Abstract
Aim: The present study was carried out to investigate the effects of fructose supplementation in glucose containing mTALP medium on motility, acrosome reaction and in vitro fertilization capability of boar spermatozoa.
Methods: Boar spermatozoa were preincubated, swum‐up, resuspended and then incubated for 6 h in mTALP medium supplemented with 0, 0.5, or 1.0 mmol fructose in the presence of 5.0 mmol glucose. After completion of the specified incubation period, motility was determined subjectively on the basis of speed of progression and on the type of forward movement of spermatozoa; acrosome status was evaluated by applying a triple staining technique; and in vitro fertilization capability was assessed by acetic–orcein staining.
Results: The combination of fructose and glucose (0.5 + 5.0 mmol) supplements in mTALP medium improved sperm motility significantly (P < 0.05), more than glucose alone (5.0 mmol) at 2–6 h of incubation. Acrosome reaction (live spermatozoa) and the sperm penetration rate was increased significantly (P < 0.05) when the spermatozoa were treated with the combination of fructose and glucose compared with glucose alone, but the incidence of polyspermy was not significantly different between the treatments.
Conclusion: These results suggest that the combination of glucose and fructose as supplements in mTALP medium improve the progressive motility, acrosome reaction and fertilization capability of boar spermatozoa. (Reprod Med Biol 2006; 5: 255–261)
Keywords: acrosome reaction, boar spermatozoa, fructose, in vitro fertilization, motility
INTRODUCTION
IN RECENT YEARS, substantial progress has been made in the development of procedures to produce pig embryos in vitro. However, polyspermic fertilization remains a major unresolved problem. Polyspermy results in early embryonic death and greatly limits the production of viable pig embryos. Further improvements are necessary to maximize embryo production through reduction of polyspermic fertilization by improving sperm activity. In mammalian spermatozoa, motility is one of the most important prerequisites for fertilization. From the functional point of view, motility is strongly related to the spermatozoa's ability to manage its energy status and a high percentage of spermatozoa energy consumption is centered on the contraction of the flagellum. 1 Thus, modulation of energy status could be translated into significant changes in motility patterns. In fact, this relationship between energy metabolism and motility patterns could be the basis for the dramatic changes observed in spermatozoa during their movement through the female reproductive tract. 2
Freshly ejaculated mammalian spermatozoa are not immediately capable of fertilizing an egg until they undergo capacitation, either within the female reproductive tract or in a suitable medium in vitro. Mammalian sperm gain the ability to undergo the acrosome reaction (AR) in response to the zona pellucida and penetrate the oocyte only after maturation in the female reproductive tract. 3 , 4 , 5 , 6 , 7 In vitro, this phenomenon can be reproduced in defined media. 8 The components of the culture media are important for sperm motility, capacitation and AR. Mammalian spermatozoa require exogenous substrates for a variety of functions; for example, to preserve intracellular energy reserves, cell components and most notably to support motility. 9 They can obtain energy through mitochondrial oxidative phosphorylation and glycolysis by the consumption of glycolysable sugars, such as glucose, fructose, mannose and maltose. 10 Fructose is thought to be a major energy source for ejaculated spermatozoa and is found in seminal plasma in many mammalian species. 11 In many species, fructose has been investigated for the different effects on gametes in terms of metabolizable energy and fertility potential, and the beneficial effects vary between species. 12 Capacitation and AR of boar spermatozoa are slightly affected when glucose is the only energy source in the culture medium. 13 Although, fresh boar semen contains 1.67 mmol fructose 14 as a nutrient for spermatozoa, it is removed when semen samples are washed for utilization in the field for in vitro fertilization (IVF) or preservation for application in artificial insemination programs. Therefore, we considered that the addition of fructose to an optimum level in basic medium is essential to prepare the spermatozoa for boar IVF programs. However, no study has been conducted on the effects of fructose on the fertilizing activities of boar spermatozoa. The present study was undertaken to investigate the effects of fructose on motility, acrosome reaction and IVF capability of boar spermatozoa.
