Abstract
Pulse wave velocity (PWV) has been used as a measurement for arterial stiffness, a predictor of cardiovascular risk. Tracking describes the stability of a measurement over time. The purpose of this study was to evaluate the tracking stability of carotid-femoral (cfPWV), carotid-radial (crPWV) and carotid-distal (cdPWV) PWV in young adults and their associations with anthropometric and hemodynamic measurements. cfPWV, crPWV and cdPWV were measured by tonometric (SphygmoCor) technique in 531 subjects (aged 23.7 ±4.9 with 42.9% African Americans and 49.9% females). Out of these subjects, 142 subjects had all these 3 PWV measurements evaluated again during their next visit with an average follow-up time of 2 years. In the tracking analysis on the data from the 142 subjects, cfPWV displayed moderate to relatively high tracking ability (r =0.61, P<0.001), whereas crPWV and cdPWV only displayed low to moderate tracking coefficients (r =0.29 and r =0.36 respectively, P<0.001). In the association test on the data from the 531 subjects, all three PWV measurements showed significant correlations with age and obesity related measurements. cfPWV displayed stronger correlations with these parameters. In addition, all three PWVs showed significant correlations with systolic blood pressure, diastolic blood pressure, mean arterial pressure and pulse pressure with the exception that no correlation existed between crPWV and pulse pressure. The higher tracking ability of cfPWV and its higher association with obesity related measurements highlights the importance of using cfPWV compared with crPWV and cdPWV for research and clinical settings.
Keywords: arterial stiffness, longitudinal study, pulse wave velocity, tracking
INTRODUCTION
Increased arterial stiffness has been shown to be an important parameter in the assessment of cardiovascular (CV) risk. Numerous methodologies exist for measuring arterial stiffness, but pulse wave velocity (PWV) in elastic arteries, such as carotid-femoral PWV (cfPWV), has been well recognized as a gold standard.1 This recognition is due to the fact that carotid-femoral PWV is easily determined, reproducible2,3 and an independent predictor of CV events in elderly patients,4 in patients with hypertension,5 diabetes,6 and end-stage renal disease7 and in the general population.8,9 Positive effects of antihypertensive treatment on carotid-femoral PWV have also been observed in patients with hypertension10 and in the general population.11 Although carotid-femoral PWV is the recommended clinical marker for CV risk stratification,1 PWV for muscular arteries, such as carotid-radial PWV (crPWV), has been used as an alternative due to ease of access. However, existing cross-sectional evidence suggest that the site (muscular vs. elastic arteries) at which PWV is measured matters, with elastic arteries (that is, carotid-femoral) being a better indicator for CV risk.1,12,13 Longitudinal studies have illustrated carotid-femoral PWV as a predictor of CV morbidity and mortality,14,15 yet limited longitudinal evidence exists that describes the tracking ability of PWV at different arterial sites. Tracking describes the ability of a characteristic to remain stable over time and facilitate the prediction of future values based on prior measurements.16 CV risk factors or subclinical measurements with high tracking stability are of considerable public health interest because subjects who are at high risk to develop CV diseases might be identified at an early age. In the present study, we aimed to compare and contrast the tracking stability of carotid-femoral, carotid-radial and carotid-distal PWV (cdPWV) in European American (EA) and African American young adults evaluated twice over a 2-year period. We further examined the effects of anthropometric measures and different hemodynamic markers such as peripheral blood pressure (BP), cardiac index and total peripheral resistance (TPR) index on carotid-femoral, carotid-radial and carotid-distal PWV values.
