa which had undergone AR.
Incorporation and oxidation of 14C‐glucose
To assess the metabolic activity of spermatozoa treated with fatty acids bound to BSA‐V by 14C‐glucose incorporation and oxidation, semen samples were prepared as above for motility evaluation. A 100 µL sample of spermatozoa (5 × 106/mL) from each treatment was incubated with 18.5 kBq/mL (specific activity 370 MBq/mmol) of 14C‐glucose and prepared for scintillation counting according to the method described by Miah et al. 12 The incorporation and oxidation of 14C‐glucose by spermatozoa was determined by a liquid scintillation counter (LS‐6500, Beckman Instruments, Fullerton, CA, USA). The value of incorporation and oxidation was expressed directly by counts per minute (cpm).
Statistical analysis
For these experiments, data were subjected to the protected Fisher's least significant difference test. The ncss (Number Crunchier Statistical System; NCSS Statistical Software, Kaysville, UT, USA) Version 5.01 computer software package was used for all statistical analysis. Each experiment was repeated four times and differences were considered significant for P < 0.05.
RESULTS
EFFECTS OF THE BSA‐V, BSA‐FAF and PVA on progressive motility, viability and AR in boar spermatozoa during 0–6 h of incubation are shown in Figure 2. BSA‐V performed better for progressive motility and AR. The percentage of progressive motility and AR were observed to be significantly higher (P < 0.05) in BSA‐V treated spermatozoa compared with BSA‐FAF from 1 to 4 h of incubation, and compared with PVA from almost all incubation periods. Compared with PVA, viability was only significantly improved (P < 0.05) by BSA‐V, not by BSA‐FAF during 1–4 h of incubation.
Figure 2.
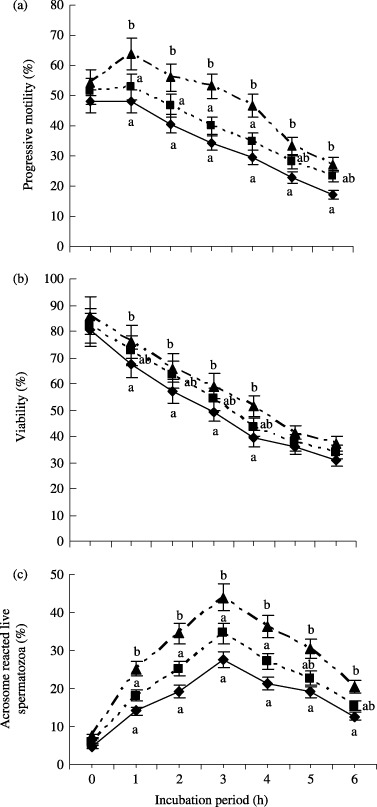
Effect of bovine serum albumin fraction V (BSA‐V), fatty acid free BSA (BSA‐FAF) and polyvinyl alcohol (PVA) on the percentage of (a) progressive motility, (b) viability and (c) acrosome reacted live spermatozoa during 0–6 h of incubation. (▴) BSA‐V; (▪) BSA‐FAF; (◆) PVA. This observation started after 1 h pre‐incubation. Each line with an error bar represents the mean ± SEM from four replicates. Data points without a common lower case letter indicate differences (P < 0.05) among the treatments.
Effects of BSA‐V, PVA + FA and PVA on motility, viability and AR of boar spermatozoa are compared in Figure 3. Compared with PVA, there was no significant difference between BSA‐V and PVA + FA treated spermatozoa with regard to improving progressive motility, viability and increasing AR.
Figure 3.
