Abstract
Background and Aims: Mammalian sperm activation and hyperactivation is regulated by protein phosphorylation. Although tyrosine phosphorylation is considered very important, several studies have investigated whether serine and threonine phosphorylation are also associated with sperm activation and hyperactivation, and that was also the aim of the present study.
Methods: Protein phosphorylation of hamster spermatozoa was detected by Western blotting using antiphospho‐amino acid monoclonal antibodies after tricine sodium dodecyl sulfate‐polyacrylamide gel electrophoresis. Amino acid sequences were analyzed using a peptide sequencer.
Results: Four proteins were phosphorylated at serine residues during hyperactivation via activation and their approximate molecular weights were 90, 38, 32 and 10 kDa, respectively. Five proteins were phosphorylated or dephosphorylated at threonine residues and their approximate molecular weights were 90, 70, 65, 35 and 10 kDa, respectively. The 10‐kDa protein corresponded to a previously reported 10‐kDa tyrosine phosphoprotein. N‐terminal sequences of the 10‐kDa protein were similar to carcinustatin, which is a neuropeptide.
Conclusions: During hyperactivation, four serine phosphorylation and five threonine phospho‐ or dephosphorylations occurred, which suggested that the 10‐kDa protein was phosphorylated at tyrosine residues when spermatozoa were activated and then dual‐phosphorylated at the serine and threonine residues during hyperactivation. (Reprod Med Biol 2004; 3: 223–230)
Keywords: dephosphorylation, hamsters, hyperactivation, phosphorylation, spermatozoa
INTRODUCTION
EJACULATED MAMMALIAN SPERMATOZOA are not able to fertilize eggs 1 until they become capacitated, which occurs after activation. Activated spermatozoa can be prepared in vitro by adding activating factors, such as calcium and/or bicarbonate, and are moved progressively with a high‐beat frequency. 1 , 2 , 3 Capacitated spermatozoa are prepared in vitro by adding calcium, albumin, glucose, etc., and capacitation involves an acrosomal reaction in the sperm head, which is very important for fertilization, and hyperactivation of motility, 1 which comprises a large bend amplitude, whiplash and frenzied flagellar movements. 4 , 5 , 6
The most recent understanding is that the changes in sperm motility are closely associated with phosphorylation of flagellar proteins, 3 particularly serine, threonine and tyrosine; for example, 175‐, 93‐, 44‐, 40‐, 38‐, 20‐kDa proteins have been detected in boar sperm, 7 90‐, 80‐, 62‐, 48‐kDa proteins in hamsters 8 , 9 and 190‐, 110‐, 94‐, 43–55‐, 35‐, 18‐kDa proteins in humans. 10 From these results, it has been assumed that tyrosine phosphorylation plays an especially important role in the hyperactivation of mammalian spermatozoa. 1 , 7 , 8 , 11 , 12 , 13 The phosphorylation at the tyrosine residues of a flagellar protein, A‐kinase anchor protein (AKAP), has also been reported in detail. 14 AKAP binds to the RII subunit of A‐kinase 15 , 16 and is a component of the fibrous sheath. 17 , 18 It has been suggested that the phosphorylation of AKAP is regulated through calcium‐calmodulin dependent signal transduction, 14 and it has been demonstrated that the phosphorylation of AKAP is also regulated by protein phosphatase. 8 However, the regulatory mechanism of capacitation is still unclear.
Because we had investigated tyrosine phosphorylation in a previous study, 9 in the present experiment we investigated serine and threonine phosphorylation associated with motility activation and hyperactivation in hamster spermatozoa.
MATERIALS AND METHODS
Reagents
ADENOSINE TRIPHOSPHATE, CYCLIC adenosine monophosphate (cAMP), antiphosphoserine monoclonal antibody and antiphosphothreonine monoclonal antibody were purchased from Sigma (St Louis, MO, USA). A Histofine SAB‐PO (M) kit was purchased from Nichirei (Tokyo, Japan). Other chemicals of reagent grade were obtained from Wako Pure Chemical Industries (Osaka, Japan).
Preparation of spermatozoa
Spermatozoa were obtained from the cauda epididymis of sexually mature (12–16 week old) male golden hamsters (Mesocricetus auratus) as described previously. 19 Immotile spermatozoa were prepared according to our previous method. 19 In brief, one volume of cauda epididymal spermatozoa were suspended directly in 10 volumes of ice‐cold pure water, and then homogenized in 50 volumes of homogenizing buffer (200 mmol/L sucrose, 25 mmol/L glutamic acid, 25 mmol/L KOH and 20 mmol/L Tris‐HCl [pH 7.9]) using a Teflon homogenizer.
