Abstract
Purpose
The fatty acid composition of rabbit blastocysts, blood serum and uterine fluids were analyzed to study embryonic lipid metabolism.
Methods
Embryos were collected from Japanese white rabbits and fatty acids were analyzed by gas chromatograph.
Results
Total amount of fatty acids in blastocysts was higher than that in serum and uterine fluid. The amount of fatty acids in blastocysts markedly decreased during days 7–13 of pregnancy, and in serum had hovered, but in uterine fluid on day 13 was nine times higher than that on day 7 of pregnancy. Palmitic acid predominates in blastocysts, serum and uterine fluid during this period.
Conclusion
Palmitic acid is the most abundant fatty acid in the blastocysts, serum and uterine fluids of rabbit during days 7–13 of pregnancy.
Keywords: Blastocysts, Fatty acid, Rabbit, Serum, Uterine fluid
Introduction
The determination of biochemical needs of embryo development and of the environment surrounding embryos in vivo and in vitro are equally helpful for the improvement of embryo culture systems. In embryonic biochemistry and metabolism, data concerning amino acids, proteins, RNA, DNA and sugars are available for numerous species. Despite the significant role of the lipid reserve in cell structure and function, there is still a paucity of knowledge on species‐specific fatty acid composition. Embryo lipid level is species‐specific in terms of its apparent abundance and utilization. In in vitro embryo development, it is reasonable to consider that the composition of the culture medium should reflect the concentration of various nutrients, electrolytes and macromolecules present in the follicular, oviductal and uterine fluids [1]. Fatty acids in the embryos might be prerequisites for the formation of membranes that are crucial during the rapid cell division after fertilization, while some fatty acids represent compact reserves of stored energy. Evidence from different animal studies suggests that individual fatty acids may influence normal oocyte maturation, fertility and early embryo development [2, 3, 4, 5]. During the cleavage of mammalian embryos, it has been shown that mouse ova synthesize steroids and lipids [6] and metabolize fatty acids [7]. It has also been shown that exogenously supplied fatty acids are beneficial for the growth and development of rabbit embryos [8].
Bovine in vitro matured and in vitro fertilized oocytes have generally been cultured up to the blastocyst stage in serum‐supplemented mediums. It has been reported that the tolerance of bovine IVM/IVF embryos to cryopreservation was increased following the removal of their cytoplasmic lipid droplets [9]. Intracellular lipids may have a critical influence on the greater sensitivity of embryos to cryopreservation. From these points of view, cytoplasmic lipid accumulation of in vitro derived embryos may be a possible cause of the production of overweight offspring after embryo transfer and of the high sensitivity of embryos to cryopreservation. Therefore, it is very important to undertake biochemical analysis of the intracellular lipids of mammalian embryos.
However, there are only a few reports on the qualitative and quantitative analysis of lipid contents present in embryonic cytoplasm [10, 11, 12]. In the present experiment, we determined the fatty acid composition of day 7–13 rabbit blastocysts, at the time they are usually transferred. Thus, the objective of this study was to obtain basic information on the fatty acid compositions of lipids in blastocysts, serum and uterine fluid during the pregnancy and their relationship during the day 7–13 developmental period.
Materials and methods
Sample collection
Embryos were collected from kbl Japanese white rabbits (Kitayama Labs Co Ltd, Ina, Japan). After normal copulation, the day 7–13 blastocysts were collected by autopsy using the pin set. Only morphologically normal blastocysts were used, and their sizes were measured with an ocular micrometer and then recorded. The calculation method of the area of the embryo shape was done by the formula: (beside + length) ÷ 2 × square × π. Uterine fluid was collected by flushing uteri of both sides and was filtered with filter paper. Blood serum was collected from each animal and, after that, blastocyst and uterine fluid of the respective animals.
