Abstract
Aim:
Theileriosis is a protozoal disease caused by Theileria spp. mostly in warm-blooded vertebrates worldwide. It is one of the common tick-borne diseases among domestic animals in tropical and sub-tropical regions, which have a variety of unlikable effects on health economy and animal welfare. In the present study, the prevalence of theileriosis among domestic farm animals in Iran was systematically evaluated.
Methods:
To identify the related papers, 10 English and Persian databases, including PubMed, Science Direct, Scopus, Web of Science, Medical Subject Headings, Google Scholar, Magiran, Barakatns (formerly Iranmedex), Elm net, and Scientific Information Database, were appraised for articles published throughout 1999-2017.
Results:
A total of 56 papers, providing the examination of 11,317 cattle, 9394 sheep, 2991 buffaloes, 1504 horses, 600 goats, and 212 donkeys were analyzed, matching for the prevalence of theileriosis from different parts of Iran were permitted for our allowing checklist. The overall prevalence of theileriosis among domestic herbivores was expected to be 19% (95% confidence interval: 15%, 22%). Our findings highlighted the average of the maximum prevalence in Razavi Khorasan (60.4%) and West Azerbaijan (49.1%) and the minimum in Mazandaran (1.1%) and East Azerbaijan provinces (2.2%), respectively. The high prevalence of Theileria infection in the herbivores (mainly sheep) verifies the well-known enzootic episode of theileriosis in Iran, predominantly in northeastern and western parts of the country.
Conclusion:
Our results suggested updated and imperative information on the true burden of theileriosis in Iran. Moreover, it could be supporting the gaps among monitoring, prevention, and control arrangements to improve the health economy, particularly among dairy farm animals.
Keywords: epidemiology, Iran, livestock, systematic review, Theileria spp
Introduction
Theileriosis is a tick-borne hemoprotozoal tropical disease in various warm-blooded vertebrates mainly domestic and wild mammals caused by protozoan parasites belonging to the Theileria spp. [1,2]. Theileria is a genus of protozoan that belongs to the phylum Apicomplexa (order: Piroplasmida and family: Theileriidae), which is transmitted by ixodid hard ticks acting as natural vectors. The parasite life cycle is well-known by extra-erythrocytic merogony in the lymphocytes and histiocytes, following by invasion of the red blood cells by the merozoites [3]. Although, Theileria parasite infect mainly a variety of both domestic and wild livestock, there is no proof that Theileria spp. are threats to human population [2,4,5].
Cattle and buffaloes can be infected by various species of Theileria and infections differ from subclinical (known as mild) to malignant (known as severe). Almost nine mild and malignant species of Theileria causing bovine theileriosis are including Theileria mutans, Theileria buffeli, Theileria orientalis, Theileria velifera, Theileria taurotragi and Theileria parva (as malignant bovine theileriosis; known as East Coast fever), Theileria annulata (known as tropical bovine theileriosis), Theileria sergenti, and T. taurotragi, respectively. Likewise, at least seven spices including Theileria lestoquardi (formerly Theileria hirci), Theileria luwenshuni, Theileria uilenbergi (causing malignant ovine theileriosis), and Theileria separata, Theileria ovis, Theileria recondita (causing mild ovine theileriosis), and T. annulata are naturally causal agents of ovine theileriosis with worldwide distribution [6]; although recently, T. annulata, as causal agent of malignant bovine theileriosis, has been reported in southern Iran [7,8].
Laboratory diagnosis of theileriosis was performed mostly by detecting schizonts in Giemsa-stained thick and thin smears from blood or lymph node fine needle aspiration. Several conventional and novel diagnostic tools vary from low to high sensitivity, such as polymerase chain reaction (PCR) test, enzyme-linked immunosorbent assays (ELISAs), or an indirect fluorescent antibody test (IFAT), were used for determining prevalence and differentiating the Theileria spp. [6,9-11]. In spite of the presence of a variety of studies concerning theileriosis among livestock in Iran, there is inapplicable data about the true burden of it in these animals to forecast the financial burden and well-known capacity for control and prevention planning.