Activated and hyperactivated spermatozoa were prepared according to the methods described previously, 9 , 19 using modified Tyrode's albumin lactate pyruvate (m‐TALP) medium. 20 An aliquot of cauda epididymal spermatozoa was placed in a test tube, several milliliters of m‐TALP medium was then carefully added and the mixture was overlayed with mineral oil. The tubes were incubated for 10 min to allow the activated spermatozoa to swim up. The supernatant containing activated spermatozoa was collected and homogenized in 50 volumes of the homogenizing buffer using a Teflon homogenizer. Spermatozoa exhibiting approximately 90% motility were used in the experiment (Table 1).
Table 1.
Motility, activation and hyperactivation of hamster spermatozoa
| Motility (%) | Activation (%) | Hyperactivation (%) | |
|---|---|---|---|
| Activated | 95 | 93 | 0 |
| Hyperactivated | 91 | 12 | 83 |
For hyperactivation, the activated sperm suspension was placed on a culture plate, covered with mineral oil and incubated for 3 h at 37°C under 5% CO2 in air. The hyperactivated spermatozoa were then collected and homogenized in 50 volumes of the homogenizing buffer using a Teflon homogenizer. Spermatozoa exhibiting approximately 80% hyperactivated motility were used in the experiment (Table 1).
Spermatozoa suspended in the m‐TALP medium were diluted 10‐fold and placed on a glass slide coated with 5% bovine serum albumin (BSA). Sperm hyperactivity was recorded on a videotape via a CCD camera (Sony, Tokyo, Japan) attached to a microscope (Olympus, Tokyo, Japan) with phase contrast illumination and warm stage (Kitazato Supply Co. Ltd, Shizuoka, Japan). Each observation was performed at 37°C, lasted for 2 min and involved analyzing the number of motile sperm or hyperactivated sperm in 10 different fields. Percent motility was defined as the number of motile spermatozoa/number of total spermatozoa, percent activation as the number of activated spermatozoa/number of motile spermatozoa, and percent hyperactivation as the number of hyperactivated spermatozoa/number of motile spermatozoa.
Preparation of demembranated sperm flagella
Demembranated sperm flagella were prepared from immotile, activated and hyperactivated spermatozoa according to the method described previously. 19 Each sperm suspension prepared as described above was centrifuged at 5500 g for 5 min at 4°C. The pelleted spermatozoa were suspended in 100‐fold of the homogenizing buffer and after centrifugation at 750 g for 5 min at 4°C, the precipitate was resuspended in a 20‐fold volume of the homogenizing buffer supplemented with 2 mmol/L phenylmethanesulfonyl fluoride (PMSF) and 20 µg/mL leupeptine. The suspension was homogenized with 100 strokes of a Teflon homogenizer in order to separate flagella from heads. The homogenate was then diluted in a fourfold volume of the homogenizing buffer supplemented with 0.5 mmol/L PMSF and 5 µg/mL leupeptine. After centrifugation at 750 g for 5 min at 4°C, the supernatant was collected and centrifuged at 5500 g for 5 min at 4°C. The precipitate, which contained isolated flagella, was suspended in a 20‐fold volume of demembranation buffer containing 200 mmol/L sucrose, 25 mmol/L glutamic acid, 25 mmol/L KOH, 1 mmol/L dithiothreitol, 0.1% (w/v) Triton X‐100 and 20 mmol/L Tris‐HCl (pH 7.9), and incubated for 30 s at room temperature. The flagellar suspension was centrifuged at 5500 g for 5 min at 4°C. The protein concentration of the pelleted flagella was determined by the method of Bradford 21 and adjusted to a final protein concentration of 1 mg/mL with homogenization buffer.
Dissolution of demembranated sperm flagella
Dissolution of demembranated sperm flagella was performed according to the method described previously. 19 Tri‐chloroacetic acid was added to the suspension of demembranated sperm flagella to a final concentration of 10% (w/v) and it was centrifuged at 15 000 g for 20 min at 4°C. The precipitate was rinsed three times with a 10‐fold volume of ice‐cold acetone and then suspended in 1 mg/mL guanidine solution containing 8 mol/L guanidine hydrochroride, 10 mmol/L sodium pyrophosphate, 10% (v/v) 2‐mercaptoethanol, 2% (v/v) Nonidet P‐40 and 500 mmol/L Tris‐HCl (pH 7.5). After centrifugation at 15 000 g for 20 min at 4°C, the suspension was dialyzed against a urea solution containing 7 mol/L urea and 1% (v/v) 2‐mercaptoethanol.