Sample preparation
The samples were prepared from pools of several embryos, the number depending on their age. Hydrolysis was performed in airtight tubes, with 3 ml of 3 N KOH + 2 ml of 95% ETOH for 3 h at 110°C. After acidification to a pH of 1–2 with 6 N HCI, fatty acids were extracted with ether (3 × 5 ml). The ether phase was then washed with water and allowed to dry at room temperature under a nitrogen atmosphere. The dry residue was solubilized in 5 ml of ether/MeOH (9:1, v/v), and then 2 ml of diazomethane was added according to the method of Schlenk and Gellerman [13] to convert the free fatty acids to methyl esters. After drying (under a nitrogen atmosphere), the residue containing the fatty acid methyl esters were solubilized in acetone (0.25–0.5 ml) for analysis.
Determination of fatty acids
After methyl esterification of the samples by 0.4 M potassium methoxide and 14 weight percentage boron trifluoride methanol, total fatty acids were measured using a gas chromatograph (Shimadzu, GC14B, Kyoto, Japan) equipped with an Omegawax 250 capillary column (30 m × 0.25 mm i.d.; 0.25 μm thickness; Supelco, Bellefonte, PA, USA). Peaks were determined using a flame‐ionization detector and were quantified with an electric integrator (Shimadzu, CR‐7A, Kyoto, Japan) using pure standard mixtures (Sigma, St. Louis, Mo, USA). We adopted the weight percentage of each fatty acid in all detected fatty acids as a measurement value.
Statistical analysis
Mean (±) fatty acid mass in the blastocyst, uterine fluid and serum total lipid samples were compared by single‐factor ANOVA and Tukey's pairwise HSD test.
Results
Changes in the size of rabbit's blastocyst from day 7 to 13 of pregnancy are shown in Fig. 1. It was observed that the size of embryos gradually increased relative to the pregnancy day.
Figure 1.
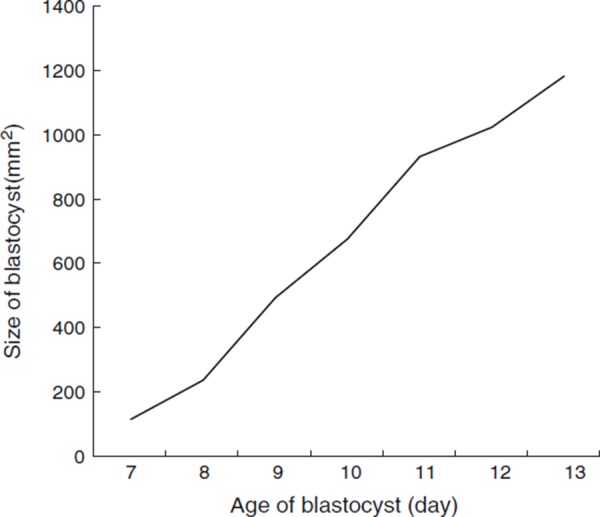
Changes in the size of rabbit's blastocyst from day 7 to 13 of pregnancy
The fatty acid composition of total lipids extracted from the blastocysts is shown in Table 1. The total amount of fatty acids markedly decreased from day 7 to 13. The fatty acid profile revealed that the most prevalent component was palmitic acid, followed by stearic, palmitoleic and oleic acids. From day 7 onwards there was a gradual decrease in the fatty acid concentration. The amount of fatty acids was slightly increased from day 7 to 8; from day 9 onwards it decreased drastically and then remained constant until day 13 of pregnancy. Interestingly, the highest concentration of linoleic acid was observed on day 9, though it was not detected on day 11.
Table 1.
