Abstract
Background
Patient participation in clinical research is low, in part due to the length and complexity of the informed consent process. Video informed consent may enhance the appeal of research and help break down barriers to participation.
Methods and Results
The Patient and Provider Assessment of Lipid Management (PALM) study enrolled 7904 patients at cardiology, endocrinology, and primary care clinics across the United States to evaluate cholesterol management practices. Of 153 participating clinics, 67 (43.8%) secured institutional review board (IRB) approval to use a tablet-based video informed consent tool that patients could select to navigate through the informed consent process instead of traditional text-based informed consent. At sites without IRB approval of video consent, all patients read a text-based informed consent document. Site activation times and enrollment volumes, as well as characteristics of enrolled patients, were compared between sites with and without video consent capability. Sites with video consent capability more often used a central IRB (89.6 vs. 73.3%), were more often rural (16.7 vs 3.8%) and tended to have fewer providers. Compared with sites without video consent capability, sites with video consent capability had shorter times from site approach to first patient enrollment (median 178 vs. 207 days, p = 0.02). Sites with video consent capability enrolled similar numbers of patients as sites without video consent capability (p = 0.48), but enrolled a greater proportion of patients who were ≥ 75 years old (27.5 vs. 23.6%, p < 0.001) and non-white (17.7 vs. 14.2%, p<0.001).
Conclusions
In this observational study of recruitment in a multicenter registry, sites approved for video consent use enrolled the same number of patients as sites with only traditional text-based informed consent, but had faster speed to first patient enrolled and more often enrolled older and non-white patients. Future randomized trials are needed to assess the impact of video consent on enrollment mechanics and demographics.
Clinical Trial Registration
INTRODUCTION
Low rates of patient participation in clinical research have led to concerns about the generalizability of study results.1–4 A key potential barrier to research participation is the informed consent process. While intended to be patient-friendly, prior studies have shown that informed consent documents used for research purposes have an average 12th grade reading level and an average length of 10 pages.5, 6 In many ways, the lengthy and complex informed consent forms currently in use are designed more to document disclosures regarding risks, benefits, and alternatives to study participation than to truly inform participants.7 Limited literacy is prevalent in the United States (U.S.), and disproportionately affects older adults and minorities, critical groups that are under-represented in clinical research studies.8 Patients who cannot read or understand complex informed consent documents may choose not to participate in clinical research.9
The Patient and Provider Assessment of Lipid Management (PALM) Study was a multicenter, cross-sectional study involving 153 primary care, endocrinology, and cardiology clinics in the U.S. The study developed and implemented a video informed consent tool, consisting of a series of video vignettes explaining the study that patients could view at their own pace. In this analysis, we aimed to 1) describe how this video consent tool was utilized across study sites, and 2) compare enrollment patterns and participant diversity between sites who did and did not utilize the video consent tool.
METHODS
The PALM study has been previously described.10 Briefly, between May and November of 2015, PALM enrolled 7904 adult patients with atherosclerotic cardiovascular disease, current statin therapy use, low density lipoprotein cholesterol (LDL-C) ≥ 130 mg/dl, diabetes, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk ≥7.5%.11 Patients were offered participation while waiting to be seen at their regularly-scheduled clinic visit. Study procedures included completion of informed consent, a 15–20 minute survey regarding patients’ experience with and beliefs regarding lipid-lowering therapy, and a blood draw for a core lab lipid panel. Study personnel screened potential subjects using a PALM study application on an electronic tablet, and patients completed the informed consent process and survey on the tablet.10 Each participating center had either local or central Institutional Review Board (IRB) approval to conduct the study, and all patients provided informed consent prior to participation. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Informed consent in the PALM Registry
Both text- and video-based consent tools were approved by the coordinating center IRB at Duke University. All participating sites attempted to gain IRB approval for the use of video consent, but the video informed consent tool was approved by IRBs at some participating sites, and not at others. At the latter sites, patients were only provided the option to complete informed consent using the text-based informed consent document on the tablet. At sites where video informed consent was approved, patients could choose either the text- or video-based tool to provide informed consent. After study coordinators determined that the patient was eligible for recruitment using the screening tool on the tablet, they handed the tablet to the patient to choose whether they would like to read the text consent form or view the informed consent videos. All consent materials were downloaded directly onto the electronic tablet, and consent could proceed without an active internet connection.
The “traditional” text-based informed consent document for the PALM study was 6 pages long, and was written at approximately a 10th grade reading level (see Supplemental Methods for the full text informed consent document template).12 It was displayed in portable document format (pdf) on the electronic tablet. Patients completed informed consent by reading and then electronically signing the document on the tablet after any questions were answered by the study coordinator or nurse.