METHODS
Subjects
The present study comprised subjects from two longitudinal cohorts: the BP stress study17,18 and the Georgia Cardiovascular Twin Study.19,20 The BP stress study was established in 1989 to study the development of CV risk factors. It included 349 African American and 396 EA youth with evaluations conducted every 1–3 years. The Georgia Cardiovascular Twin Study was established in 1996 including roughly equal numbers of African Americans and EAs (>500 twin pairs) with evaluations conducted every 2–3 years. Subjects in both data sets were recruited from the southeastern United States and were overtly healthy and free of any acute or chronic illness based on parental report. Study design and selection criteria for these two studies have been described previously.17–20
A total of 531 subjects were available for this study, 210 individuals (mean ±s.d. age, 27.8 ±2.8 years; range, 21.3–34.8 years; 54.8% EAs and 53.5% males) from the BP stress study and 321 twins (mean ±s.d. age, 21.0 ±3.97 years; range, 15.1–32.4 years; 58.6% EAs and 47.7% males) from the Georgia Cardiovascular Twin study, who had cfPWV, crPWV and cdPWV measured from 2008 to 2010 during a routine visit. The correlations between the three PWV measurements, and anthropometric and hemodynamic measurements were calculated in these 531 subjects. Out of these subjects, 142 subjects (73 from BP stress study and 69 from the twin study) had all these three PWV measurements evaluated again during their next visit with an average follow-up time of 2 years (ranging from 1 to 4 years). The two time point measurements on PWV from these 142 subjects were used for the tracking coefficient analysis. The Institutional Review Board at the Medical College of Georgia had given approval for this study. Informed consent was provided by all subjects and by parents if subjects were <18 years of age.
Measures
cfPWV, crPWV and cdPWV were measured non-invasively via applanation tonometry and the SphygmoCor CPV analysis software (SphygmoCor, AtCor Medical, Sydney, Australia). Pressure waves were recorded at the common carotid and femoral arteries for the carotid-femoral PWV, at the common carotid and radial arteries for the crPWV, and at the common carotid and dorsalispedis arteries for the cdPWV. The SphygmoCor system then calculated PWV from measurements of pulse transit time and distance traveled by the pulse between two arterial sites: PWV=distance (meters)/transit time (seconds).21
Anthropometrics and body composition assessment were obtained during the examination. Height and weight were measured by standard methods using a wall-mounted stadiometer and a digital scale, respectively. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Waist circumference (in cm) was measured twice at the center of the umbilicus over the T-shirt and the values were averaged. Skinfold thicknesses (that is, triceps, subscapular and suprailiac) were measured on the right side of the body with Lange calipers according to established protocols.22 Three sets of measurements for each skinfold were recorded and averaged. The inter-correlations were >99%. BMI and the sum of the three skinfold thicknesses were used as measures of general adiposity, whereas waist circumference was used as a measure of central adiposity.
Hemodynamic measurements were conducted using established protocols.20 Participants were instrumented for the recording of BP and heart rate by Dinamap (model 1864 SX) and of stroke volume and cardiac output by bioimpedance cardiography (BioZ, CardioDynamics, San Diego, CA, USA). Pulse pressure (PP) was calculated as (systolic blood pressure (SBP)-diastolic blood pressure (DBP)). Cardiac output was indexed by body surface area (that is, cardiac index). TPR index was calculated as mean arterial pressure/cardiac index. Measurements were taken at 11, 13 and 15 min while the subjects lay (supine) on a hospital bed. The average of the three measurements were used.
Statistical analysis
The following multivariate linear regression model was use to estimate the PWV tracking coefficients: .17 Yit2 is the dependent variable for individual i at t = 2 (t =time) and in this study, it is carotid-femoral, carotid-radial or cdPWV. Yit1 is the initial observation of individual i at t =1. Xijt is the time-dependent covariate j for individual i. Zik is the time-independent covariate k for individual i. εit is the measurement error for individual i. The dependent variable Y at time t1 is regressed on the same variable Y at time t2. Then the standardized β1 regression coefficient can be interpreted as the tracking coefficient between the two time periods.17 This tracking coefficient can range between −1 and +1. However, assuming the correlations between the PWVs at the two visits to be positive, this tracking coefficient takes values between 0 and 1, with 1 indicating perfect tracking and 0 indicating no tracking. Although there is no clear definition in terms of the interpretation of the magnitude of the tracking coefficients, it is generally considered that a variable with tracking coefficient >0.7 tracks well and a variable with tracking coefficient <0.3 tracks poorly. If the value lies between these two, then the variable displays moderate tracking.23 The advantages of this model are: (1) A balanced data set is not necessary because it can run with missing values of the dependent variable and (2) possible confounders can be adjusted for with the use of covariates. In the present analysis, ethnicity, gender, cohort, baseline BMI, baseline age and age difference between initial visit and the follow-up visit were included as covariates. To control for the non-independence of twins, we used generalized estimating equations, which yielded unbiased standard errors and P-values.