Gel electrophoresis
Tricine sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (tricine SDS‐PAGE) was carried out according to the method of Schagger and Jagow. 22 The separating gel was 10% polyacrylamide containing 0.1% SDS.
Western blotting
Western blotting was performed according to the method of Towbin et al. 23 The blotted membrane was blocked with 5% (w/v) BSA in Tris‐buffered saline (TBS) containing 0.15 mol/L NaCl and 20 mmol/L Tris‐HCl (pH 7.5) for 1 h at 20°C, and incubated with first antibodies (1:1000 dilution with 5%[w/v] BSA in TBS) for 1 h at 20°C. Color reaction was carried out with the Histofine SAB‐PO (M) kit and H2O2 and 3,3′‐diaminobenzidine tetrahydrochloride as the substrate for peroxidase.
Amino acid sequence analysis with a peptide sequencer
Amino‐acid sequence analysis with a peptide sequencer (PPSQ‐21, Shimadzu Co. Ltd, Kyoto, Japan) was carried out according to the method used in our previous study. 24 After tricine SDS‐PAGE, proteins were transferred to a polyvinylidene fluoride membrane (Immobilone Psq; Millipore Corp., Bedford, MA, USA) with 10 mmol/L Caps buffer containing 10 mmol/L Caps‐NaOH, pH 8.0. After the transfer, the membrane was stained with a Coomassie Brilliant Blue (CBB) solution containing 0.025% CBB and 40% methanol, and destained with a destaining solution containing 40% methanol and 10% acetic acid. Bands were cut out of the membrane and subjected to amino‐acid sequence analysis, from which the 10‐kDa protein was identified by means of searching the FASTA database (http://fasta.genome.ad.jp/).
RESULTS
IN ORDER TO examine the successive changes in serine and threonine phosphorylation that are related to sperm motility, we prepared three suspensions of spermatozoa: immotile, activated and hyperactivated spermatozoa. The immotile spermatozoa were obtained by dilution of mature epididymal spermatozoa in pure water, 19 activation occurred after dilution of spermatozoa in m‐TALP (they moved progressively with a high beat frequency of approximately 10 Hz) 8 , 19 and hyperactivated spermatozoa were obtained after 3 h incubation in m‐TALP; they exhibited a beat frequency of approximately 10 Hz, large bend amplitude, whiplash and frenzied flagellar movements. 4 , 5 , 6 , 8 We therefore redefined the activation of spermatozoa as the change from immotile to active and hyperactivation as the change from active to hyperactive.
Serine and threonine phosphorylation associated with hyperactivation via activation
The first aim was to detect serine phosphorylation of flagellar proteins, particularly during activation and hyperactivation. As shown in Figure 1, we detected four proteins that were phosphorylated at serine residues during either activation or hyperactivation, and these were designated as 90‐, 38‐, 32‐ and 10‐kDa proteins from their apparent molecular weights. Phosphorylation of the 90‐kDa protein occurred on activation and was maintained or increased during the change in sperm motility from activation to hyperactivation. Conversely, phosphorylation of the 38‐, 32‐ and 10‐kDa proteins occurred when the activated spermatozoa were hyperactivated.
Figure 1.
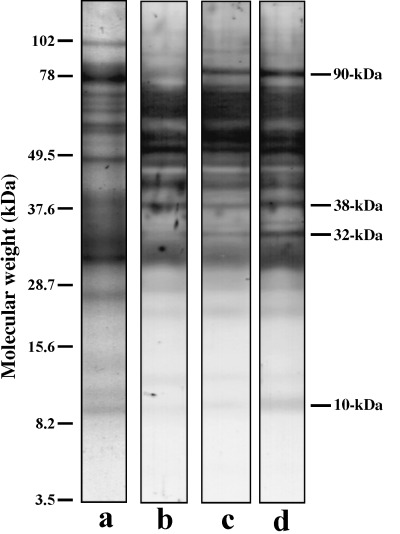
Phosphorylation of serine in immotile, activated and hyperactivated sperm flagella. Protein stain (lane a), Western blotting of immotile spermatozoa (lane b), activated spermatozoa (lane c) and hyperactivated spermatozoa (lane d) are shown. Bars on the right side indicate phosphoproteins associated with activation and hyperactivation. Bars on the left side show molecular weight standards.