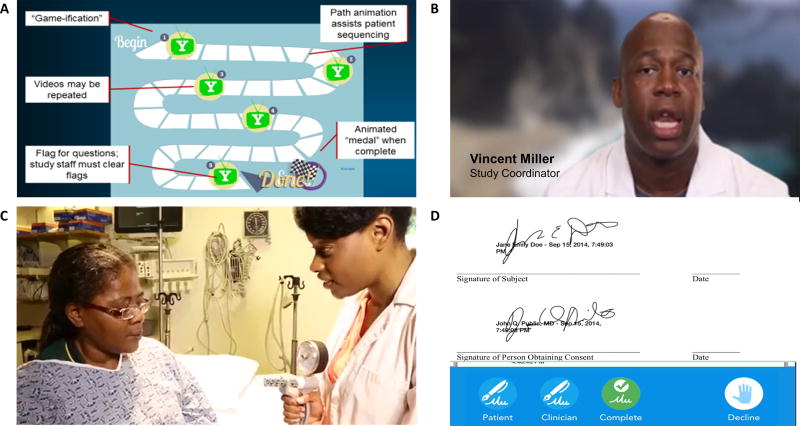
The video-based consent tool distilled the content of the traditional text-based informed consent into a series of 6 video vignettes totaling 8 minutes in length (see Supplemental Methods for the script of the informed consent videos). The vignettes captured the essential elements of informed consent in compliance with U.S. regulations,13 including sections describing research studies in general, the rationale for the PALM study, detailed study procedures, study duration, risks and benefits to participants, maintenance of confidentiality, alternatives to participating, costs and compensation for participating, assurances that participants would be contacted if relevant new information arose, instructions for stopping participation in the study, and instructions for whom to contact with any problems. The video modules were gamified to promote completion, and allowed patients to proceed at their own pace (Figure 1). Video content was not alterable after coordinating center IRB approval, and therefore could not be tailored to individual sites. Site-specific information, such as who to contact for problems, was provided to the patient in their printed copy of the signed informed consent document.
Figure 1.
Screen captures from the video consent process. Participants navigated through a series of video vignettes, each lasting 30 to 140 seconds, using a gamified interface (Panel A). Participants were able to flag vignettes that they had additional questions about, and study personnel were required to clear the flags after answering patient questions before proceeding to the signature screen. In the vignettes, study personnel described key elements of the informed consent process (Panel B), and B-roll footage showed patients participating in clinical research (Panel C). Patients and study coordinators both signed the consent form using their finger or a stylus on the tablet (Panel D) before patients proceeded to the PALM study’s patient survey. Patients were provided with a paper or electronic copy of the signed consent form.
A prototype of the video consent tool was pilot-tested in 14 patients at Duke University Medical Center between October and November of 2014 prior to implementation in the PALM study. Participants in the pilot study were shown a 15.5-minute video narration of a typical informed consent form, then asked to provide survey feedback regarding the video consent process. The PALM video consent tool was built based on the feedback from this pilot as well as iterative feedback from the Duke University IRB.
Statistical analysis
We described characteristics of sites that approved and did not approve video informed consent, and characteristics of the patients enrolled at each group of sites. Site and patient characteristics were compared between groups using the Mantel-Haenszel Chi-square test for categorical variables and Wilcoxon’s rank sum test for continuous variables.
To evaluate the association of video informed consent capability with enrollment mechanics, we compared the time from site approach to IRB approval, IRB approval to site activation, site activation to first patient enrollment, and site approach to first patient enrollment for sites with and without video consent capability using the Wilcoxon rank sum test. Site approach was defined as the date that the site received the packet of materials, including protocol and consent template, to begin study start-up activities. Site activation was defined as the date that a site had completed all required steps of the start-up process (including IRB approval, site contract execution, and completion of protocol and data entry training) and was able to begin screening and enrollment of patients. We repeated these analyses separately for sites that used local and central IRBs. Local IRB sites submitted their own application to their local IRB of record, usually the one affiliated with their associated healthcare organization but, in some cases, a non-affiliated fee-for-service IRB with which the site has a contract to perform IRB services. Central IRB sites either did not have an IRB of record, or their IRB of record agreed to abdicate oversight to a central IRB. At central IRB sites, the coordinating center submitted a protocol-level IRB application to the central IRB, and sites submitted additional documentation particular to the sites once the central IRB approved the study. We used multivariable linear regression, adjusting for site location (rural vs. non-rural) and IRB type (central vs. local), to evaluate the adjusted association between video consent capability and enrollment mechanics.
We also compared the total number of patients enrolled at sites with and without video informed consent capability and the number of patients enrolled per week using Wilcoxon rank sum test, and used a generalized linear model to perform Poisson regression,14 adjusting for site location (rural vs. non-rural) and IRB type (central vs. local), to evaluate the adjusted association between video consent and number of patients enrolled per week.
The investigators had full access to all of the data. Faculty and staff statisticians at the Duke Clinical Research Institute performed all analyses using SAS version 9.4 (Cary, NC, USA).