MATERIALS AND METHODS
Semen collection and preparation
DILUTED SEMEN FROM Duroc boars was collected from Nagano Animal Industry Experiment Station, Nagano, Japan. The semen was diluted according to the method of Johnson et al. 15 with Modena extender to give a sperm concentration of 1 × 108/mL at room temperature. The vial of semen was carried in a cork box to the laboratory. The upper most 10 mL of the semen sample was taken from the vial and incubated for 10 min in a water bath at 37°C for anabiosis of spermatozoa. After anabiosis, 3 mL of sperm suspension was washed three times with mTALP 16 medium by centrifugation at 900 × g for 5 min each time. The sperm pellet was suspended in mTALP medium containing 5.0 mmol glucose and incubated at 37°C for 1 h to swim‐up.
Semen treatments
To evaluate the effects of fructose on sperm motility and acrosome reaction, 0, 0.5, or 1.0 mmol fructose (Sigma Chemical, St Louis, MO, USA) was added to the mTALP medium. A 50 µL sample of spermatozoa obtained from swim‐up separation was suspended in prepared drops of the media to give a final sperm concentration of 5 × 106/mL and incubated at 37°C for 0–6 h to evaluate the sperm motility.
To evaluate acrosome status, swim‐up spermatozoa was resuspended separately in mTALP medium containing 0, 0.5, or 1.0 mmol fructose at a concentration of 20 × 106/mL and incubated at 37°C for 0–6 h.
Progressive motility
After completion of the specified incubation period, three subsamples of each sample were placed on warm glass slides (37°C) for motility and movement quality evaluation. The slides were examined under a light microscope (×400). Sperm motility was assessed by determining the percentage of spermatozoa in four categories of movement: (i) rapid progressive motility; (ii) slow or sluggish progressive motility; (iii) non‐progressive motility; and (iv) immotility and was expressed as progressive motility percentage.
Acrosome status
To evaluate acrosome reaction, spermatozoa were stained after incubation by applying the triple staining technique described previously. 17 Briefly, a 100 µL aliquot (20 × 106 spermatozoa/mL) was diluted with an equal volume of trypan blue, incubated at 39°C for 15 min and then fixed for 60 min in 2.5% glutaraldehyde. Fixed spermatozoa were stained with 0.8% Bismark brown at 40°C for 5 min and then with 0.8% Rose Bengal at 24°C for 30 min. The triple stained slides were examined by light microscopy (×1000). Acrosome status of the spermatozoa was evaluated from randomly selected fields of the triple stained slides until 400 spermatozoa had been examined. Dead spermatozoa were stained by trypan blue in the post acrosomal region and showed dark blue pattern, whereas live spermatozoa were not stained by this dye but were stained by Bismark brown and showed a light brown color in the post acrosomal region. The acrosome intact or unreacted spermatozoa showed a pink color in the acrosomal cap but spermatozoa with reacted acrosome or in the absence of the acrosome had unstained acrosomal caps. Therefore, spermatozoa could be categorized based on the staining patterns into the following characteristics: (i) live and acrosome unreacted; (ii) live and acrosome reacted; (iii) dead and acrosome unreacted; and (iv) dead and acrosome reacted.