Fatty acid composition of lipids in rabbit blastocysts from day 7 to 13
| Fatty acid | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 |
|---|---|---|---|---|---|---|---|
| Palmitic | 3.70 ± 1.39a | 3.41 ± 0.59a | 1.96 ± 0.34ab | 0.64 ± 0.05b | 0.55 ± 0.06b | 0.51 ± 0.05b | 0.49 ± 0.00b |
| Palmitoleic | 0.80 ± 0.50a | 0.81 ± 0.21a | 0.29 ± 0.10b | 0.14 ± 0.02b | 0.13 ± 0.01b | 0.08 ± 0.00b | 0.12 ± 0.01b |
| Stearic | 2.81 ± 0.95a | 2.32 ± 0.40ab | 1.30 ± 0.35bc | 0.47 ± 0.02c | 0.42 ± 0.06c | 0.36 ± 0.03c | 0.34 ± 0.01c |
| Oleic | 0.79 ± 0.44a | 0.72 ± 0.09a | 0.61 ± 0.09a | 0.15 ± 0.02b | 0.11 ± 0.01b | 0.09 ± 0.01b | 0.07 ± 0.01b |
| Linoleic | 0.43 ± 0.15b | 1.06 ± 0.06a | 1.17 ± 0.36a | 0.21 ± 0.02b | ND | 0.15 ± 0.04b | 0.09 ± 0.01b |
| Total fatty acid | 12.05 ± 3.42ab | 17.60 ± 2.52a | 8.15 ± 1.37b | 2.70 ± 0.29b | 2.37 ± 0.17b | 1.95 ± 0.19b | 2.05 ± 0.18b |
Values are expressed as 10−1 mg/ml. Data are presented as mean ± SEM, n = 10. Values with different superscripts in the same row are significantly different (P < 0.05)
ND Not detected
The fatty acid composition of total lipids extracted from the serum during the pregnancy is shown in Table 2. There was much palmitic acid, stearic acid and linoleic acid, and an increase and decrease of fatty acids was seen in the serum during days 7–13 of pregnancy. On day 9, the total fatty acid content in the serum was higher than on day 11. The most abundant fatty acid in the serum was palmitic acid. The palmitoleic acid content remained almost unchanged during the experimental period. From day 7 onwards a gradual increase in the oleic and linoleic acid concentrations was observed.
Table 2.
Fatty acid composition of lipids in rabbit serum from day 7 to 13
| Fatty acid | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 |
|---|---|---|---|---|---|---|---|
| Palmitic | 1.04 ± 0.21a | 0.72 ± 0.12b | 0.86 ± 0.34ab | 0.86 ± 0.05ab | 0.63 ± 0.01b | 0.95 ± 0.04ab | 1.09 ± 0.05a |
| Palmitoleic | 0.24 ± 0.11a | 0.12 ± 0.00b | 0.10 ± 0.02b | 0.09 ± 0.01b | ND | 0.08 ± 0.02b | 0.07 ± 0.00b |
| Stearic | 0.79 ± 0.12a | 0.44 ± 0.06c | 0.52 ± 0.11bc | 0.67 ± 0.07ab | 0.46 ± 0.02bc | 0.59 ± 0.03abc | 0.71 ± 0.05ab |
| Oleic | 0.16 ± 0.03b | 0.19 ± 0.05b | 0.61 ± 0.17a | 0.24 ± 0.02b | 0.20 ± 0.02b | 0.29 ± 0.02b | 0.37 ± 0.01b |
| Linoleic | 0.33 ± 0.05c | 0.70 ± 0.20bc | 1.68 ± 0.52a | 0.70 ± 0.09bc | 0.47 ± 0.02bc | 1.00 ± 0.14b | 1.10 ± 0.01ab |
| Total fatty acid | 4.61 ± 1.29 | 3.24 ± 0.48 | 4.84 ± 0.72 | 3.57 ± 0.26 | 2.47 ± 0.18 | 4.10 ± 0.13 | 4.39 ± 0.17 |
Values are expressed as 10−1 mg/ml. Data are presented as mean ± SEM, n = 10. Values with different superscripts in the same row are significantly different (P < 0.05)
ND Not detected
The fatty acid composition of the total lipids extracted from the uterine fluid during the pregnancy is shown in Table 3. The total amount of fatty acids in the uterine fluid was higher than that of the blastocysts and serum during the pregnancy. On day 13, the content of fatty acid was nine times higher than that on day 7. The palmitic and stearic acid content were higher on day 12, and were very low on days 7 and 8. A consistent fluctuation in palmitic acid content was observed during the study period. Oleic and linoleic acids were almost undetected except on day 13.