To contrast the tracking abilities amongst carotid-femoral, carotid-radial and cdPWVs, the following test statistic was used: , where β1 and β2 are tracking coefficients for the different PWVs, and s.e1 and s.e2 are the respective s.e for β1 and β2.17 A Bonferroni correction (n =3) was applied and a value of P<0.017 was considered statistically significant.
Partial correlation analysis was used to assess the correlations between PWV and anthropometric/hemodynamic measurements. The covariates were age, ethnicity, gender and cohort for all the correlation tests and BMI was further included for the correlation tests between PWV and hemodynamic measurements. The P-values were obtained from the generalized estimating equations, which takes account of the dependence between twins. Steiger’s Z test24 for calculating the difference between correlations that involve a common variable (the anthropometric/hemodynamic measurements) was used to test the significance of the difference between the correlation coefficients. Owing to the multiple testing, false discovery rate was applied and a false discovery rate<0.05 was considered statistically significant. All analyses were performed using STATA 12.0 (StataCorp, College Station, TX, USA).
RESULTS
Table 1 shows the general characteristics of the overall sample (n =531) and the subset sample for tracking analysis (n =142). Apart from a relatively small percentage of females (41.6 vs. 49.9%, P =0.02), we did not find any significant differences between the subset sample for tracking analysis and the overall sample in the distribution of age, ethnicity, anthropometric, hemodynamic measurements or the PWV measurements.
Table 1.
General characteristics of the subjects
| Overall sample | Subset sample | |
|---|---|---|
| N | 531 | 142 |
| Age (years) | 23.7 ±4.9 | 23.6 ±4.8 |
| African Americans (%) | 42.9 | 47.2 |
| Female (%) | 49.9 | 41.6a |
| BMI (kg m−2) | 25.4 ±5.1 | 25.1 ±4.5 |
| Waist circumference (cm) | 84.9 ±12.6 | 84 ±11.7 |
| Sum of skinfolds (mm) | 54.7 ±24.5 | 50.8 ±24.3 |
| SBP (mm Hg) | 113.8 ±13.2 | 112.1 ±12.5 |
| DBP (mm Hg) | 64.5 ±8.4 | 63.4 ±7.6 |
| Mean arterial pressure (mm Hg) | 81.9 ±9.5 | 80.8 ±9 |
| Pulse pressure (mm Hg) | 49.4 ±10.8 | 48.7 ±10.9 |
| TPR index | 29.3 ±5.7 | 29 ±5.7 |
| Cardiac index | 2.9 ±0.5 | 2.9 ±0.4 |
| PWV (m/s) | ||
| Carotid-femoral | 5.6 ±1.2 | 5.5 ±1 |
| Carotid-radial | 7.5 ±1.4 | 7.3 ±1.2 |
| Carotid-distal | 7.9 ±1 | 7.9 ±1 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; PWV, pulse wave velocity; SBP, systolic blood pressure; TPR, total peripheral resistance.
P<0.05 in comparison with the overall sample.