We then investigated threonine phosphorylation of flagellar proteins and as shown in Figure 2, we detected three proteins that were phosphorylated at threonine residues, during either activation or hyperactivation and these were designated as 90‐, 65‐ and 10‐kDa proteins from their apparent molecular weights. Moreover, two proteins were dephosphorylated at threonine residues on activation and these were designated as 70‐ and 35‐kDa proteins from their apparent molecular weights. Phosphorylation of the 10‐kDa protein occurred when the activated spermatozoa were hyperactivated, whereas dephosphorylation of the 70‐ and 35‐kDa proteins occurred on activation. Moreover, the 90‐ and 10‐kDa proteins were dual‐phosphorylated at serine and threonine residues when the spermatozoa were activated or hyperactivated (1, 2).
Figure 2.
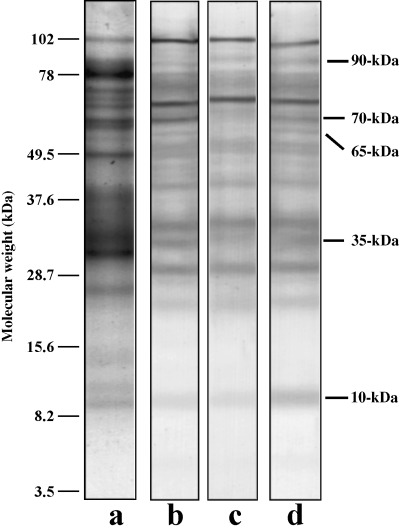
Phosphorylation of threonine in immotile, activated and hyperactivated sperm flagella. Protein stain (lane a), Western blotting of immotile spermatozoa (lane b), activated spermatozoa (lane c) and hyperactivated spermatozoa (lane d) are shown. Bars on the right side indicate phosphorylated or dephosphorylated proteins associated with activation and hyperactivation. Bars on the left side show molecular weight standards.
Phosphorylation of the 10‐kDa protein
When activated spermatozoa were hyperactivated, the 10‐kDa protein was phosphorylated at both serine and threonine residues (1, 2). In our previous study, 9 we found that the 10‐kDa protein was phosphorylated at tyrosine residues on activation and hyperactivation, and as shown in Figure 3, this also occurred in the present experiment. Because the same protein band of 10‐kDa was phosphorylated at the tyrosine, serine and threonine residues (Fig. 3), it is likely that multiphosphorylation of the 10‐kDa protein is associated with sperm motility.
Figure 3.
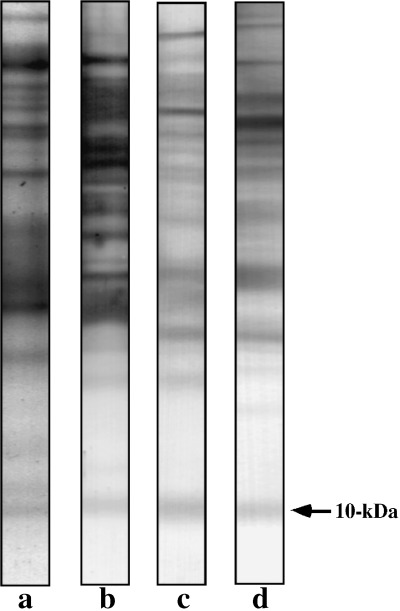
Phosphorylation of the 10‐kDa protein. Protein stain (lane a), phosphoserine (lane b), phosphothreonine (lane c) and phosphotyrosine (lane d) are shown. Bars on the right side indicate the 10‐kDa protein.
Identification of the 10‐kDa protein
In order to identify the 10‐kDa protein, its N‐terminal sequence was analyzed by peptide sequencer and was found to be MYNRRRPQLYA. A search of the FASTA database revealed that the N‐terminal sequence of the 10‐kDa protein was similar to the N‐terminal sequence (5th to 15th) of carcinustatin 25 (Fig. 4).
Figure 4.

N‐terminal amino acid sequences of the 10‐kDa protein and carcinustatin.