Oocytes collection and in vitro maturation
Ovaries were collected from prepubertal cross‐bred gilts (Landrace, Large White and Duroc) at a local abattoir and transported to the laboratory in 0.9% (w/v) NaCl containing 75 µg/mL potassium penicillin G and 50 µg/mL streptomycin sulfate maintained at 30°C. Cumulus‐oocyte complexes (COC) were aspirated from antral follicles of 3–5 mm in diameter with an 18‐gauge needle fixed to a 10 mL disposable syringe. COC were washed three times with modified NCSU‐37 medium containing 10% (v/v) porcine follicular fluid, 0.6 mmol cystein, and 1 mmol dibutyl cyclic AMP (dbc AMP; Nacalai Tesque, Kyoto, Japan). Groups of 20 COC were suspended in 50 µL of modified NCSU‐37 medium in a four‐well culture dish and cultured at 39°C for 22 h in an atmosphere of 5% CO2 in air. Hormonal supplementation, 10 ng/mL epidermal growth factor (Sigma Chemical), 10 IU/mL hCG (Sankyo Zoki, Tokyo, Japan), and 10 IU/mL PMSG (Sankyo Zoki) were added to the medium just before culture. The medium had been previously covered with warm paraffin oil in polystyrene cell culture dishes (35 × 10 mm2, Iwaki Glass, Tokyo, Japan) and equilibrated for at least 6 h. After completion of 22 h maturation, the oocytes were washed three times in hormone‐free basic maturation medium and transferred in 50 µL of modified NCSU‐37 medium to a four‐well culture dish for an additional 22 h of culture. After maturation, oocytes were stripped of cumulus cells by pipetting with 0.1% (w/v) hyaluronidase.
In vitro fertilization
Cumulus free matured oocytes were washed three times with fertilization medium and 30 oocytes were transferred into 50 µL wells with fertilization medium covered with mineral oil that had been equilibrated overnight at 39°C in 5% CO2 in air. The culture dishes were kept in a CO2 incubator until spermatozoa were added for fertilization. The IVF medium was mTALP medium containing 3 mg/mL BSA (Sigma Chemical), 2 mmol caffeine (Sigma Chemical) and 0, 0.5, or 1.0 mmol fructose with 5.0 mmol glucose (Sigma Chemical). After washing twice by centrifugation at 900 × g (5 min each time), the sperm pellet was resuspended in 100 µL of mTALP to give a final concentration of 5 × 106 spermatozoa/mL. The 50 µL spermatozoa sample was added to the 50 µL of the fertilization medium containing the oocytes. The oocytes and spermatozoa were cocultured for 6 h at 39°C in an atmosphere of 5% CO2 in air. After 6 h of spermatozoa‐oocyte coincubation, putative embryos were washed three times in oocyte culture medium NCSU‐37, transported in 50 µL of the same medium to a four‐well culture dish and incubated for 18 h at 39°C in an atmosphere of 5% CO2 in air.
Assessment of oocytes fertilization
At 24 h after IVF, oocytes were mounted on a glass slide and fixed for 10 min in 25% (v/v) acetic acid in ethanol. Fixation was carried out on a warm plate (33°C) for the removal of lipid within 10 min. Then oocytes were stained with 1% (w/v) orcein in 45% (v/v) acetic acid solution and examined under a phase‐contrast microscope at ×400. Oocytes at the metaphase II stage were regarded as mature. Oocytes were considered to be penetrated when they had one or more swollen sperm heads or male pronuclei with corresponding sperm tails in the vitellus. Only oocytes containing male and female pronuclei with intact nuclear membrane were defined as having formed pronuclei. When an oocyte contained one female and one male pronucleus, it was considered as monospermy fertilization and oocytes with one female and more than one male pronucleus were considered as polyspermy fertilization.
Statistical analysis
The mean value with SEM of sperm motility, acrosome status and penetration rate, monospermy and polyspermy for each treatment at different incubation times were calculated and statistical significance between the spermatozoa treated with 0, 0.5, or 1.0 mmol fructose and 5.0 mmol glucose was carried out using the protected Fisher least difference test. The NCSS (Number Crunchier Statistical System) Version 5.01 computer software package was used for all statistical analysis. Each experiment was repeated five times. Differences were considered significant at P < 0.05.
RESULTS
THE EFFECT OF fructose (1.0 mmol) and glucose (5.0 mmol) supplementation in mTALP medium on progressive motility is shown in Figure 1. The percentage of spermatozoa showing progressive motility and hyperactivation (data not shown) were observed to be significantly (P < 0.05) higher in fructose treated spermatozoa than only glucose treated spermatozoa.