RESULTS
Video consent development and implementation
The video consent application was first pilot tested in 14 patients at Duke University Medical Center. The median age of the pilot population was 73 years, 43% were female, 29% were of non-white race, and 33% patients had previously participated in a research study. When surveyed, 83% of patients reported good understanding of study goals and 92% of patients reported good understanding of what was expected from their participation in the study. A third of patients reported that the video was too long and only 1 patient rated the ease of application use as below average. After incorporating patient feedback, the PALM video consent tool was submitted to the coordinating center IRB on December 2, 2014 and approved on February 5, 2015 after an iterative process to ensure that the videos developed for the consent were clear and contained all key information necessary for informed consent. Key revisions to the consent tool included a) shortening the total video length to 8 minutes; b) addition of options for patients to replay videos and to flag any questions (Figure 1); c) the need for study staff to verbally confirm patient understanding of the study before proceeding to e-signature; and d) ability to transmit a signed copy of the consent form from the device to a wireless printer and/or secured account for record-keeping.
Characteristics of sites with and without video consent
Of 153 sites that participated in the PALM study, 67 (43.8%) ultimately secured IRB approval to use the video informed consent, and 86 sites (56.2%) were approved to use text-based consent only. Sites that did not approve use of the video consent tool had institution- or state-specific IRB requirements – such as language pertaining to injury related to research, HIPAA information, and patient bill of rights – that could not be individualized in the consent videos. Sites with and without video consent availability were distributed throughout the United States, with representation of each in counties with high and low proportions of non-white people and older adults (Figure 2). Sites with video consent capability were more likely to be rural and to use a central IRB; they also tended to be smaller practices with fewer providers (Table 1).
Figure 2.
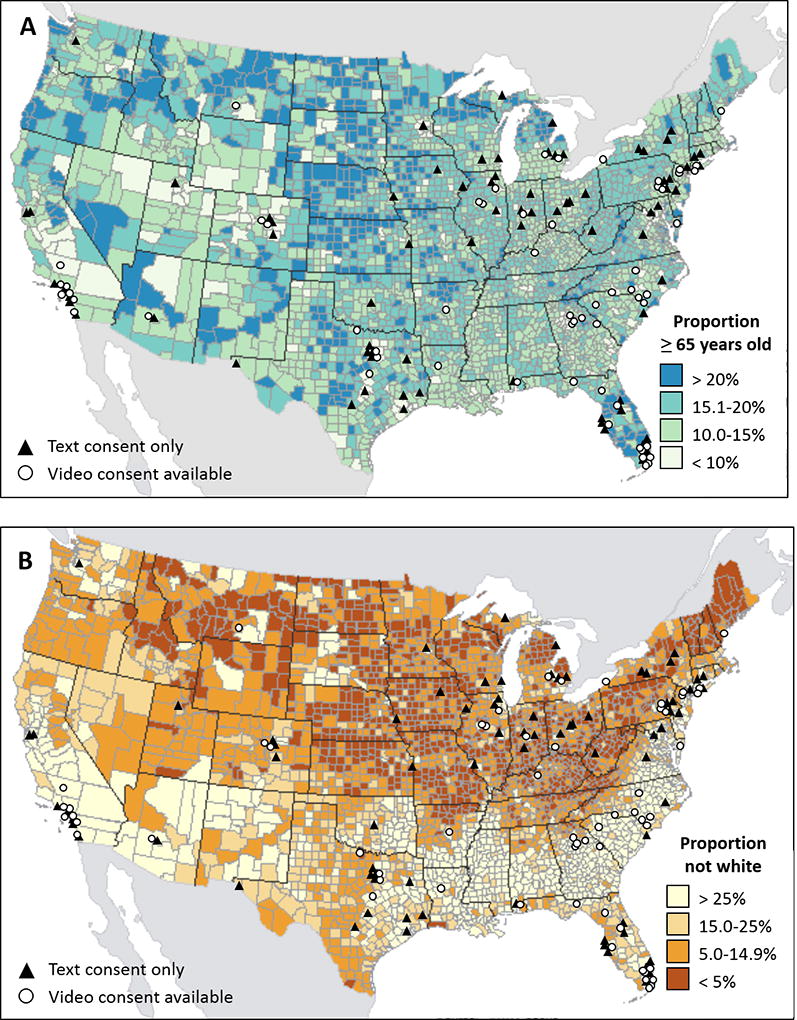
Geographic distribution of sites with and without video consent capability. Locations of enrolling sites are shown overlaid on U.S. maps showing county-level proportions of older adults and non-white people
Table 1.
Site characteristics by video consent capability
| All PALM Sites | PALM Sites With At Least 1 Enrolled Patient | |||||
|---|---|---|---|---|---|---|
| Video consent approved (n = 67) |
Video consent not approved (n = 86) |
P-value | Video consent approved (n = 60) |
Video consent not approved (n = 80) |
P-value | |
| Number of providers at site | 5 (3, 12) | 10 (4, 22) | 0.09 | 5 (3, 11) | 10 (4, 22) | 0.12 |
| Central IRB | 60 (89.6%) | 63 (73.3%) | 0.01 | 55 (91.7%) | 58 (72.5%) | 0.005 |
| Rural location* | 10 (14.9%) | 3 (3.5%) | 0.01 | 10 (16.7%) | 3 (3.8%) | 0.01 |
| Training facility† | 9 (13.4%) | 14 (16.3%) | 0.61 | 7 (11.7%) | 14 (17.5%) | 0.35 |
| Cardiology practice | 38 (56.7%) | 47 (54.7%) | 0.80 | 35 (58.3%) | 42 (52.5%) | 0.49 |
Rural location based on site zip code metropolitan statistical area;
At least one provider sees patients with trainees (residents, fellows, etc). Continuous variables represented as median (25th, 75th percentiles); categorical variables represented as frequency (percent).