DISCUSSION
IT HAS BEEN suggested that protein phosphorylation is a very important event in the regulation of sperm motility, 1 , 2 , 3 and many protein phosphorylations associated with sperm motility have been detected in several species of animals, for example, bovine, 26 , 27 dog, 28 hamster, 8 , 9 , 19 , 29 , 30 human, 10 mouse, 11 , 12 , 13 , 31 ascidian, 32 salmon and trout. 33 , 34 , 35 , 36 As for capacitation, it has been suggested that protein phosphorylation is also a very important event, 1 particularly tyrosine phosphorylation. 9 , 12 , 13 , 29 , 30 Although many tyrosine phosphorylations have been detected, it is now accepted that tyrosine phosphorylation of AKAP, a major fibrous sheath protein, is the most essential event associated with sperm capacitation. 14 , 16 , 17 , 18 , 29 , 30
Recently, it was shown that serine and threonine phosphorylation are also associated with sperm capacitation. 10 Several previous studies have shown that the tyrosine phosphorylation of AKAP is regulated through a serine/threonine phosphorylation cascade, 13 , 14 , 29 and it is likely that the same mechanism regulates sperm capacitation. Moreover, it has been shown that sperm capacitation is regulated through the classical MAP kinase cascade, 37 , 38 and that tyrosine phosphorylation of AKAP is also associated with this cascade. 38 Although the dual serine/tyrosine kinase is associated with the MAP kinase cascade, the cascade is basically one of serine/threonine phosphorylation.
Capacitated spermatozoa show an acrosomal reaction in the head and hyperactivation in the flagellum. 1 Many protein phosphorylations have been detected in the acrosomal reaction when it is used as a capacitation event, 10 , 37 , 38 , 39 and several have been detected during hyperactivation as a capacitation event. 9 , 29 , 30 The results of these studies suggest that tyrosine phosphorylation of AKAP is also associated with sperm hyperactivation and therefore, it is speculated that tyrosine phosphorylation of AKAP is not only related to the acrosomal reaction, but also to hyperactivation.
In the present experiments, we detected four serine phosphorylations (Fig. 1) and five threonine phosphorylations (Fig. 2) associated with sperm motility activation and hyperactivation. Although we detected four serine phosphoproteins associated with sperm motility initiation and activation in a previous study, 19 the four serine phosphoproteins detected in the present experiment did not correspond to the previous ones 19 because their approximate molecular weights differed.
Serine and threonine phosphorylations of hamster sperm proteins during capacitation have been reported on the basis of detecting four serine phosphoproteins and five threonine phosphoproteins, 39 but the protein phosphorylations detected by us in the present experiments using hamster sperm flagella differed from those detected by Kula Nand and Shivaji. 39 We think the discrepancies are caused by sample preparation and electrophoresis. We used a lysate of isolated sperm flagella, whereas Kula Nand and Shivaji used a whole sperm lysate. 39 We carried out tricine SDS‐PAGE according to the method of Schagger and Jagow, 22 whereas Kula Nand and Shivaji carried out SDS‐PAGE according to the method of Laemmli. 40 However, it is likely that the 65‐kDa protein detected in the present experiment (Fig. 2) corresponds to the 61‐kDa protein detected by Kula Nand and Shivaji, 39 according to the molecular weights and threonine phosphorylation of both proteins.