Figure 1.
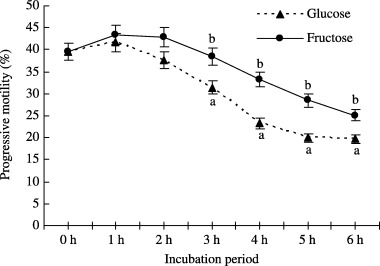
Effect of fructose (1.0 mmol) and glucose (5.0 mmol) supplementation in mTALP medium on progressive motility of boar spermatozoa incubated for 0–6 h. Values are expressed as means ± SEM values from five replicates. Different letters indicate significant differences (P < 0.05) in the same incubation period.
Progressive motility of boar spermatozoa treated with 0.5 mmol fructose and 5.0 mmol glucose is shown in Figure 2. The percentage of progressive motility and hyperactivation with 0.5 mmol fructose + 5.0 mmol glucose and 1.0 mmol fructose + 5.0 mmol glucose was significantly higher than the 5.0 mmol glucose treated sample from 2 to 6 h of incubation.
Figure 2.
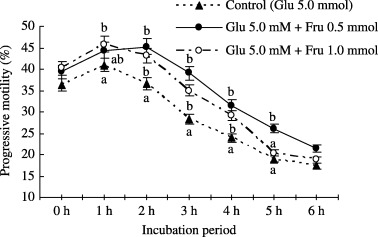
Effect of 0, 0.5, or 1.0 mmol fructose and/or 5.0 mmol glucose supplementation in mTALP medium on progressive motility of boar spermatozoa incubated for 0–6 h. Values are expressed as means ± SEM values from five replicates. Different letters indicate significant differences (P < 0.05) in the same incubation period.
The effect of glucose and different levels of fructose supplemention in mTALP medium on sperm acrosome reaction (live and dead) after 5 h of incubation is shown in Figure 3. The percentage of acrosome reacted spermatozoa was observed to be significantly (P < 0.05) higher when spermatozoa were incubated in 0.5 mmol fructose + 5.0 mmol glucose and 1.0 mmol fructose + 5.0 mmol glucose supplemented medium than that of spermatozoa incubated in 5.0 mmol glucose supplemented medium.
Figure 3.
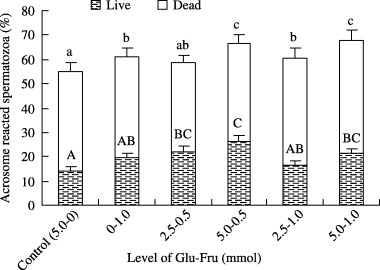
Effect of fructose and glucose combination at different concentrations to supplement mTALP medium on acrosome reaction (live and dead) of boar spermatozoa at 5 h of incubation. Values are expressed as means ± SEM values from five replicates. Different letters (capital for acrosome reacted live and lower case for acrosome reacted dead spermatozoa) indicate significant differences (P < 0.05) among the levels of glucose and fructose (mmol).
Acrosome reaction of boar spermatozoa was influenced by supplementation of 5.0 mmol glucose, 0.5 mmol fructose + 5.0 mmol glucose, and 1.0 mmol fructose + 5.0 mmol glucose in medium incubated up to 6 h is shown in Figure 4. The proportion of acrosome reacted live spermatozoa treated with 1.0 mmol fructose + 5.0 mmol glucose and 0.5 mmol fructose + 5.0 mmol glucose containing mTALP medium was significantly (P < 0.05) higher than the spermatozoa treated with only 5.0 mmol glucose at 1–5 h of incubation.
Figure 4.