Time to first patient enrolled at sites with and without video consent capability
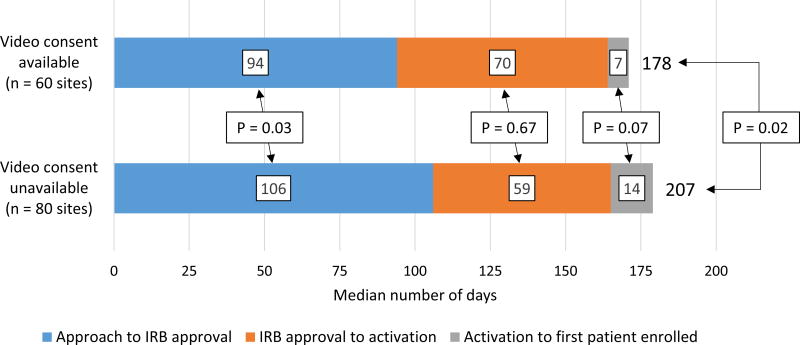
Among the 140 sites that enrolled at least 1 patient, 60 (42.8%) had video consent capability and 80 (57.1%) did not. The median time from site approach to first patient enrolled was 29 days shorter in sites with video consent capability compared with sites without (178 vs. 207 days, p = 0.02, Figure 3). This difference was primarily driven by shorter median time from approach to IRB approval (94 vs. 106 days, p = 0.03) in the video consent sites. The time from site activation to first patient enrolled was numerically shorter in the sites with video consent capability but this did not reach statistical significance (median 7 vs. 14 days, p = 0.07).
Figure 3.
Time from site contact to first patient enrolled at sites with and without video consent capability. Sites with video informed consent capability had shorter time from contact to first patient enrolled, driven by shorter time from site contact to IRB approval, and shorter time from site activation to first patient enrolled. IRB, institutional review board. Median time from contact to first patient enrolled was calculated separately, and thus does not equal the sum of the medians of its components (contact to IRB approval, IRB approval to site activation, site activation to first patient enrolled).
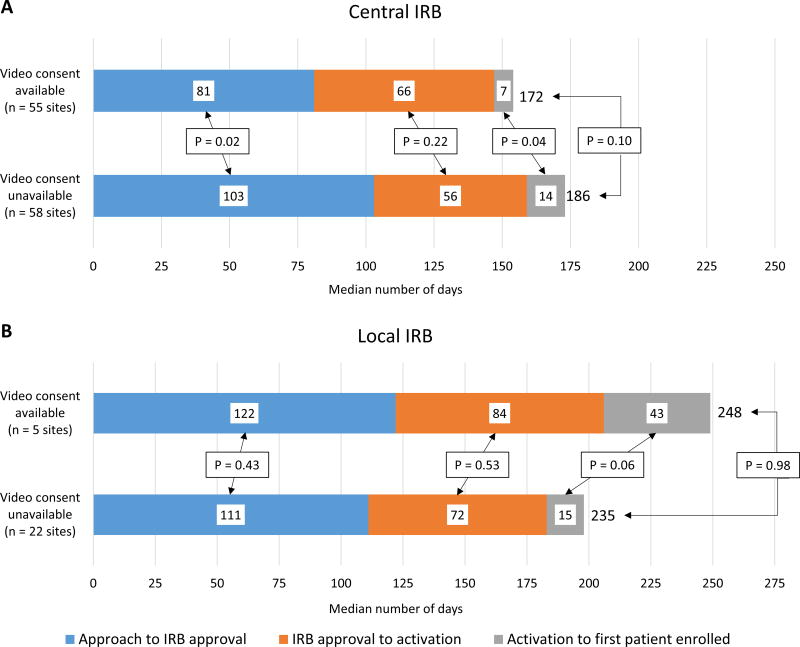
At sites using central IRBs, the time from site approach to first patient enrolled was 14 days shorter in sites with video consent capability compared with sites without, though this difference was not significant (median 172 vs. 186 days, p = 0.10) (Figure 4). Local IRB sites that had video consent capability had numerically longer duration from site approach to first patient enrolled than sites that did not have video consent capability (median 248 vs. 235 days, p = 0.98). After adjusting for site location and type of IRB used, sites with video consent capability had an estimated time from site approach to first patient enrolled that was 12.7 days shorter than sites without video consent availability, though this difference was not significant (p = 0.12).
Figure 4.
Time from site contact to first patient enrolled at sites with and without video consent capability with central IRBs (A) and local IRBs (B). At central IRB sites, sites with video informed consent available had numerically shorter time from contact to first patient enrolled. At local IRB sites, sites with video informed consent capability had numerically longer time from contact to first patient enrolled.