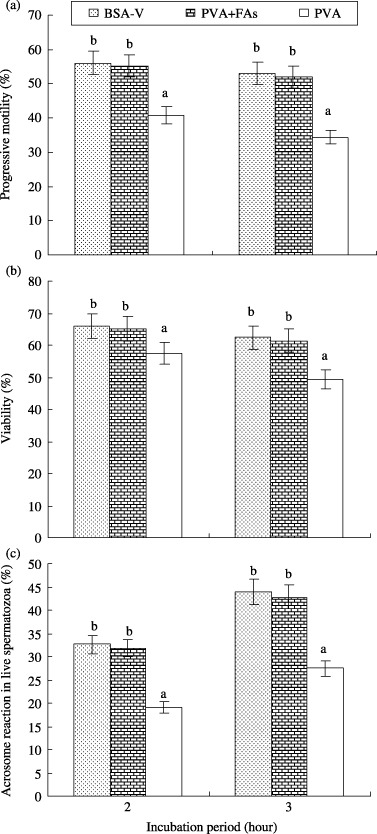
Effect of bovine serum albumin fraction V (BSA‐V), polyvinyl alcohol + main fatty acids bound to BSA‐V (PVA + FA) and PVA on the percentage of (a) progressive motility, (b) viability and (c) acrosome reacted live spermatozoa at 2 and 3 h of incubation. This observation started after 1 h pre‐incubation. Each line with an error bar represents the mean ± SEM from four replicates. Data points without a common lower case letter indicate differences (P < 0.05) among the treatments.
The rates of incorporation and oxidation of 14C‐glucose by spermatozoa is shown in Figure 4. The incorporation rate of 14C‐glucose was higher (P < 0.05) in the BSA‐V treated spermatozoa than in the BSA‐FAF and PVA from 1 to 4 h of incubation. The trend of 14C‐glucose oxidation was also the same as incorporation; where performance of BSA‐V was greater (P < 0.05) than BSA‐FAF and PVA from 1 to 4 h incubation periods. The incorporation and oxidation rate of 14C‐glucose in the spermatozoa treated by BSA‐V, BSA‐FAF and PVA increased up to 3 h of incubation and then decreased up to 6 h of the incubation period.
Figure 4.
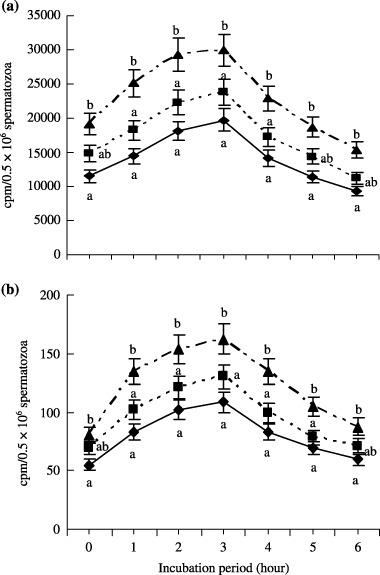
Effect of bovine serum albumin fraction V (BSA‐V), fatty acid free BSA (BSA‐FAF) and polyvinyl alcohol (PVA) on (a) incorporation and (b) oxidation of 14C‐glucose in pre‐incubated boar spermatozoa for 0–6 h. (▴) BSA‐V; (▪) BSA‐FAF; (◆) PVA. This observation started after 1 h pre‐incubation. Each line with an error bar represents the mean ± SEM from four replicates. Data points without a common lower case letter indicate differences (P < 0.05) among the treatments.
DISCUSSION
IN THE PRESENT study, motility was increased by fatty acids bound to BSA‐V compared with fatty acid free BSA or PVA. This study showed that fatty acids play an important role in improving sperm motility. Motility of rabbit 1 and hamster 13 spermatozoa was improved by fatty acids bound to BSA; boosting of motility might have been the result of supplying sufficient energy from fatty acids bound to BSA. The viability was also improved by fatty acids bound to BSA‐V. Lipids bound to BSA improved boar sperm viability during cooling and after cryopreservation. 14 In the present study, BSA‐V increased sperm motility, viability and acrosome reaction more than the BSA‐FAF and PVA. Sperm functions were also stimulated by PVA but not greatly in a phenomenon observed by Bavister. 15 From the first experiment it appears that fatty acids of BSA‐V significantly improved sperm functions, but this BSA‐V might have contained some contaminants. To avoid the risk of this inconvenience, main fatty acid compounds of BSA‐V were added to PVA and observed for sperm functions; it was found that motility, viability and AR were again improved by PVA + FA almost as much as with BSA‐V. The main fatty acids of BSA‐V were oleic, linoleic, palmitic and arachidonic acid, 9 which have important roles in improving sperm motility, viability and inducing AR.