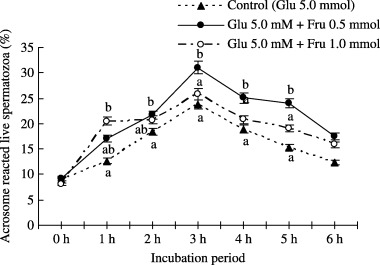
Effect of 0, 0.5, or 1.0 mmol fructose and/or 5.0 mmol glucose supplementation in mTALP medium on acrosome reaction (live) of boar spermatozoa at 5 h of incubation. Values are expressed as means ± SEM values from five replicates. Different letters indicate significant differences (P < 0.05) in the same incubation period.
In IVF studies, the sperm penetration rate observed in 5.0 mmol glucose and 0.5 mmol fructose + 5.0 mmol glucose supplemented medium is shown in the Table 1. The sperm penetration rate was significantly (P < 0.05) higher in spermatozoa treated with 0.5 mmol fructose + 5.0 mmol glucose supplemented medium than that of 5.0 mmol glucose. There was no significant difference in monospermy and polyspermy fertilization when coincubating with the spermatozoa treated with 5.0 mmol glucose or the combination of 0.5 mmol fructose + 5.0 mmol glucose.
Table 1.
Effect of fructose on in vitro fertilization of boar spermatozoa
| Treatment | No. oocytes | Fertilized oocytes number (%) | Penetrated sperm/oocyte | ||
|---|---|---|---|---|---|
| Penetrated | Monospermy | Polyspermy | |||
| Control (Glu 0.5.0 mmol) | 266 | 154 (58.1 ± 1.8)* | 60 (38.8 ± 0.7) | 94 (61.2 ± 0.7) | 1.71 ± 0.07 |
| Glu + Fru (0.5 + 0.5 mmol) | 265 | 176 (66.7 ± 1.8)* | 75 (42.2 ± 1.8) | 101 (58.2 ± 2.0) | 1.82 ± 0.05 |
Fru, fructose; Glu, glucose.
Significantly different (P < 0.05). Values are expressed as means ± SEM from three replicates.
DISCUSSION
MAMMALIAN SPERMATOZOA REQUIRE metabolic energy for a variety of functions, most notably to support motility. The principal sources of adenosine triphospate (ATP) production are mitochondrial oxidative phosphorylation and glycolysis. 18 Sugars in the external medium can be used by spermatozoa in two ways: first, for energy obtained by metabolism of the sugars through glycolysis and the Krebs cycle; and second, for the storage of sugars in the form of glycogen, to obtain middle‐ to long‐term energy reserves for maintaining motility when the environment does not provide an external energy source. 19 Thus, boar spermatozoa are almost totally reliant on the glycolytic pathway, not only for the direct production of ATP by substrate level phosphorylation, but also for the production of lactate, which is the primary source of mitochondrial acetyl CoA. 20 Fructose is an essential nutrient for spermatozoa, which is metabolized and converted to pyruvate and lactate. The metabolism of fructose by spermatozoa proceeds by way of the Embden–Meyerh pathway. Hexose phosphates, triose phosphates and pyruvic acid are involved as transient intermediates leading to the formation of lactic acid, which tends to accumulate although it is further oxidized in the presence of oxygen to carbon dioxide and water by the Krebs cycle. 14 In boar spermatozoa, glycolysis is the main pathway of glucose utilization producing lactate/pyruvate, limited glycogen synthesis through the direct pathway and some incorporation of pyruvate into the Krebs cycle also take place. 21 In boar sperm, the metabolic profile is characterized by a high glycolytic rate with low glucose 6‐phosphate levels. Boar spermatozoa are capable of utilizing lactate when it is the major carbon source, 20 but it is currently unclear whether lactate driven motility can be sustained for prolonged periods of time, or is sufficiently vigorous to achieve optimal fertilization. In the present study, based on the progressive motility observed after incubation with glucose alone or with glucose and fructose, these sugars might play different roles in regulating in vitro spermatozoa function. It is worth noting that glucose plays a specific role in spermatozoa hyperactvation in several species 21 where our results show that the combination of glucose and fructose has a more positive effect on progressive motility than glucose alone. The specific steps for which glucose is necessary might include hyperactivated motility 22 , 23 and sperm‐oocyte fusion, 24 which are dependent on the provision of nicotinamide adenine dinuclaotide phosphate (NADPH) by glucose metabolism through the pentose phosphate pathway. 25 However, the present findings show greater progressive motility when spermatozoa were incubated in mTALP medium supplemented with a combination of fructose and glucose compared with that in glucose alone.