Table 3.
Fatty acid composition of lipids in rabbit uterine fluid from day 7 to 13
| Fatty acid | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 |
|---|---|---|---|---|---|---|---|
| Palmitic | 0.34 ± 0.03c | 0.65 ± 0.00c | 2.60 ± 0.31ab | 2.40 ± 0.31b | 2.72 ± 0.16ab | 3.76 ± 0.33a | 2.99 ± 0.15ab |
| Palmitoleic | ND | 0.18 ± 0.05 | 0.68 ± 0.08 | 1.08 ± 0.36 | 1.26 ± 0.16 | 1.00 ± 0.17 | 0.85 ± 0.11 |
| Stearic | 0.23 ± 0.01c | 0.44 ± 0.01c | 1.91 ± 0.14b | 1.76 ± 0.22b | 2.26 ± 0.04ab | 2.88 ± 0.23a | 2.35 ± 0.07ab |
| Oleic | ND | ND | 0.39 ± 0.05 | ND | 0.56 ± 0.10 | ND | 0.69 ± 0.04 |
| Linoleic | ND | ND | ND | ND | ND | ND | 0.29 ± 0.08 |
| Total fatty acid | 1.76 ± 0.22b | 2.52 ± 0.07b | 10.32 ± 2.33ab | 12.10 ± 2.60a | 13.69 ± 0.82a | 17.57 ± 1.85a | 15.75 ± 0.60a |
Values are expressed as × 10−2 mg/ml. Data are presented as mean ± SEM, n = 10. Values with different superscripts in the same row are significantly different (P < 0.05)
ND Not detected
Discussion
This study demonstrates the fatty acid composition of rabbit blastocysts from days 7 to 13 of pregnancy. The rabbit blastocysts implant at 7–7.5 days post‐coitum, and the volume of the extra‐embryonic fluid compartments of the rabbit increase to about 1 ml by 10 days post‐coitum [14]. The epithelium then becomes syncytial and fusion becomes general about 8.5 or 9 days post coitum [15]. During these developmental phases, differential fatty acid metabolism occurs in the blastocysts. An abundant amount of palmitic acid during days 7 and 8 was identified in all the blastocysts. It is of interest that the high level of palmitic acid found in the blastocysts may serve as a storage pool of metabolic precursors for fatty acid elongation and desaturation [16]. We reported previously that the constituents of fatty acids in 1‐cell to day 9 blastocysts [17]. The fatty acids of day 6 and 9 blastocysts of this experiment got the same result as the previous report [17]. In the previous report, 1‐cell stage embryos had the highest concentration of arachidonic acid, followed by lignoceric acid. In morula, linoleic acid composition was highest, followed by oleic and palmitic acid. But palmitoleic, stearic, arachidic, docosahexaenoic and lignoceric acid content was less [17]. Haggarty [18] reported that human preimplantation embryos which developed beyond the 4‐cell stage had significantly higher concentrations of the unsaturates, particularly linoleic acid and oleic acid, and a lower concentration of total saturates. The long chain polyunsaturates, especially arachidonic acid and docosahexaenoic acid were also present at higher concentrations in the later stages of development but these differences were not statistically significant. Compared with the reported fatty acid composition for oocytes from cattle, pigs and sheep, early stage human embryos had a similar proportion of the important unsaturated fatty acids, especially linolenic, arachidonic and eicosapentaenoic and the saturated palmitic [19]. Moreover, it was reported that oleic, palmitic and linoleic acids are capable of supporting growth of 1‐cell rabbit embryos to viable morulae [17]. Kane [8] reported that long chain fatty acids, myristic, palmitic, stearic, oleic and linoleic acid, supported rabbit embryo growth. The polyunsaturated fatty acids in general, and linoleic acid in particular, support a number of key developmental processes in mammalian embryos [20]. Linoleic acid acts as precursors for eicosanoids and regulates the processes of endocytosis or exocytosis, ion‐channel modulation, DNA polymerase inhibition and gene expression. Linoleic acid stimulates protein kinase C [21], which is critical for cell growth and differentiation [22], and is probably the fatty acid that is most implicated in animal fertility studies [23].