Enrollment and study completion at sites with and without video consent capability
Sites with video consent capability enrolled a median of 33 patients (25th, 75th percentiles: 11, 94; range 1–247), whereas sites without video consent capability enrolled a median of 23 patients (25th, 75th percentiles: 11, 83; range 1–254; p = 0.48). There was no significant difference in the number of patients enrolled per week between sites with and without video consent capability (median 2.9 patients at sites with video consent capability vs. 3.0 patients at sites without video consent capability, p = 0.91), and there remained no difference after adjusting for site location and type of IRB (p = 0.62).
In the PALM study application, sites needed to ensure patient eligibility using the screening tool on the electronic tablet prior to handing the tablet over to the patient to proceed with consent. Among patients who were eligible and approached for enrollment, sites with video consent capability successfully consented and enrolled 79.1% of these patients while sites without video consent capability enrolled 79.6% of these patients (p = 0.61). Sites with video consent capability had no significant difference in the proportion of their enrolled patients who completed the core lab blood draw compared with sites with text-based consent only (95% vs. 97%, p = 0.28). The proportion of patients who completed > 80% of patient survey questions were also not significantly different between groups (91% vs. 93%, p = 0.99).
Patient Characteristics at sites with and without video consent capability
The 140 sites consented a total of 7904 patients, ranging in age from 20 to 99 years old. Compared with text-only sites, sites with video consent capability enrolled more patients ≥ 75 years old, more African American patients, and more patients without college degrees. They enrolled fewer patients with prior atherosclerotic vascular disease or diabetes, and more patients who were not taking statin therapy at the time of enrollment (Table 2). The median LDL-C level was higher in patients consented at sites with video consent capability.
Table 2.
Patient characteristics by site video consent capability
| Video consent approved (n = 3485 patients; 60 sites) |
Video consent not approved (n = 4419 patients; 80 sites) |
P- value | |
|---|---|---|---|
| Demographics | |||
| Age | 68 (60, 75) | 67 (59, 74) | < 0.001 |
| Age ≥ 75 years old | 959 (27.5%) | 1041 (23.6%) | < 0.001 |
| Female gender | 1809 (51.9%) | 2348 (53.1%) | 0.26 |
| Race | < 0.001 | ||
| White | 2868 (82.3%) | 3793 (85.8%) | |
| African American | 537 (15.4%) | 534 (12.1%) | |
| Asian | 66 (1.9%) | 84 (1.9%) | |
| American Indian/Alaskan | 7 (0.2%) | 4 (0.1%) | |
| Insurance | < 0.001 | ||
| Private | 2113 (60.6%) | 2458 (55.6%) | |
| Government | 1277 (36.6%) | 1850 (41.9%) | |
| Other | 3 (0.1%) | 0 (0.0%) | |
| None | 71 (2.0%) | 108 (2.4%) | |
| College graduate or higher | 1069 (30.7%) | 1573 (35.6%) | <0.001 |
| Clinical Characteristics | |||
| Atherosclerotic vascular disease | 1450 (41.6%) | 1977 (44.7%) | 0.005 |
| Coronary artery disease | 1206 (34.6%) | 1624 (36.8%) | 0.05 |
| Peripheral vascular disease | 284 (8.2%) | 419 (13.4%) | 0.04 |
| Cerebrovascular disease | 437 (12.5%) | 593 (13.4%) | 0.25 |
| History of MI | 342 (9.8%) | 666 (15.1%) | < 0.001 |
| Diabetes | 1216 (34.9%) | 1847 (41.8%) | < 0.001 |
| Hypertension | 2679 (76.9%) | 3446 (78.0%) | 0.25 |
| Current/recent smoker | 463 (13.3%) | 597 (13.5%) | 0.77 |
| Chronic kidney disease | 360 (10.3%) | 393 (8.9%) | 0.03 |
| Currently taking statin | 2399 (68.8%) | 3253 (73.6%) | < 0.001 |
| Core lab LDL-C level | 99 (77, 126) | 94 (73, 120) | <0.001 |
Continuous variables expressed as median (25th, 75th percentile); categorical variables expressed as number (%).
DISCUSSION
This study describes one of the largest experiences of using videos as an alternative to text to conduct informed consent in a multicenter clinical trial. The video consent tool was approved for use in 44% of participating sites. Sites that had video informed consent capability did not experience a delay in activation, and in fact progressed more rapidly to enrolling their first patient. This was primarily observed in sites that used a central IRB. The availability of video informed consent was not associated with enrollment of a significantly greater number of patients, nor a faster enrollment rate. However, sites with video consent capability enrolled more older and non-white patients than sites with only traditional text-based consent.