The acrosome reaction is a morphological change that occurs in live mammalian spermatozoa before they enter the egg in the process of fertilization. 26 Because actively motile spermatozoa can fertilize eggs only after losing their acrosomal caps, 27 an acrosome reaction can be considered an indication that the spermatozoa have completed capacitation and acquired the ability to fertilize eggs. 28 Because the acrosome reaction is primarily a membrane phenomenon, one logical possibility is that in the presence of glucose, fructose somehow interacts with the plasma membrane or the outer acrosomal membrane to it break down. The presence of glucose and fructose could influence membrane function, which is characteristic of the acrosome reaction. In our pre‐experiment, fructose significantly enhanced the ability of boar spermatozoa to undergo acrosome reaction up to 6 h of incubation in comparison with the glycolysable sugar, glucose. The possibility of the sugars acting as a control system is also supported by the fact that responses to both glucose and fructose occur rapidly. When fructose is available in the medium, the spermatozoa appear to undergo acrosome reaction in preference to when glucose supplemented alone. If glucose alone is present as the sole source of energy, the rate of initiation of the acrosome reaction is lower than that with the combination of glucose and fructose. The present results indicate that a combination of energy substrates could render better effects on sperm motility, capacitation, and subsequently acrosome reaction and fertilization than the addition of a single substrate into the media. This supports speculation that the combination of glucose and fructose might be metabolized to lactate or pyruvate until the intracellular concentration of these metabolites builds up to a certain level that then becomes capable of inducing acrosome reaction. It is attractive to postulate that acrosome reaction of spermatozoa is controlled by the environment. In the presence of a high concentration of glucose and/or fructose, which exist in semen, 11 spermatozoa might take longer for the acrosome reaction to begin. However, when the spermatozoa are at the site of fertilization in the oviduct, the microenvironment is rich in lactate and pyruvate so the acrosome reaction would be initiated much sooner.
Rogers and Perreault examined both fertilization and acrosome reaction in the presence of glucose or other sugars. 29 If glucose or other sugars are present during the fertilization step it is possible that they might influence the eggs rather than the sperm or, alternatively, that enhanced sperm acrosome reaction in the presence of sugars might promote a higher penetration rate through a higher collision frequency. Glucose is required for successful fertilization by boar sperm. 30 Furthermore, in the present study, the combination of glucose and fructose was found to have a significant effect on fertilization capability of boar spermatozoa.
An optimal number of fully capacitated spermatozoa at the site of fertilization is extremely important for reducing the incidence of polyspermy in pigs, 31 , 32 , 33 because it not only maintains a high proportion of oocytes being fertilized but also avoids high rates of polyspermic penetration. The present result indicates that glucose, or the combination of glucose and fructose, is not able to reduce the incidence of polyspermy fertilization in pigs.
In conclusion, the present findings indicate that the combination of glucose and fructose enhances motility, acrosome reaction and the penetration rate of spermatozoa. Therefore, it is suggested that the combination of glucose and fructose can be used to supplement mTALP medium to improve the progressive motility, acrosome reaction and fertilization capability of boar spermatozoa.