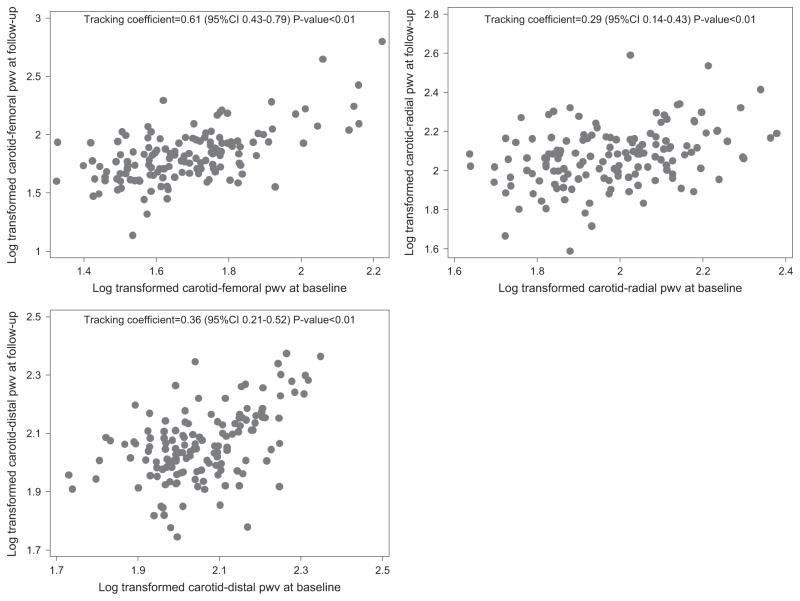
The tracking abilities of cfPWV, crPWV, and cdPWV are illustrated in Table 2 and Figure 1. The tracking coefficients of cfPWV, crPWV, cdPWV were 0.61, 0.29, and 0.36, respectively with P<0.001. These findings indicate that the tracking coefficient is significant for all three PWV measurements. However, cfPWV exhibited relatively high tracking ability, whereas crPWV and cdPWV exhibited only moderate tracking ability. We used the test statistic Z as a method of comparison amongst the three PWVs. In all, cfPWV tracked significantly better than crPWV and cdPWV (Bonferroni corrected P-value<0.01). No significant difference existed in the tracking of crPWV and cdPWV. Further adjustment of mean arterial pressure and PP did not change the results with the tracking coefficients of cfPWV, crPWV and cdPWV being 0.54, 0.23 and 0.27, respectively.
Table 2.
Tracking coefficients of PWV
| PWVa | Tracking coefficient (95% CI) | P-value |
|---|---|---|
| Carotid-femoral | 0.61 (0.43, 0.79) | <0.001 |
| Carotid-radial | 0.29 (0.14, 0.43) | <0.001 |
| Carotid-distal | 0.36 (0.21, 0.52) | <0.001 |
Abbreviations: BMI, body mass index; CI, confidence interval; GEE, generalized estimating equation; PWV, pulse wave velocity.
All the tracking coefficients were calculated by GEE, and adjusted for baseline age, age difference, baseline BMI, gender, ethnicity and cohort.
Figure 1.
Scatter plots for carotid-femoral pulse wave velocity (cfPWV), carotid-radial PWV (crPWV) and carotid-distal PWV (cdPWV) at two different time points (n =142).
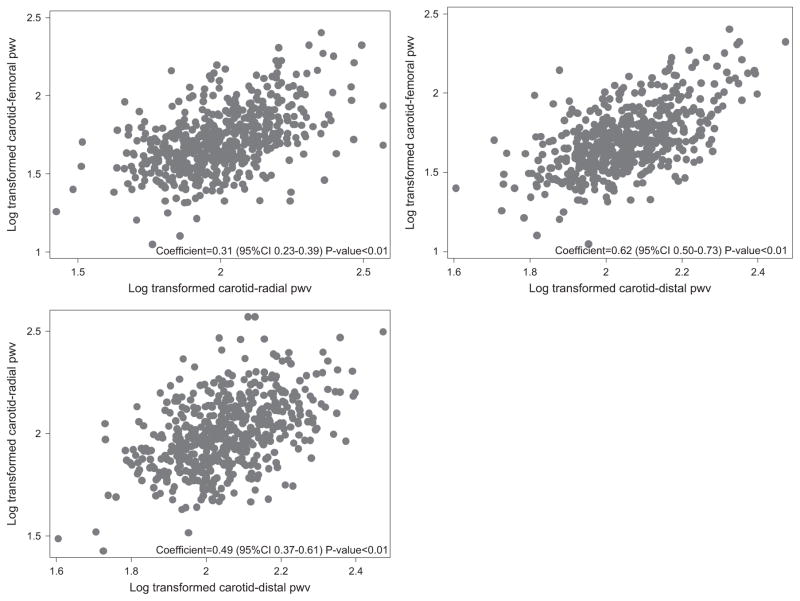
Figure 2 shows the correlations between different PWV measurements in the overall sample (n =531). All measurements were significantly correlated (carotid-femoral and carotid-radial: r =0.31, P<0.001, carotid-distal and carotid-radial: r =0.49, P<0.001, carotid-femoral and carotid-distal: r =0.62, P<0.001).