The 90‐kDa phosphoprotein detected in the present experiments (1, 2) was dual‐phosphorylated at the serine and threonine residues during sperm activation and maintained during hyperactivation. Therefore, it is likely that phosphorylation of the 90‐kDa protein is associated with motility activation. In human spermatozoa, it has been shown that the 94‐kDa protein is dual‐phosphorylated at the serine and threonine residues during capacitation. 10 Therefore, the 90‐kDa protein in hamster spermatozoa may correspond to the 94‐kDa protein from human spermatozoa, according to the molecular weights and dual‐phosphorylations of both proteins. 10
The 10‐kDa protein detected in the present experiments (1, 2) was also dual‐phosphorylated at the serine and threonine residues during hyperactivation (1, 2). In our previous study, 9 the 10‐kDa protein was identified as the protein phosphorylated at tyrosine residues in a cAMP‐dependent manner when hamster spermatozoa were activated. By its molecular weight, the 10‐kDa protein detected in the present experiments corresponds to that previous 10‐kDa protein 9 (Fig. 3). Serine phosphorylation and threonine phosphorylation of the 10‐kDa protein occurred during hyperactivation, whereas tyrosine phosphorylation occurred during activation and was maintained during hyperactivation. 9 Therefore, it seems that the 10‐kDa protein was triple‐phosphorylated at the serine, threonine and tyrosine residues in association with sperm activation and hyperactivation. Because the 10‐kDa protein is phosphorylated at the tyrosine residues in a cAMP‐dependent manner, 9 we first considered that it corresponded to the 15‐kDa protein detected in salmonid spermatozoa by Morisawa et al., 33 , 41 but the present study showed that the 10‐kDa protein included carcinustatin‐like sequences 25 (Fig. 4), rather than the tubulin‐like sequences of the 15‐kDa protein. 42
In the present study, two proteins, the 70‐kDa and 35‐kDa proteins, were dephosphorylated at threonine residues (Fig. 2) when hamster spermatozoa were activated. Protein phosphorylation is proposed as one of the events regulating mammalian sperm motility. 2 , 43 In mammalian spermatozoa, protein phosphatase activity is regulated by calcium via calmodulin. 43 , 44 Because an activation factor of hamster spermatozoa is calcium iron, 29 it seems that the dephosphorylation of the 70‐kDa and 35‐kDa proteins depends on calmodulin‐dependent protein phosphatase. In our previous study, 9 we detected dephosphorylation of a 20‐kDa protein at the tyrosine residues when hamster spermatozoa were activated and moreover, protein tyrosine phosphatase activity has been detected in hamster spermatozoa. 45 Therefore, it is likely that three proteins are dephosphorylated at threonine or tyrosine residues when hamster spermatozoa are activated, and this is regulated by calmodulin‐dependent protein phosphatase. Recently, it was demonstrated that the dynein light chain is phosphorylated in a cAMP‐dependent manner during activation of salmonid fish sperm motility, 46 and dephosphorylated by a type 2A protein phosphatase. 47 Moreover, it has been also suggested that the regulatory subunit of cAMP‐dependent protein kinase is phosphorylated in a cAMP‐dependent manner 34 and dephosphorylated by other types of protein phosphatase. 47 Therefore, it is likely that sperm motility activation is regulated not only by protein phosphorylation, but also by protein dephosphorylation.
Although we did not always identify and characterize the proteins detected in the present experiments, they were phosphorylated and/or dephosphorylated at serine and/or threonine residues in association with hamster sperm activation and hyperactivation. Future studies should investigate the role of those proteins in motility.
ACKNOWLEDGMENTS
WE THANK STAFF of the Laboratory Animal Research Center of Dokkyo University School of Medicine for their support. This study was supported by a Grant‐in‐Aid for Scientific Research (No. 15790860) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1. Yanagimachi R. Mammalian fertilization In: Neill K, Pfaff GM, eds. The Physiology of Reproduction, 2nd edn New York: Raven Press, 1994; 189–317. [Google Scholar]
- 2. Morisawa M. Cell signaling mechanisms for sperm motility. Zoolog Sci 1994; 11: 647–662. [PubMed] [Google Scholar]