In this study, the fatty acid composition from day 7–9 blastocysts show that palmitic acid concentration was highest, followed by stearic, palmitoleic and oleic acids. Obviously the composition of fatty acids of day 7–9 blastocysts was different with the 1‐cell embryos. Dokko et al. [24] reported that stearic, oleic, linoleic, and linolenic acid were taken up by Hep‐G2 cells at almost identical rates, demonstrating that differences in the cellular synthesis of lipids and their secretion are attributable to the metabolic specificity of those fatty acids, rather than to variable rates of their uptake. It may be that these fatty acids are of lesser importance in the congenital environment; further investigations are required. Polyunsaturated fatty acids such as arachidonic and docosahexaenoic acids in the embryo lipid were identified in rat [20], cattle, sheep and pig [19], and reported to have functional significance for embryo development. These results were not found in rabbit embryos in the present study. This difference might be due to species variation. However, embryos which developed from day 7 to 13 had significantly higher concentrations of the saturated fatty acids than the unsaturated fatty acids. This may indicate the selective channeling of previously accumulated saturated fatty acids towards oxidation for energy production at the blastocyst stage in rabbit embryos.
Although there is a rough correspondence between pools of uterine fluids and blastocysts, concentration gradients exist at all stages, and involve levels of fatty acids.
It is evident that before and after implantation blastocysts can establish autonomy from their uterine environment. Serum and uterine fluids play important role in the development of blastocysts. Among the unsaturated fatty acids, linoleic acid concentration in the serum was much higher than the others; though only a scant amount was detected in the uterine fluid. A higher proportion of palmitic and linoleic acids in the lipids of embryo and secretion of reproductive tracts have also been observed in other species [20, 25]. Oleic acid was mainly found in the embryo lipid, but negligibly detected in the uterine fluid. A similar trend for oleic acid concentration was reported for bovine [26], pig and sheep [19]. Palmitoleic acid concentration was very low in serum and uterine fluid samples. This is consistent with the findings of Yao et al. [27] and with our earlier studies [17, 20].
Further, it was revealed that fatty acid composition of lipids in embryos and uterine fluid changed considerably even during implantation and this change is thought to have multiple advantages at the beginning of the implantation. Proportions of various fatty acids in lipid and their changes during early stages of embryo development differed largely among different samples. Killian et al. [28] reported that lipid composition in the fluid recovered from the lumen of the bovine oviduct differed considerably from that in the blood. These differences must be ascribed to differences in the process of synthesis, secretion and transduction of lipids and fatty acids in various organs and tissues, even between the oviduct and uterus [29, 30].
Lipids may act as a reservoir of latent energy [31], although metabolic studies indicate that ATP requirements of embryos are met by utilization of carbohydrates [31]. Nevertheless, oxygen consumption by one‐cell mouse embryos indicates that there is scope for utilization of endogenous fatty acids without precluding alternative endogenous or exogenous fuel sources [32]. One function attributed to fatty acid utilization is the generation of water for blastocoele fluid by mitochondrial ‐oxidation [33]; in mice, this is preceded by cortical localization of randomly dispersed cytoplasmic droplets and mitochondria to apposed cell surfaces [33].
The data presented on the developmental changes in fatty acid composition in rabbit embryos add useful basic information to the growing body of knowledge of early embryo metabolism. These data may also be useful practically in helping to develop non‐destructive tests of embryo quality and improving culture media, cryopreservation and the success of IVF treatment.