Figure 2.
Scatter plots for pulse wave velocity (PWV) between different measurement sites (n =531).
Table 3 shows the correlations of cfPWV, crPWV and cdPWV with anthropometric (BMI, waist circumference and skinfolds) and hemodynamic parameters (SBP, DBP, mean arterial blood pressure (MAP), PP, TPR index and cardiac index). Overall, all three PWV measurements showed significant correlations with age and obesity related measurements including BMI, waist circumference and skinfold, but cfPWV displayed significantly stronger correlations (ps<0.001 & false discovery rate<0.05) with obesity related measurements in comparison with crPWV and cdPWV. All three PWV measurements also showed significant correlations with SBP, DBP, MAP and PP with the exception that no correlation existed between crPWV and PP. cdPWV displayed significantly stronger correlations (ps<0.008 & false discovery rate<0.05) with SBP, DBP and MAP than cfPWV and crPWV. Both crPWV and cdPWV showed significant correlations with TPR index but not cardiac index, while cfPWV showed significant correlation with cardiac index but not TPR index. None of the difference between these correlation coefficients reached statistical significance.
Table 3.
Correlations between PWVs and anthropometric and hemodynamic values, n =531
| Carotid-femoral | Carotid-radial | Carotid-distal | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | |
| Agea (years) | 0.3358 | <0.001 | 0.2599 | <0.001 | 0.2612 | <0.001 |
| BMIb (kg/m2) | 0.2827c | <0.001 | 0.0985 | 0.023 | 0.1625 | <0.001 |
| Waist circumferenceb (cm) | 0.3079c | <0.001 | 0.0853 | 0.048 | 0.1582 | <0.001 |
| Sum of skinfoldb (mm) | 0.2681c | <0.001 | 0.0961 | 0.036 | 0.1355 | 0.002 |
| SBPd (mm Hg) | 0.2761 | <0.001 | 0.1973 | <0.001 | 0.3780e | <0.001 |
| DBPd (mm Hg) | 0.2957 | <0.001 | 0.3548 | <0.001 | 0.4587e | <0.001 |
| MAPd (mm Hg) | 0.2999 | <0.001 | 0.2836 | <0.001 | 0.4554e | <0.001 |
| PPd (mm Hg) | 0.1385 | 0.001 | 0.0468 | 0.256 | 0.1328 | 0.002 |
| TPR indexd | 0.0930 | 0.054 | 0.1976 | <0.001 | 0.1841 | <0.001 |
| Cardiac indexd | 0.0834 | 0.03 | −0.0419 | 0.391 | 0.0834 | 0.051 |
Abbreviations: BMI, body mass index; crPWV, carotid-radial pulse wave velocity; DPB, diastolic blood pressure; MAP, mean arterial blood pressure; PP, pulse pressure; SBP, systolic blood pressure; TPR index, total peripheral resistance index.
P-value calculated by generalize estimate equation.
adjusted for ethnicity, gender, BMI and cohort.
adjusted for age, ethnicity, gender and cohort.