- 3. Inaba K. Molecular architecture of the sperm flagella: molecules for motility and signaling. Zool Sci 2003; 20: 1043–1056. [DOI] [PubMed] [Google Scholar]
- 4. Shivaji S, Peedicayil J, Girija Devi L. Analysis of the motility parameters of in vitro hyperactivated hamster spermatozoa. Mol Reprod Dev 1995; 42: 233–247. [DOI] [PubMed] [Google Scholar]
- 5. Yanagimachi R. In vitro capacitation of hamster spermatozoa by follicular fluid. J Reprod Fertil 1969; 18: 275–286. [DOI] [PubMed] [Google Scholar]
- 6. Yanagimachi R. The movement of golden hamster spermatozoa before and after capacitation. J Reprod Fertil 1970; 23: 193–196. [DOI] [PubMed] [Google Scholar]
- 7. Kaláb P, Pêknicová J, Geussová G, Moos J. Regulation of protein tyrosine phosphorylation in boar sperm through a cAMP‐dependent pathway. Mol Reprod Dev 1998; 51: 304–314. [DOI] [PubMed] [Google Scholar]
- 8. Si Y, Okuno M. Role of tyrosine phosphorylation of flagellar proteins in hamster sperm hyperactivation. Biol Reprod 1999; 61: 240–246. [DOI] [PubMed] [Google Scholar]
- 9. Fujinoki M, Ohtake H, Okuno M. Tyrosine phosphorylation and dephosphorylation associated with motility of hamster spermatozoa. Biomed Res 2001; 22: 147–155. [Google Scholar]
- 10. Naz RK. Involvement of protein serine and threonine phosphorylation in human sperm capacitation. Biol Reprod 1999; 60: 1402–1409. [DOI] [PubMed] [Google Scholar]
- 11. Tash JS, Bracho GE. Identification of phosphoproteins coupled to initiation of motility in live epididymal mouse sperm. Biochem Biophy Res Commun 1998; 251: 557–563. [DOI] [PubMed] [Google Scholar]
- 12. Visconti PE, Bailey JL, Moore GD, Pan D, Olds‐Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I: Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995; 121: 1129–1137. [DOI] [PubMed] [Google Scholar]
- 13. Visconti PE, Moore GD, Bailey JL et al. Capacitation of mouse spermatozoa. II: Protein tyrosine phosphorylation and capacitation are regulated by a cAMP‐dependent pathway. Development 1995; 121: 1139–1150. [DOI] [PubMed] [Google Scholar]
- 14. Carrera A, Moos J, Ning XP et al. Regulation of protein tyrosine phosphorylation in human sperm by a calcium/calmodulin‐dependent mechanism: Identification of A‐kinase anchor proteins as major substrates for tyrosine phosphorylation. Dev Biol 1996; 180: 284–296. [DOI] [PubMed] [Google Scholar]
- 15. Vijayaraghavan S, Goueli SA, Davey MP, Carr DW. Protein kinase A‐anchoring inhibitor peptides arrest mammalian sperm motility. J Biol Chem 1997; 272: 4747–4752. [DOI] [PubMed] [Google Scholar]
- 16. Visconti PE, Johnson LR, Oyaski M et al. Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev Biol 1997; 192: 351–363. [DOI] [PubMed] [Google Scholar]
- 17. Carrera A, Gerton GL, Moss SB. The major fibrous sheath polypeptide of mouse sperm: Structural and functional similarities to the A‐kinase anchoring proteins. Dev Biol 1994; 165: 272–284. [DOI] [PubMed] [Google Scholar]
- 18. Johnson LR, Foster JA, Haig‐Ladewig L et al. Assembly of AKAP82, a protein kinase A anchor protein, into the fibrous sheath of mouse sperm. Dev Biol 1997; 192: 340–350. [DOI] [PubMed] [Google Scholar]
- 19. Fujinoki M, Ohtake H, Okuno M. Serine phosphorylation of flagellar proteins associated with the motility activation of hamster spermatozoa. Biomed Res 2001; 22: 45–58. [Google Scholar]
- 20. Maleszewski M, Kline D, Yanagimachi R. Activation of hamster zona‐free oocytes by homologous and heterologous spermatozoa. J Reprod Fertil 1995; 105: 99–107. [DOI] [PubMed] [Google Scholar]
- 21. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 22. Schagger H, Jagow G. Tricine‐sodium dodecyl sulfate‐polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 1987; 166: 369–379. [DOI] [PubMed] [Google Scholar]
- 23. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 1979; 76: 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fujinoki M, Kawamura T, Toda T et al. Identification of 36‐kDA flagellar phosphoproteins associated with hamster sperm motility. J Biochem, 2003; 133: 361–369. [DOI] [PubMed] [Google Scholar]
- 25. Dircksen H, Skiebe P, Abel B et al. Structure, distribution, and biological activity of novel members of the allatostatin family in the crayfish Orconectes limosus . Peptides 1999; 20: 695–712. [DOI] [PubMed] [Google Scholar]
- 26. Brandt H, Hoskins DD. A cAMP‐dependent phosphorylated motility protein in bovine epididymal sperm. J Biol Chem 1980; 255: 982–987. [PubMed] [Google Scholar]