References
- 1. Tsujii H, Khandoker MAMY, Hamano K. Lipid in mammalian embryo development. J Mamm Ova Res, 2001, 18, 73–80 10.1274/jmor.18.73 [Google Scholar]
- 2. Waterman RA, Wall RJ. Lipid interactions with in vitro development of mammalian zygotes. Gamete Res, 1988, 21, 243–254 10.1002/mrd.1120210306 [DOI] [PubMed] [Google Scholar]
- 3. Nonogaki T, Noda Y, Goto Y, Kishi J, Mori T. Developmental blockage of mouse embryos caused by fatty acids. J Assist Reprod Genet, 1994, 11, 482–488 10.1007/BF02215713 [DOI] [PubMed] [Google Scholar]
- 4. Khandoker MAMY, Tsujii H. Effect of exogenous fatty acids on in vitro development of rat embryos. Asian Australas J Anim Sci, 1999, 12, 169–173 [Google Scholar]
- 5. Kim JY, Kinoshita M, Ohnishi M, Fukui Y. Lipid and fatty acid analysis of fresh and frozen‐thawed immature and in vitro matured bovine oocytes. Reproduction, 2001, 122, 131–138 10.1530/rep.0.1220131 [PubMed] [Google Scholar]
- 6. Pratt HPM Johnson MH. Lipids and transitions in embryos. Development in mammals, 1977. Amsterdam: North‐Holland; 83–129 [Google Scholar]
- 7. Hillman N, Flynn TJ. The metabolism of exogenous fatty acids by preimplantation mouse embryos developing in vitro. J Embryol Exp Morphol, 1980, 56, 157–168 [PubMed] [Google Scholar]
- 8. Kane MT. Fatty acids as energy sources for culture of one‐cell rabbit ova to viable morulae. Biol Reprod, 1979, 20, 323–332 10.1095/biolreprod20.2.323 [DOI] [PubMed] [Google Scholar]
- 9. Ushijima H, Yamakawa H, Nagashima H. Cryopreservation of bovine pre‐morula‐stage in vitro matured/in vitro fertilized embryos after delipidation and before use in nucleus transfer. Biol Reprod, 1999, 60, 534–539 10.1095/biolreprod60.2.534 [DOI] [PubMed] [Google Scholar]
- 10. Menezo Y, Renard JP, Delobel B, Pageaux JF. Kinetic study of fatty acid composition of day 7 to day 14 cow embryos. Biol Reprod, 1982, 26, 787–790 10.1095/biolreprod26.5.787 [DOI] [PubMed] [Google Scholar]
- 11. Homa ST, Racowsky C, McGaughey RW. Lipid analysis of immature pig oocytes. J Reprod Fertil, 1986, 77, 425–434 [DOI] [PubMed] [Google Scholar]
- 12. Youngs CR, Knight TJ, Batt SM, Beitz DC. Phospholipid, cholesterol, triacylglycerol, and fatty acid composition of porcine blastocysts. Theriogenology, 1994, 41, 343 10.1016/S0093‐691X(05)80253‐5 [Google Scholar]
- 13. Schlenk H, Gellerman JL. Esterification of fatty acids with diazomethane on a small scale. Anal Chem, 1960, 32, 1412–1416 10.1021/ac60167a011 [Google Scholar]
- 14. DeSesso JM Hood R. Comparative embryology. Handbook of developmental toxicology, 1997. Boca Raton: CRC Press; 111–174 [Google Scholar]
- 15. Boving BG. Anatomical analysis of rabbit trophoblast invasion. Contrib Embryol, 1962, 254, 33–35 [Google Scholar]
- 16. Jeffcoat R. The biosynthesis of unsaturated fatty acids and its control in mammalian liver. Essays Biochem, 1979, 15, 1–36 [PubMed] [Google Scholar]
- 17. Khandoker MAMY, Tsujii H, Karasawa D. A kinetic study of fatty acid composition of embryos, oviductal and uterine fluids in the rabbit. Asian Australas J Anim Sci, 1998, 11, 60–64 [Google Scholar]
- 18. Haggarty P, Wood M, Ferguson E, Hoad G, Srikantharajah A, Milne E, Hamilton M, Bhattacharya S. Fatty acid metabolism in human preimplantation embryos. Hum Reprod, 2006, 21, 766–773 10.1093/humrep/dei385 [DOI] [PubMed] [Google Scholar]