Clinical research in the U.S. is hampered by a limited number of research-capable sites and low patient enrollment at select centers,1 curtailing investigators’ ability to answer a number of critical public health questions and calling into question the representativeness of study populations.15, 16 Despite efforts to increase the enrollment of minorities and older adults in clinical research – including a statutory mandate to address the inclusion of these groups in the design phase of federally-funded research17 and U.S. Food and Drug Administration expectations that clinical trial participants will be similar to the drug’s intended target population18 – older adults and non-white people continue to be underrepresented in cardiovascular clinical trials.19 For this reason, proposed solutions to low enrollment have focused on simplifying the research process to enhance trial participation among clinical practice sites and patients, and especially focused on reducing barriers to enrollment for potential older adult and non-white participants.15, 16, 20
The video informed consent process used in PALM has the potential to make clinical research more attractive to both sites and participants. At the site level, the application was designed to guide patients through the informed process in a user-friendly fashion that allowed the patient to proceed at his/her own comfortable pace, but did not necessitate hands-on shepherding by the study nurse or coordinator. Site coordinators were thus available to enroll multiple patients simultaneously, which permitted enrollment to be more facilely integrated into sites’ clinical workflow. The PALM application seamlessly led patients from informed consent directly to the patient survey on the tablet, thus eliminating the need for study coordinators to transcribe data from paper into the study database. Sites with video informed consent capability were distributed across the US geographically, and were more likely to be rural practices without local IRBs, suggesting that the availability of video informed consent may have attracted sites that have not traditionally been able to participate in clinical research due to limited resources or staff training, though sites’ prior experience with research was not collected.
On the patient level, video informed consent puts a human face on study personnel, uses more colloquial verbiage to communicate study concepts simply, and breaks down health literacy or physical barriers (e.g., forgot reading glasses) to understanding the written word. Inclusion of non-white, non-male members of the study team in the informed consent video may have fostered trust in the clinical research enterprise.21 The gamified interface, as well as B-roll footage showing patients participating in the research process, may stimulate patient engagement and more active participation in the research process. Although sites with video informed consent capability did not have significantly higher or faster enrollment, these sites enrolled more older adults and non-white participants than sites without video informed consent. Sites with video informed consent capability also enrolled more participants without college degrees than sites without video consent, suggesting that it may also help overcome educational/literacy-related barriers to participation in research. Video decision support tools have been demonstrated to help seriously ill patients make difficult clinical decisions regarding advanced care, overcoming knowledge barriers better than verbal descriptions,22–24 and may inform ways to increase patients’ comfort with clinical research. Baseline characteristics of patients who were eligible for but did not enroll in the PALM study were not collected, so it is not clear whether the greater participation of older adults and non-white people at sites with video informed consent capability is due to site demographics or greater likelihood of participation. Importantly, however, the mechanism by which video informed consent could potentially increase participation of older adults and non-white people includes both increasing enrollment of these groups at individual sites and attracting sites with greater proportions of these groups to clinical research. The proportion of African American patients enrolled in PALM is in-line with U.S. population estimates,25 and is greater than the proportion of African American patients (as a fraction of total U.S. enrollment) in recent cardiovascular clinical trials.26, 27 Nevertheless, future clinical trials are needed to assess the effect of video informed consent on enrollment of older and non-white patients into clinical trials by randomizing sites to video- or text-based informed consent.
These results also demonstrate the feasibility of incorporating video informed consent (and more broadly, innovative clinical trial procedures) into a large, multisite clinical research program. A key concern was that using the novel consent format might delay IRB approval and site activation to enroll patients. This was our institutional IRB’s first experience with video consent, and upfront engagement with IRB leadership was required to design the tool and shorten the video length to preserve patient interest in the study while adequately conveying all necessary elements of consent. Despite their lack of prior IRB experience with video informed consent, sites with video informed consent capability in fact had shorter lag time to IRB approval and to enrollment of the first patient. Part of the difference in time to IRB approval and first patient enrollment may be explained by more frequent use of central IRBs by sites with video informed consent capability, since the difference in time from site approach to first patient enrolled was no longer significant after adjusting for IRB type. Among sites that used central IRBs, those with video informed consent capability had a significantly shorter lag time to IRB approval, which was not observed for sites that used local IRBs. The longer time to IRB approval in the sites that ultimately did not approve use of video consent may reflect back and forth dialogue in attempts to meet institution- or state-specific IRB requirements. The National Institutes of Health recently announced a policy establishing the expectation that a single, central IRB should be used in multi-site clinical research occurring in the United States.28 The policy’s goal was to enhance and streamline the IRB approval process, avoiding duplicative review and administrative burden without jeopardizing patient safety. Video informed consent would not have been feasible without the ability to use identical informed consent documents at multiple sites. The learning curve of each IRB with regards to the video informed consent tool is unlikely to differ much between sites, but once familiar with the relevant ethical and patient safety issues, efficiency may be enhanced by a central IRB’s ability to approve its use for the multiple sites it oversees.
Several limitations should be acknowledged. First, this is an observational analysis subject to both measured and unmeasured confounding. Sites approving video informed consent may have been more interested in innovative clinical trial methods, for example, and thus more invested in demonstrating that video informed consent could boost clinical research enrollment. Second, PALM did not collect data on the populations participating sites served, so it is therefore difficult to tell whether sites with video consent capability more effectively recruited older and non-white patients, or whether the availability of video consent enabled recruitment of sites that serve a greater proportion of older and non-white patients. Third, the PALM consent tool did not record how individual patients completed the informed consent process; patients at sites with video consent availability may have chosen to read the traditional informed consent document rather than viewing the video. Lastly, neither patients nor site personnel in PALM were formally surveyed to assess their experience with the video informed consent process.