During mammalian fertilization, spermatozoa undergo physiological and functional modifications that are known as capacitation. 16 After capacitation, spermatozoa undergo an exocytotic process called AR; 17 multiple fusions occur between the plasma membrane and the outer acrosomal membrane. It has been known for some years that free or esterified fatty acids added to media can induce fusion of ordinary cells, but the effect of fatty acids on sperm cell fusion is still a matter of controversy. In the present study, at the onset of incubation, the improvement of AR by fatty acids bound to BSA‐V was very mild but it increased significantly up to 3 h of incubation. Compared with PVA, 1.6‐fold AR of live spermatozoa was increased by fatty acids bound to BSA‐V and 1.2‐fold by fatty acids free BSA at 3 h of incubation. As spermatozoa gradually dies, from 3 h upward there was a decline in AR. In the present study, fatty acids bound to BSA‐V triggered higher induction of AR compared with fatty acids free BSA and PVA in all incubation periods. When fatty acids were added to PVA, AR was almost the same as BSA‐V, whereas PVA without fatty acid failed in both experiments. Thus, the results of the second experiment showed that the additional effect of BSA‐V on inducing AR is a result of the presence of fatty acids in BSA‐V. Stewart 18 found that capacitation in golden hamster spermatozoa was increased more in the presence of fatty acids bound to BSA than the fatty acid free BSA. Creutz 19 reported that exogenous unsaturated fatty acids promoted the fusion of chromaffin granules, which are associated with AR. Peter 20 and Meizel 21 suggested that a variety of molecules, such as fatty acids and amines, are associated with BSA which possibly act on the sperm plasma membrane and trigger AR. In contrast, oleic, arachidonic and docosahexanoic acid triggered AR in hamster spermatozoa. 22 High amounts of fatty acids in mammalian sperm plasma membrane play a major role in membrane fusions and increasing AR. 23 The findings of these studies are consistent with the results of the present study, showing that fatty acids of BSA‐V provided an adequate environment for boar sperm AR. Thus, the present study is the first report on the effect of fatty acids bound to BSA‐V on AR in boar spermatozoa.
The present study shows that fatty acids bound to BSA‐V stimulate the rate of incorporation and oxidation of 14C‐glucose in boar spermatozoa in almost all incubation periods. Although, under normal physiological circumstances, oxidation of glucose has an inverse correlation with oxidation of fatty acid. Wolfe 24 stated that oxidation of glucose increases oxidation of fatty acids. The trend of incorporation and oxidation of glucose being the same as AR is supported by the findings of Miah et al. 12 and suggests that incorporation and oxidation of glucose increased up to 3 h for more ATP for AR at that time. Tsujii et al. 25 also reported that fructose stimulated induction of the AR in boar spermatozoa. Mayes 26 reported that fatty acids which are bound to BSA have metabolic significance in sperm cells and most of the long chain fatty acids derived from these fatty acids by chain elongation. The function of fatty acid oxidation is, of course, to generate metabolic energy. 27 Octanoate metabolism indicates that inside the mitochondria, there is a stimulatory effect of glucose on β‐oxidation of fatty acids. 24 Each round of β‐oxidation produces one NADH, one FADH2, and one acetyl‐CoA. Oxidation of acetyl‐CoA through the citric acid cycle generates additional FADH2 and NADH, which are reorganized through oxidative phosphorylation to form ATP. Complete oxidation of a fatty acid molecule is, therefore, a highly exergonic process which yields numerous ATP. 27 However, ATP is necessary for sperm motility. Boar sperm has a complex system to optimize its ATP levels through a sophisticated regulation of its energy fluxes. 28 It is noteworthy that the control of boar sperm pyruvate kinase and LDH is greatly dependent on the intracellular ATP/ADP ratio. 29 , 30 This seems to show that one of the most important mechanisms by which boar sperm can simultaneously control separate metabolic pathways is through the regulation of its intracellular ATP levels and ATP/ADP ratio, which directly modulates the consumption of separate energy substrates. 28 Obtaining energy substrates could allow boar sperm to increase their survival rate and metabolic strategy that might play an important role in improving sperm functions. The relevance of such metabolic activity in spermatozoa is not yet firmly established. In contrast, Neill 31 reported that metabolism of fatty acid is correlated with bovine sperm motility, but we speculate that such metabolism is not only associated with motility but also with acrosome reaction.