REFERENCES
- 1. Roldan ERS. Signal transduction during mammalian sperm acrosomal exocytosis In: Lauria A, Gandolfi F, Enne G, Gianaroli L, eds. Gametes: Development and Function. Serono Symposia, Rome, 1998; 219–228. [Google Scholar]
- 2. Mortimer S. A critical review of the physiological importance and analysis of sperm movement in mammals. Hum Reprod Update 1997; 3: 403–439. [DOI] [PubMed] [Google Scholar]
- 3. Boatman DE, Robbins RS. Bicarbonate: carbon‐dioxide regulation of sperm capacitation, hyperactivated motility, and acrosome reactions. Biol Reprod 1991; 44: 806–813. [DOI] [PubMed] [Google Scholar]
- 4. Fraser LR. Minimum and maximum extracellular Ca2+ requirements during mouse sperm capacitation and fertilization in vitro . J Reprod Fertil 1987; 81: 77–89. [DOI] [PubMed] [Google Scholar]
- 5. Fraser LR, Abeydeera LR, Niwa K. Ca2+‐regulating mechanisms that modulate bull sperm capacitation and acrosomal exocytosis as determined by chlortetracycline analysis. Mol Reprod Dev 1995; 40: 233–241. [DOI] [PubMed] [Google Scholar]
- 6. Shi QX, Roldan ER. Bicarbonate/CO2 is not required for zona pellucida‐ or progesterone‐induced acrosomal exocytosis of mouse spermatozoa but is essential for capacitation. Biol Reprod 1995; 52: 540–546. [DOI] [PubMed] [Google Scholar]
- 7. Harrison RAP, Ashworth PJ, Miller NG. Bicarbonate/CO2, an effect or of capacitation, induces a rapid and reversible change in the lipid architecture of boar sperm plasma membranes. Mol Reprod Dev 1996; 45: 378–391. [DOI] [PubMed] [Google Scholar]
- 8. Harrison RAP. Capacitation mechanisms, and the role of capacitation as seen in eutherian mammals. Reprod Fertil Dev 1996; 8: 581–594. [DOI] [PubMed] [Google Scholar]
- 9. Salisbury GW, VanDemark NL, Lodge JR. Extenders and extension of unfrozen semen In: Salisbury GW, ed. Physiology of Reproduction and Artificial Insemination of Cattle. W.H. Freeman and Company, San Francisco, 1978; 442–493. [Google Scholar]
- 10. Salisbury GW, VanDemark NL. Principles and technique of spermatozoa preservation In: Salisbury GW, Crampton EW, eds. Physiology of Reproduction and Artificial Insemination of Cattle. W.H. Freeman and Company, San Francisco, 1961; 380–402. [Google Scholar]
- 11. Nagai T, Yamaguchi K, Moriwaki C. Studies on the effects of sugars on washed human sperm motility. J Pharmacol Dynamics 1982; 5: 564–567. [DOI] [PubMed] [Google Scholar]
- 12. Williams AC, Ford WCL. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl 2001; 22: 680–695. [PubMed] [Google Scholar]
- 13. Medrano A, Pena A, Rigau T, Rodriguez‐Gil JE. Variations in the proportion of glycolytic/non‐glycolytic energy substrates modulate sperm membrane integrity and function in diluted boar samples stored at 15–17°C. Reprod Domest Anim 2005; 40: 448–453. [DOI] [PubMed] [Google Scholar]
- 14. Mann T. The Biochemistry of Semen and of the Male Reproductive Tract. Methuen, London, 1964; 94–110. [Google Scholar]
- 15. Johnson LA, Aalbers JG, Grooten HJG. Artificial insemination of swine: fecundity of boar semen stored in Beltsville TS (BTS), modified Modena (MM), or MR‐A and inseminated on one, three and four days after collection. Zuchthyg 1988; 23: 49–55. [Google Scholar]