CONCLUSION
In this early experience with video consent in a multicenter study, availability of video informed consent was associated with greater enrollment of older and non-white patients, and faster speed to first patient enrollment. Video informed consent may represent an attractive method to increase participation in clinical research and representativeness of patient populations enrolled, and its effect should be formally evaluated in randomized clinical trials.
Supplementary Material
What is known
The informed consent process typically involves reading a lengthy document, and represents a key potential barrier to research participation, perhaps especially for older adults and minorities, who are disproportionately affected by limited literacy.
Innovative approaches to make informed consent simpler and more patient friendly, like video informed consent, may help overcome these barriers.
What the study adds
In the multi-center, prospective PALM study, investigators created a video informed consent tool; 44% of participating sites’ IRBs approved use of video informed consent
Compared with sites with only traditional text-based informed consent, sites with video consent capability enrolled the same number of patients, but had faster speed from site approach to first patient enrolled, and more often enrolled older and non-white patients.
Acknowledgments
SOURCES OF FUNDING
The PALM Registry was supported by Sanofi Pharmaceuticals and Regeneron Pharmaceuticals. Pilot testing for the video informed consent tool was supported by a grant from the Agency for Healthcare Research and Quality (U19HS021092). Dr. Fanaroff is supported by a career development grant from the American Heart Association (17FTF33661087).
Dr. Fanaroff reports research support from the American Heart Association (17FTF33661087) and Gilead Sciences. Dr. Navar reports research support from Regeneron/Sanofi Amgen, Inc., and Janssen Pharmaceuticals; consultant for Sanofi and Amgen, Inc.; also funded by National Institutes of Health, K01HL133416-01. Dr Peterson reports research support from Eli Lilly, Janssen, Merck, consulting from AstraZeneca, Bayer, Boehringer Ingelheim, Genentech, Janssen, Merck, and Sanofi Aventis. Dr. Wang reports research support from AstraZeneca, Boston Scientific, Bristol Myers Squibb, Cryolife, Daiichi Sankyo, Eli Lilly, Gilead Sciences, Novartis, Pfizer, and Regeneron; consultant/advisory/education from Merck, Gilead, and Pfizer.
Footnotes
DISCLOSURES
All other authors report no relevant disclosures.
References
- 1.Califf RM, Harrington RA. American industry and the US cardiovascular clinical research enterprise. J Am Coll Cardiol. 2011;58:677–680. doi: 10.1016/j.jacc.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 2.Getz KA, Campo RA. Trial watch: Trends in clinical trial design complexity. Nat Rev Drug Discov. 2017;16:307. doi: 10.1038/nrd.2017.65. [DOI] [PubMed] [Google Scholar]
- 3.Sung NS, Crowley WF, Jr, Genel M, Salber P, Sandy L, Sherwood LM, Johnson SB, Catanese V, Tilson H, Getz K. Central challenges facing the national clinical research enterprise. J Am Med Assoc. 2003;289:1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 4.Lee S, Monz BU, Clemens A, Brueckmann M, Lip GY. Representativeness of the dabigatran, apixaban and rivaroxaban clinical trial populations to real-world atrial fibrillation patients in the United Kingdom: a cross-sectional analysis using the General Practice Research Database. BMJ Open. 2012;2:e001768. doi: 10.1136/bmjopen-2012-001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larson E, Foe G, Lally R. Reading level and length of written research consent forms. Clin Transl Sci. 2015;8:355–356. doi: 10.1111/cts.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonds VW, Garroutte EM, Buchwald D. Health Literacy and Informed Consent Materials: Designed for Documentation, Not Comprehension of Health Research. J Health Comm. 2017;22:682–691. doi: 10.1080/10810730.2017.1341565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grady C. Enduring and emerging challenges of informed consent. N Engl J Med. 2015;372:855–862. doi: 10.1056/NEJMra1411250. [DOI] [PubMed] [Google Scholar]
- 8.Goodman M, Finnegan R, Mohadjer L, Krenzke T, Hogan J, U.S. Department of Education National Center for Education Statistics [Access date: 12 March 2018];Literacy, Numeracy, and Problem Solving in Technology-Rich Environments among US Adults: Results from the Program for the International Assessment of Adult Competencies. https://nces.ed.gov/pubs2014/2014008.pdf.