However, intracellular signaling cascades induced by fatty acids bound to BSA‐V in the stimulation of AR are still not clearly understood. We suggest that fatty acids present in BSA‐V supplemented the sperm incubation medium thought to increase intracellular Ca2+ and cAMP concentration, which are responsible for inducing AR. In another experiment, Tsai 32 found that high‐lipid BSA induces more Ca2+ influx in human osteoblast‐like cells than the low lipid BSA. Dominguez 33 stated that arachidonic acid induces acrosome reaction in human spermatozoa by increasing Ca2+ influx. The increase in protein tyrosine phosphorylation during AR is regulated by increasing intracellular cAMP and Ca2+ influx. 34 Thus, the effect of individual fatty acids of BSA‐V on sperm functions and intracellular concentration of cAMP, Ca2+ influx and protein tyrosine phosphorylation by fatty acids in boar spermatozoa needs to be studied further for a complete understanding of the mechanism by which fatty acids bound to BSA improve motility and induce AR. Fatty acid content of BSA‐V can enhance sperm motility and AR. Fatty acids bound to BSA‐V are able to access the spermatozoa easily, be absorbed by them and are highly activated for sperm metabolism. In conclusion, fatty acids bound to BSA‐V are more capable than fatty acid free BSA to improve AR and enhance the utilization of glucose in boar spermatozoa.
REFERENCES
- 1. Harrison RAP, Dott HM, Foster GC. Bovine serum albumin, sperm motility and the dilution effect. J Exp Zool 1982; 222: 81–88. [DOI] [PubMed] [Google Scholar]
- 2. Chen FR. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem 1967; 242: 173–181. [PubMed] [Google Scholar]
- 3. Ravnik SE, Albers JJ, Muller CH. A novel view of albumin supported sperm capacitation: role of lipid transfer protein‐1. Fertil Steril 1993; 59: 629–638. [DOI] [PubMed] [Google Scholar]
- 4. Mann T. The Biochemistry of Semen and of the Male Reproductive Tract. New York: John Wiley and Sons, 1964. [Google Scholar]
- 5. Scott TW, Dawson RMC. Metabolism of phospholipids by spermatozoa and seminal plasma. Biochem J 1968; 108: 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fleming AD, Yanagimachi R. Evidence suggesting the importance of fatty acids and the fatty acid moieties of sperm membrane phospholipids in the acrosome reaction of guinea pig spermatozoa. J Exp Zool 1984; 229: 485–489. [DOI] [PubMed] [Google Scholar]
- 7. Parrish JJ, Susko‐Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod 1988; 38: 1171–1180. [DOI] [PubMed] [Google Scholar]
- 8. Khandoker MAMY, Nishioka M, Tsujii H. Effect of BSA binding fatty acids on mouse and rat embryo development. J Mamm Ova Res 1995; 12: 113–118. [Google Scholar]
- 9. Khandoker MAMY, Tsujii H. Effect of exogenous fatty acids on in vitro development of rat embryos. Asian-Aus J Anim Sci 1999; 12: 169–173. [Google Scholar]
- 10. Johnson LA, Aalbers JG, Grooten HJG. Artificial insemination of swine: fecundity of boar semen stored in Beltsville TS (BTS), modified Modena (MM), or MR‐A and inseminated on one, three and four days after collection. Zuchthyg 1988; 23: 49–55. [Google Scholar]
- 11. Ooba T, Sricharoen P, Areekijsree M, Kitiyanat Y, Pavasuthipaisit K. Evaluation of acrosome reaction in bovine sperm by a triple staining technique. J Physiol Sci 1990; 3: 91–104. [Google Scholar]
- 12. Miah AG, Hossain MS, Tareq KMA et al Effect of relaxin on motility, acrosome reaction and viability of cryopreserved boar spermatozoa. Reprod Med Biol 2006; 5: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uto N, Yamahama Y. The motility and fertility of golden hamster sperm cultured in BSA‐free medium. Biol Cell 1996; 88: 23–28. [DOI] [PubMed] [Google Scholar]