Significantly higher than the correlation coefficients observed in crPWV and cdPWV.
Adjusted for age, ethnicity, gender, BMI and cohort.
Significantly higher than the correlation coefficients observed in carotid-femoral PWV and crPWV.
DISCUSSION
There are two major findings in the present study. First, PWV, a measurement for arterial stiffness, demonstrates significantly different tracking stabilities in different arterial systems. Elastic arteries, as measured by carotid-femoral PWV, exhibited moderate to high tracking stability, whereas in muscular arteries, as measured by crPWV, tracking stability tended to be poorer. cdPWV, which measures both elastic and muscular arteries, exhibited the second highest tracking ability. Second, carotid-femoral PWV showed a higher correlation with obesity related measurements including BMI, waist circumference and sum of skinfolds while cdPWV showed a higher correlation with BP related measurements including SBP, DBP and MAP.
To the best of our knowledge, no previous studies exist that explore the tracking stability of PWV, although short-term (ranging from 2 min to 2.5 weeks) reproducibility of PWV measured at different arterial sites have been examined. Asmar et al.25 found good reproducibility of carotid-femoral PWV in 56 subjects, showing its application for longitudinal clinical studies. Liang et al.26 found the carotid-femoral PWV to be highly reproducible with a coefficient of variation of 3.2% in 50 healthy adults. On the other hand, Wilkinson et al. found carotid-femoral PWV to be less reproducible than crPWV. However, their group also found low within-observer and between-observer variability for both carotid-femoral and crPWVs in this heterogeneous population of healthy, hypertensive and hypercholestrolaemics, and believed both PWV measures to be simple and reproducible techniques for population studies investigating the clinical relevance of arterial stiffness.2 The reproducibility of cdPWV has not been reported yet although two studies explored the reproducibility of femoral-distal PWV and observed that it showed less reproducibility in comparison with carotid-femoral PWV.12,26 In this study, we found that although still significant, crPWV had much lower tracking coefficients in comparison with carotid-femoral PWV. This might be one of the underlying reasons for the previous findings that peripheral arteries such as brachial and radial arteries, as measured by crPWV, is not associated with CV events and mortality.14,15,27 On the other hand, longitudinal studies have illustrated carotid-femoral PWV as a predictor of CV morbidity and mortality. Our findings that carotid-femoral PWV tracked better than crPWV and cdPWV, further highlights the importance of using carotid-femoral PWV over carotid-radial and cdPWV in clinical practice and in predicting future CV events.
The associations of PWV with CV risk factors have been extensively studied; however, majority of the studies used PWV measurement at one site. In the present study with PWV measured at three sites in over 500 subjects from a general population, we found that carotid-femoral PWV was more strongly correlated with obesity related measures such as BMI, waist circumference and sum of skinfolds in comparison with crPWV and cdPWV. The association between PWV and adiposity is highly controversial. A systematic review conducted in 200928 suggests that obesity shows small association with carotid-femoral PWV and accounts for little of the variability in PWV after adjustment of BP. On the other hand, several recent studies29–32 support the association between obesity and PWV and a recently meta-analysis in 201533 shows that modest weight loss can significantly improve PWV, suggesting a casual role of obesity on PWV. Important considerations when interpreting these discrepancy findings include the characteristics of the study populations and the coexistence of other CV risk factors as well as the controlling of them in the data analysis. Obesity is strongly related to many CV risk factors including type 2 diabetes and high BP. Over-adjustment might happen when controlling these factors in the analysis. Our study population is youth and young adult with high prevalence of obesity (46% subjects are overweight or obese) but yet limited prevalence of hypertension (4.7% subjects have either SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg), increasing the power to identify the effect of obesity on PWV. Abnormal biophysical properties have been observed in the aorta of obese children34 and might be a potential explanation for the higher association of obesity with carotid-femoral PWV. In this study, we also observed that cdPWV showed a higher correlation with BP related measurements including SBP, DBP and MAP than carotid-femoral PWV and crPWV. BP is determined both by cardiac output and TPR. Physiologically, cardiac output is related to the arterial tone in the elastic arteries, whereas TPR is related to the arterial tone in muscular conduit arteries. As measurement for cdPWV encompasses almost the entire arterial tree including elastic and muscular artery, its correlation with both cardiac output and TPR as well as higher correlation with BP measurements are expected.