- 27. Vijayarabhavan S, Trautman KD, Goueli SA, Carr DW. A tyrosine‐phosphorylated 55‐kilodalton motility associated bovine sperm protein is regulated by cyclic adenosine 3′,5′‐monophosphates and calcium. Biol Reprod 1997; 56: 1450–1457. [DOI] [PubMed] [Google Scholar]
- 28. Tash JS, Kakar SS, Means AR. Flagellar motility requires the cAMP‐dependent phosphorylation of a heat‐stable NP‐40‐soluble 56 kd protein, axokinin. Cell 1984; 38: 551–559. [DOI] [PubMed] [Google Scholar]
- 29. Si Y, Okuno M. Regulation of microtubule sliding by a 36‐kDa phosphoprotein in hamster sperm flagella. Mol Reprod Dev 1999; 52: 328–334. [DOI] [PubMed] [Google Scholar]
- 30. Si Y. Hyperactivation of hamster sperm motility by temperature‐dependent tyrosine phosphorylation of an 80‐kDa protein. Biol Reprod 1999; 61: 247–252. [DOI] [PubMed] [Google Scholar]
- 31. Si Y, Okuno M. Activation of mammalian sperm motility by regulation of microtubule sliding via cyclic adenosine 5′‐monophosphate‐dependent phosphorylation. Biol Reprod 1995; 53: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 32. Nomura M, Inaba K, Morisawa M. Cyclic AMP‐ and calmodulin‐dependent phosphorylation of 21 and 26 kDa proteins in axoneme is a prerequisite for SAAF‐induced motile activation in ascidian spermatozoa. Dev Growth Differ 2000; 42: 129–138. [DOI] [PubMed] [Google Scholar]
- 33. Morisawa M, Hayashi H. Phosphorylation of a 15K axonemal protein is the trigger initiating trout sperm motility. Biomed Res 1985; 6: 181–184. [Google Scholar]
- 34. Inaba K, Morisawa S, Morisawa M. Proteasomes regulate the motility of salmonid fish sperm through modulation of cAMP‐dependent phosphorylation of an outer arm dynein light chain. J Cell Sci 1998; 111: 1105–1115. [DOI] [PubMed] [Google Scholar]
- 35. Itoh A, Inaba K, Fujinoki M, Morisawa M. Motility‐associated and cyclic AMP‐dependent protein phosphorylation in the sperm of the chum salmon, Oncorhynchus keta . Biomed Res 2001; 22: 241–248. [Google Scholar]
- 36. Itoh A, Fujinoki M, Kawamura T et al. Purification and characterization of the 15‐kDa protein from the sperm flagella of salmonid fishes. Biomed Res 2003; 24: 153–164. [Google Scholar]
- 37. Du Plessis SS, Page C, Franken DR. The zona pellucida‐induced acrosome reaction of human spermatozoa involves extracellular signal‐regulated kinase activation. Andrologia 2001; 33: 337–342. [DOI] [PubMed] [Google Scholar]
- 38. De Lamirande E, Gagnon C. The extracellular signal‐regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol Hum Reprod 2002; 8: 124–135. [DOI] [PubMed] [Google Scholar]
- 39. Kula Nand J, Shivaji S. Protein serine and threonine phosphorylation, hyperactivation and acrosome reaction in in vitro capacitated hamster spermatozoa. Mol Reprod Dev 2002; 63: 119–130. [DOI] [PubMed] [Google Scholar]
- 40. LaemmLi UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 41. Hayashi H, Yamamoto K, Yonekawa H, Morisawa M. Involvement of tyrosine protein kinase in the initiation of flagellar movement in rainbow trout spermatozoa. J Biol Chem 1987; 262: 16692–16698. [PubMed] [Google Scholar]
- 42. Itoh A, Fujinoki M, Kawamura T et al. Purification and characterization of the 15‐kDa protein from the sperm flagella of salmonid fishes. Biomed Res 2003; 24: 153–164. [Google Scholar]
- 43. Tash JS, Krinks M, Patel J, Means RL, Klee CB, Means AR. Identification, characterization, and functional correlation of calmodulin‐dependent protein phosphatase in sperm. J Cell Biol 1988; 106: 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tash JS, Bracho GE. Regulation of sperm motility: emerging evidence for a major role for protein phosphatases. J Androl 1994; 15: 505–509. [PubMed] [Google Scholar]
- 45. Uma Devi K, Kula Nand J, Shivaji S. Plasma membrane‐associated protein tyrosine phosphatase activity in hamster spermatozoa. Mol Reprod Dev 1999; 53: 42–50. [DOI] [PubMed] [Google Scholar]
- 46. Inaba K, Kagami O, Ogawa K. Tctex2‐related outer arm dynein light chain is phosphorylated at activation of sperm motility. Biochem Biophys Res Commun 1999; 256: 177–183. [DOI] [PubMed] [Google Scholar]
- 47. Inaba K. Dephosphorylation of Tctex2‐related dynein light chain by type 2A protein phosphatase. Biochem Biophys Res Commun 2002; 297: 800–805. [DOI] [PubMed] [Google Scholar]