- 19. McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JS, Speake BK. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod Fertil, 2000, 118, 163–170 10.1530/reprod/118.1.163 [PubMed] [Google Scholar]
- 20. Khandoker MAMY, Tsujii H, Karasawa D. Fatty acid composition of blood serum, oocytes, embryos and reproductive tract fluids of rat and comparison with BSA. Anim Sci Technol Jpn, 1997, 68, 1070–1074 [Google Scholar]
- 21. Murakami K, Chan SY, Routtenberg A. Protein kinase C activation by cis‐fatty acid in the absence of Ca2+ and phospholipids. J Biol Chem, 1986, 261, 15424–15429 [PubMed] [Google Scholar]
- 22. Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature, 1988, 334, 661–665 10.1038/334661a0 [DOI] [PubMed] [Google Scholar]
- 23. Homa ST, Brown CA. Changes in linoleic acid during follicular development and inhibition of spontaneous breakdown of germinal vesicles in cumulus‐free bovine oocytes. J Reprod Fertil, 1992, 94, 153–160 [DOI] [PubMed] [Google Scholar]
- 24. Dokko RC, Cho BH, Chung BH. Cellular uptake of stearic, oleic, linoleic, and linolenic acid and their effects on synthesis and secretion of lipids in Hep‐G2 cells. Int J Biochem Cell Biol, 1998, 30, 65–76 10.1016/S1357‐2725(97)00097‐6 [DOI] [PubMed] [Google Scholar]
- 25. Pinter E, Reece EA, Ogburn PL, Turner S, Hobbins JC, Mchoney MS, Naftolin F. Fatty acid content of yolk sac and embryo in hyperglycemia‐induced embryopathy and effect of arachidonic acid supplementation. Am J Obstet Gynecol, 1988, 159, 1484–1490 [DOI] [PubMed] [Google Scholar]
- 26. Sata R, Tsujii H, Abe H, Yamashita S, Hoshi H. Fatty acid composition of bovine embryos cultured in serum‐free and serum‐containing medium during early embryonic development. J Reprod Dev, 1999, 45, 97–103 10.1262/jrd.45.97 [Google Scholar]
- 27. Yao JK, Ryan RJ, Dyck PJ. The porcine ovarian follicle. VI. Composition of fatty acid. Composition of serum and follicular fluid at different developmental stages. Biol Reprod, 1980, 22, 141–147 10.1095/biolreprod22.2.141 [DOI] [PubMed] [Google Scholar]
- 28. Killian GJ, Chapman DA, Kavanaugh JF, Deaver DR, Wiggin HB. Changes in phospholipids, cholesterol and protein content of oviduct fluid of cows during the estrous cycle. J Reprod Fertil, 1989, 86, 419 10.1530/jrf.0.0860419 [DOI] [PubMed] [Google Scholar]
- 29. Grippo AA, Anderson SH, Chapman DA, Henault MA, Killian GJ. Cholesterol, phospholipid and phospholipase activity of ampullary and isthmic fluid from the bovine oviduct. J Reprod Fertil, 1994, 102, 87–93 10.1530/jrf.0.1020087 [DOI] [PubMed] [Google Scholar]
- 30. Henault MA, Killian GJ. Synthesis and secretion of lipids by bovine oviduct mucosal explants. J Reprod Fertil, 1993, 98, 431–438 [DOI] [PubMed] [Google Scholar]
- 31. Thompson JG, Partridge RG, Houghton FD, Cox CI, Leese HJ. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fertil, 1996, 106, 299–306 [DOI] [PubMed] [Google Scholar]
- 32. Leese HJ Milligan SR. Metabolism of the pre‐implantation mammalian embryo. Oxford reviews of reproductive biology, 1991. Oxford: Oxford University Press; 35–72 [PubMed] [Google Scholar]
- 33. Wiley LM Bavister BD. Development of the blastocyst: role of cell polarity in cavitation and cell differentiation. The mammalian preimplantation embryo: regulation of growth and differentiation in vitro, 1987. New York: Plenum Press; 65–93 [Google Scholar]