- 16. Rath D, Long CR, Dbrinsky JR, Welch GR, Schreir LL, Johnson LA. In vitro production of sexed embryos for gender pre selection: high‐speed sorting of X‐chromosome bearing sperm to produce pigs after embryo transfer. J Anim Sci 1999; 77: 3346–3352. [DOI] [PubMed] [Google Scholar]
- 17. Ooba T, Sricharoen P, Areekijseree M, Kitiyanant Y, Pavasuthipaisit K. Evaluation of acrosome reaction in bovine sperm by a triple staining technique. J Physiol Sci 1990; 3: 91–104. [Google Scholar]
- 18. Williams AC, Ford WC. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl 2001; 22: 680–695. [PubMed] [Google Scholar]
- 19. Rigau T, Farre M, Ballester J, Mogas T, Pena A, Rodriguez‐Gil JE. Effects of glucose and fructose on motility patterns of dog spermatozoa from fresh ejaculates. Theriogenology 2001; 56: 801–815. [DOI] [PubMed] [Google Scholar]
- 20. Jones AR, Milmlow D. Endogenous energy production by mature boar spermatozoa. J Reprod Fert 1997; 111: 285–290. [DOI] [PubMed] [Google Scholar]
- 21. Marin S, Chiang K, Bassilian S et al. Metabolic strategy of boar spermatozoa revealed by a metabolomic characterization. FEBS Lett 2003; 554: 342–346. [DOI] [PubMed] [Google Scholar]
- 22. Fraser LR, Quinn PJ. A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whipiash motility required for fertilization in the mouse. J Treprod Fertil 1981; 61: 25–35. [DOI] [PubMed] [Google Scholar]
- 23. Urner F, Sakkas D. Glucose is not essential for the occurrence of sperm binding and zona‐pellucida‐induced acrosome reaction in the mouse. Int J Androl 1996a; 19: 91–96. [DOI] [PubMed] [Google Scholar]
- 24. Urner F, Sakkas D. Glucose participates in sperm‐oocyte fusion in the mouse. Biol Reprod 1996b; 55: 917–922. [DOI] [PubMed] [Google Scholar]
- 25. Urner F, Sakkas D. A possible role for the pentose phosphate pathway of spermatozoa in gamete fusion in the mouse. Biol Reprod 1999; 60: 733–739. [DOI] [PubMed] [Google Scholar]
- 26. Bedford JM. Sperm capacitation and fertilization in mammals. Biol Reprod 1970; 2: 128–158. [PubMed] [Google Scholar]
- 27. Yanagimachi R. Behavior and functions of the structural elements of the mammalian sperm head in fertilization. In: Segal SJ, Crozier R, Corfman PA, Condliffe PG, Thomas CC, eds. The Regulation of Mammalian Reproduction. Springfield, Illinois, 1973; 215–230.
- 28. Barros C. Capacitation of mammalian spermatozoa In: Coutinho EM, Fuchs F, eds. Physiology and Genetics of Reproduction. Plenum Press, New York, 1974; 3–24. [Google Scholar]
- 29. Rogers BJ, Perreault SD. Importance of glycolysable substrates for in vitro capacitation of human spermatozoa. Biol Reprod 1990; 43: 1064–1069. [DOI] [PubMed] [Google Scholar]
- 30. Jelinkova P, Liberda J, Manaskova P, Ryslava H, Jonakova V, Ticha M. Mannan‐binding proteins from boar seminal plasma. J Reprod Immunol 2004; 62: 167–182. [DOI] [PubMed] [Google Scholar]
- 31. Rath D. Experiments to improve in vitro fertilization techniques for in vivo‐matured porcine oocytes. Theriogenology 1992; 37: 885–896. [DOI] [PubMed] [Google Scholar]
- 32. Coy P, Martinez E, Ruiz S, Vazquez JM, Roca J, Gadea C. Sperm concentration influences fertilization and male pronuclear formation in vitro in pigs. Theriogenology 1993; 40: 539–546. [DOI] [PubMed] [Google Scholar]
- 33. Xu X, Seth PC, Harbison DS, Cheung AP, Foxcroft GR. Semen dilution for assessment of boar ejaculated quality in pig IVM and IVF systems. Theriogenology 1996; 46: 1325–1337. [Google Scholar]