The strength of the present study is that this is the first study evaluating the tracking stability of PWV at different arterial sites. However, findings in this study need to be carefully interpreted within the context of its limitations. First, the population selected for evaluation of tracking stability was relatively small, which provide limited statistical power to test the potential ethnic differences in the tracking stability of the PWV measurements. We tested whether there was ethnic difference in the correlations between PWV measurements and anthropometric or hemodynamic variables and did not observe significantly ethnicity-dependent effects. Second, the population was restricted to only young adults with a small age variation with relatively low prevalence of hypertension, diabetes and dyslipidemia, and thus a more diverse population in terms of age may be needed to be applicable to the general population.
In summary, we observed that carotid-femoral PWV had a high tracking stability whereas carotid-radial and cdPWV demonstrated low to moderate tracking stability. In addition, our results were also consistent with previous publications of the associations between CV risk factors (that is, obesity) and carotid-femoral PWV. In combination with established evidence of the predictive value of carotid-femoral PWV in CV events, it supports the use of carotid-femoral PWV in future CV risk assessment and daily clinical practice.
Acknowledgments
The BP stress cohort study was supported by grant HL69999 from the National Heart Lung and Blood Institute. The Georgia Cardiovascular Twin Study was supported by grant HL56622 from the National Heart Lung and Blood Institute.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 3.Davies JI, Struthers AD. Pulse wave analysis and pulse wave velocity: a critical review of their strengths and weaknesses. J Hypertens. 2003;21:463–472. doi: 10.1097/00004872-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 5.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 6.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 7.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 8.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 9.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 10.Peng F, Pan H, Wang B, Lin J, Niu W. The impact of angiotensin receptor blockers on arterial stiffness: a meta-analysis. Hypertens Res. 2015;38:613–620. doi: 10.1038/hr.2015.51. [DOI] [PubMed] [Google Scholar]
- 11.Seidlerova J, Filipovsky J, Mayer O, Wohlfahrt P, Cifkova R. Positive effects of antihypertensive treatment on aortic stiffness in the general population. Hypertens Res. 2014;37:64–68. doi: 10.1038/hr.2013.113. [DOI] [PubMed] [Google Scholar]
- 12.Tillin T, Chambers J, Malik I, Coady E, Byrd S, Mayet J, Wright AR, Kooner J, Shore A, Thom S, Chaturvedi N, Hughes A. Measurement of pulse wave velocity: site matters. J Hypertens. 2007;25:383–389. doi: 10.1097/HJH.0b013e3280115bea. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Agnoletti D, Protogerou AD, Topouchian J, Wang JG, Xu Y, Blacher J, Safar ME. Characteristics of pulse wave velocity in elastic and muscular arteries: a mismatch beyond age. J Hypertens. 2013;31:554–559. doi: 10.1097/HJH.0b013e32835d4aec. discussion 9. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Sloten TT, Schram MT, van den Hurk K, Dekker JM, Nijpels G, Henry RM, Stehouwer CD. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: the Hoorn study. J Am Coll Cardiol. 2014;63:1739–1747. doi: 10.1016/j.jacc.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 16.Ware JH, Wu MC. Tracking: prediction of future values from serial measurements. Biometrics. 1981;37:427–437. [Google Scholar]