- 14. He L, Bailey JL, Buhr MM. Incorporating lipids into boar sperm decreases chilling sensitivity but not capacitation potential. Biol Reprod 2001; 64: 69–79. [DOI] [PubMed] [Google Scholar]
- 15. Bavister BD. Substitution of a synthetic polymer for protein in a mammalian gamete culture system. J Exp Zool 1981; 217: 45–51. [DOI] [PubMed] [Google Scholar]
- 16. Chang MC, Hunter RHF. Capacitation of mammalian sperm. Biological and experimental aspects In: Hamilton DW, Greep RO, eds. Handbook of Physiology. Washington: American Physiology Society, 1975; 339–351. [Google Scholar]
- 17. Barros CJ, Bedford M, Franklin LE, Austin CR. Membrane vesiculation as a feature of the mammalian acrosome reaction. J Cell Biol 1967; 34: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stewart SJ. Effect of bovine serum albumin concentration and source on sperm capacitation in the golden hamster. Biol Reprod 1993; 49: 74–81. [DOI] [PubMed] [Google Scholar]
- 19. Creutz CE. Cis‐unsaturated fatty acids induce the fusion of chromaffin granules aggregated by synexin. J Cell Biol 1981; 91: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peter TT. Serum albumin In: Putnam FW, ed. The Plasma Proteins: Structure, Function, and Genetic Control. New York: Academic Press, 1975; 1133–1181. [Google Scholar]
- 21. Meizel S. The mammalian sperm acrosome reaction, a biochemical approach In: Johnson MH, ed. Development in Mammals. Amsterdam: North‐Holland Publishing Co, 1979; 1–64. [Google Scholar]
- 22. Meizel S, Turner KO. Stimulation of an exocytotic event, the hamster sperm acrosome reaction, by cis‐unsaturated fatty acids. FEBS Lett 1983; 161: 315–318. [DOI] [PubMed] [Google Scholar]
- 23. Wolf DE, Lipscomb AC, Maynard VM. Causes of nondiffusing lipid in the plasma membrane of mammalian spermatozoa. Biochemistry 1988; 27: 860–865. [DOI] [PubMed] [Google Scholar]
- 24. Wolfe RR. Metabolic interactions between glucose and fatty acids in humans. Am J Clin Nutr 1998; 67: 519–526. [DOI] [PubMed] [Google Scholar]
- 25. Tsujii H, Ohta E, Miah AG, Hossain MS, Salma U. Effect of fructose on motility, acrosome reaction and in vitro fertilization capability of boar spermatozoa. Reprod Med Biol 2006; 5: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayes PA. Metabolism of unsaturated fatty acids and eicosanoids In: Barnes DA, ed. Harper's Biochemistry. USA: McGraw‐Hill, 2000; 250–258. [Google Scholar]
- 27. Voet D, Voet J. Biochemistry. New York: John Wiley and sons, 1995. [Google Scholar]
- 28. Medrano A, Pena A, Rigau T, Rodriguez‐Gil JE. Variations in the proportion of glycolytic/non‐glycolytic energy substrates modulate sperm membrane integrity and function in diluted boar samples stored at 15–17°C. Reprod Dom Anim 2005; 40: 448–453. [DOI] [PubMed] [Google Scholar]
- 29. Medrano A, Pena A, Rigau T, Rodriguez‐Gil JE. Citrate and lactate as feasible energy sources for boar spermatozoa. Reprod Dom Anim 2003; 38: 340. [Google Scholar]
- 30. Medrano A, Pena A, Rivera del Alamo M, Ramio L, Rigau T, Rodriguez‐Gil JE. Utilization of different monosaccharide by boar sperm. Reprod Dom Anim 2004; 39: 259.
- 31. Neill AR. Metabolism of fatty acids by bovine spermatozoa. Biochem J 1972; 127: 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsai JA, Lagumdzija A, Stark A, Kindmark H. Albumin‐bound lipids induce free cytoplasmic calcium oscillations in human osteoblast‐like cells. Cell Biochem Funct 2006. [DOI] [PubMed]
- 33. Dominguez L, Yunes RMF, Fornes MW, Burgos M, Mayorga LS. Calcium and phospholipase A2 are both required for the acrosome reaction mediated by G‐proteins stimulation in human spermatozoa. Mol Reprod Dev 1999; 52: 297–302. [DOI] [PubMed] [Google Scholar]
- 34. Visconti PE, Moore GD, Bailey JL et al Capacitation in mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by cAMP‐dependent pathway. Development 1995; 121: 1139–1150. [DOI] [PubMed] [Google Scholar]