- 9.Brown BA, Long HL, Gould H, Weitz T, Milliken N. A conceptual model for the recruitment of diverse women into research studies. J Womens Health Gend Based Med. 2000;9:625–632. doi: 10.1089/15246090050118152. [DOI] [PubMed] [Google Scholar]
- 10.Navar AM, Wang TY, Goldberg AC, Robinson JG, Roger VL, Wilson PF, Virani SS, Elassal J, Lee LV, Webb LE. Design and rationale for the Patient and Provider Assessment of Lipid Management (PALM) registry. Am Heart J. 2015;170:865–871. doi: 10.1016/j.ahj.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Stone N, Robinson J, Lichtenstein A, Bairey Merz C, Blum C, Eckel R, Goldberg A, Gordon D, Levy D, Lloyd-Jones D. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 12.Text Readability Consensus Calculator. [Access date: 12 March 2018];Readability Formulas. 2018 http://www.readabilityformulas.com/free-readability-formula-tests.php.
- 13.U.S. Department of Health and Human Services. Code of Federal Regulations. 21CFR50.25. Elements of informed consent. 2017. [Google Scholar]
- 14.McCullagh P, Nelder JA. Generalized Linear Models, Second Edition. London: Chapman and Hall; 1989. [Google Scholar]
- 15.Eapen ZJ, Vavalle JP, Granger CB, Harrington RA, Peterson ED, Califf RM. Rescuing clinical trials in the United States and beyond: a call for action. Am Heart J. 2013;165:837–847. doi: 10.1016/j.ahj.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor CM, Psotka MA, Fiuzat M, Lindenfeld J, Abraham WT, Bristow MR, Canos D, Harrington RA, Hillebrenner M, Jessup M. Improving Heart Failure Therapeutics Development in the United States: The Heart Failure Collaboratory. J Am Coll Cardiol. 2018;71:443–453. doi: 10.1016/j.jacc.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health. [Access date: 12 March 2018];NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research. 2001 https://grants.nih.gov/grants/funding/women_min/guidelines.htm.
- 18.U.S. Food and Drug Administration. [Access date: 12 March 2018];Guidance for industry. Collection of race and ethnicity data in clinical trials. 2013 https://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126396.pdf.
- 19.Downing NS, Shah ND, Neiman JH, Aminawung JA, Krumholz HM, Ross JS. Participation of the elderly, women, and minorities in pivotal trials supporting 2011–2013 US Food and Drug Administration approvals. Trials. 2016;17:199. doi: 10.1186/s13063-016-1322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones WS, Roe MT, Antman EM, Pletcher MJ, Harrington RA, Rothman RL, Oetgen WJ, Rao SV, Krucoff MW, Curtis LH. The Changing Landscape of Randomized Clinical Trials in Cardiovascular Disease. J Am Coll Cardiol. 2016;68:1898–1907. doi: 10.1016/j.jacc.2016.07.781. [DOI] [PubMed] [Google Scholar]
- 21.Sherber NS, Powe NR, Braunstein JB. Personal physicians as study investigators: Impact on patients' willingness to participate in clinical trials. Contemp Clin Trials. 2009;30:227–232. doi: 10.1016/j.cct.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 22.El-Jawahri A, Paasche-Orlow MK, Matlock D, Stevenson LW, Lewis EF, Stewart G, Semigran M, Chang Y, Parks K, Walker-Corkery ES. Randomized, Controlled Trial of an Advance Care Planning Video Decision Support Tool for Patients With Advanced Heart FailureClinical Perspective. Circulation. 2016;134:52–60. doi: 10.1161/CIRCULATIONAHA.116.021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain A, Corriveau S, Quinn K, Gardhouse A, Vegas DB, You JJ. Video decision aids to assist with advance care planning: a systematic review and meta-analysis. BMJ Open. 2015;5:e007491. doi: 10.1136/bmjopen-2014-007491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volandes AE, Ferguson LA, Davis AD, Hull NC, Green MJ, Chang Y, Deep K, Paasche-Orlow MK. Assessing end-of-life preferences for advanced dementia in rural patients using an educational video: a randomized controlled trial. J Palliat Med. 2011;14:169–177. doi: 10.1089/jpm.2010.0299. [DOI] [PubMed] [Google Scholar]
- 25.United States Census Bureau. [Access date: 12 March 2018];United States Quick Facts. 2018 https://www.census.gov/quickfacts/fact/table/US/PST045217.
- 26.Mathews R, Wang TY, Honeycutt E, Henry TD, Zettler M, Chang M, Fonarow GC, Peterson ED. Persistence with secondary prevention medications after acute myocardial infarction: Insights from the TRANSLATE-ACS study. Am Heart J. 2015;170:62–9. doi: 10.1016/j.ahj.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahaffey KW, Wojdyla DM, Carroll K, Becker RC, Storey RF, Angiolillo DJ, Held C, Cannon CP, James S, Pieper KS. Ticagrelor Compared With Clopidogrel by Geographic Region in the Platelet Inhibition and Patient Outcomes (PLATO) Trial. Circulation. 2011;124:544–554. doi: 10.1161/CIRCULATIONAHA.111.047498. [DOI] [PubMed] [Google Scholar]
- 28.National Institutes of Health. [Access date: 12 March 2018];Final NIH Policy on the Use of a Single Institutional Review Board for Multi-Site Research. 2018 https://grants.nih.gov/grants/guide/notice-files/NOT-OD-16-094.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.