- 17.Li Z, Snieder H, Harshfield GA, Treiber FA, Wang X. A 15-year longitudinal study on ambulatory blood pressure tracking from childhood to early adulthood. Hypertens Res. 2009;32:404–410. doi: 10.1038/hr.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 19.Ge D, Dong Y, Wang X, Treiber FA, Snieder H. The Georgia Cardiovascular Twin Study: influence of genetic predisposition and chronic stress on risk for cardiovascular disease and type 2 diabetes. Twin Res Hum Genet. 2006;9:965–970. doi: 10.1375/183242706779462877. [DOI] [PubMed] [Google Scholar]
- 20.Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension. 2003;41:1196–1201. doi: 10.1161/01.HYP.0000072269.19820.0D. [DOI] [PubMed] [Google Scholar]
- 21.Ge D, Young TW, Wang X, Kapuku GK, Treiber FA, Snieder H. Heritability of arterial stiffness in black and white American youth and young adults. Am J Hypertens. 2007;20:1065–1072. doi: 10.1016/j.amjhyper.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dekkers JC, Podolsky RH, Treiber FA, Barbeau P, Gutin B, Snieder H. Development of general and central obesity from childhood into early adulthood in African American and European American males and females with a family history of cardiovascular disease. Am J Clin Nutr. 2004;79:661–668. doi: 10.1093/ajcn/79.4.661. [DOI] [PubMed] [Google Scholar]
- 23.Twisk JW, Kemper HC, Mellenbergh GJ. Mathematical and analytical aspects of tracking. Epidemiol Rev. 1994;16:165–183. doi: 10.1093/oxfordjournals.epirev.a036149. [DOI] [PubMed] [Google Scholar]
- 24.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–251. [Google Scholar]
- 25.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 26.Liang YL, Teede H, Kotsopoulos D, Shiel L, Cameron JD, Dart AM, Target R, Levy BI. Non-invasive measurements of arterial structure and function: repeatability, interrelationships and trial sample size. Clin Sci (Lond) 1998;95:669–679. doi: 10.1042/cs0950669. [DOI] [PubMed] [Google Scholar]
- 27.Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Stiffness of capacitive and conduit arteries: prognostic significance for end-stage renal disease patients. Hypertension. 2005;45:592–596. doi: 10.1161/01.HYP.0000159190.71253.c3. [DOI] [PubMed] [Google Scholar]
- 28.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. doi: 10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- 29.Shah AS, Wadwa RP, Dabelea D, Hamman RF, D’Agostino R, Jr, Marcovina S, Daniels SR, Dolan LM, Fino NF, Urbina EM. Arterial stiffness in adolescents and young adults with and without type 1 diabetes: the SEARCH CVD study. Pediatr Diabetes. 2015;16:367–374. doi: 10.1111/pedi.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strasser B, Arvandi M, Pasha EP, Haley AP, Stanforth P, Tanaka H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr Metab Cardiovasc Dis. 2015;25:495–502. doi: 10.1016/j.numecd.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Castro JM, Garcia-Espinosa V, Curcio S, Arana M, Chiesa P, Giachetto G, Zócalo Y, Bia D. Childhood obesity associates haemodynamic and vascular changes that result in increased central aortic pressure with augmented incident and reflected wave components, without changes in peripheral amplification. Int J Vasc Med. 2016;2016:3129304. doi: 10.1155/2016/3129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryder JR, Dengel DR, Jacobs DR, Jr, Sinaiko AR, Kelly AS, Steinberger J. Relations among adiposity and insulin resistance with flow-mediated dilation, carotid intima-media thickness, and arterial stiffness in children. J Pediatr. 2016;168:205–211. doi: 10.1016/j.jpeds.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen KS, Blanch N, Keogh JB, Clifton PM. Effect of weight loss on pulse wave velocity: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35:243–252. doi: 10.1161/ATVBAHA.114.304798. [DOI] [PubMed] [Google Scholar]
- 34.Harris KC, Al Saloos HA, De Souza AM, Sanatani S, Hinchliffe M, Potts JE, Sandor GG. Biophysical properties of the aorta and left ventricle and exercise capacity in obese children. Am J Cardiol. 2012;110:897–901. doi: 10.1016/j.amjcard.2012.05.019. [DOI] [PubMed] [Google Scholar]