Concerning the influences of theileriosis on economy and animal welfare, more deliberations would be crucial for the epidemiological aspects and the approaches to screening panels in Iran. To the best of our knowledge, there is no systematic review and meta-analysis on the subject of animal theileriosis in Iran; thus, the main goal of our study was to find out the present status of ovine, bovine, and equine theileriosis in the country.
Methods
Ethical approval
Ethical approval is not needed for this kind of study.
Searching approach
For searching purpose, 10 English and Persian databases, including PubMed, Science Direct, Scopus, Web of Science, Medical Subject Headings, Google Scholar, Magiran, Barakatkns (formerly Iranmedex), Elm net, and Scientific Information Database, were chosen during 1999-2017. To exploring the articles, the terms including: Theileria, Theileriosis, T. orientalis, T. annulata, T. hirci, T. lestoquardi, T. ovis, Theileria equi, cattle, buffalo, bovine, sheep, goat, ovine, caprine, horse, donkey, equine, and “Iran” alone or in combination were used. To avoid the risk of selection bias in this study, the inclusion criteria were clearly classified and studied. Experimental studies, clinical trials, duplicates, case reports, monkey, carnivores, camel, and studies out of Iran were expelled. All descriptive studies corresponding to the prevalence of ovine, bovine, and equine theileriosis were reviewed. The stages of the study plan are briefly explained in Figure-1.
Figure-1.
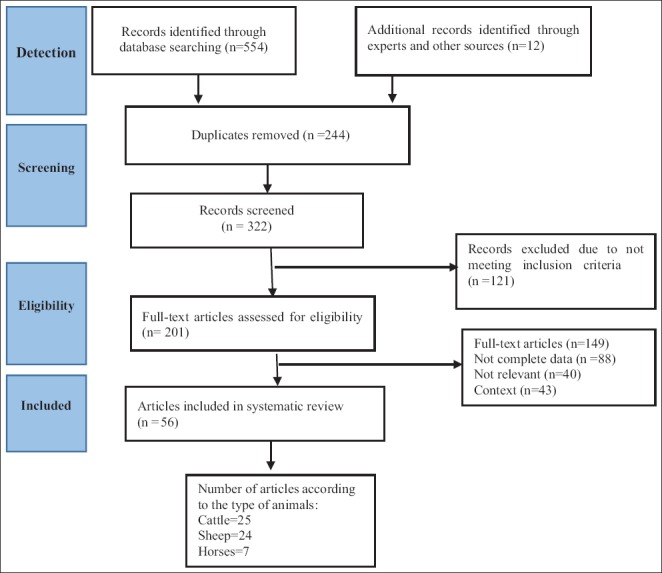
Flow diagram of classification of papers for inclusion in this systematic review and meta-analysis.
Data extraction
The data were extracted from the included studies by four reviewers (M. Soosaraei, M. Motavalli Haghi, F. Etemadifar, and Sh.Asfaram), who used a standard form. Any disagreement was resolved by discussion between the four reviewers. If consensus could not be reached, two reviewers were consulted (M. Fakhar and H. Ziaei Hezarjaribi). The kappa index showed an agreement of 89% between the fives reviewers. The standard form consisted of the following variables: First author; year of publication, year of study, type of animal, place of conducted survey (Province), sample size, number of positive, and diagnostic laboratory methods (Table-1) [7,12-65]. The quality of selected studies was assessed using the STROBE scale (score under 7.75 low quality, 7.76-15.5 moderate and 15.6-23.5 moderate to height, and upper 23.6 height quality).
Table-1.
Baseline features of included studies.
| Animal | No. of examined | No. of positive | Species | Laboratory method | Place (province) | References |
|---|---|---|---|---|---|---|
| Sheep | 1000 | 92 | T. lestoquardi | Microscopy | Fars | [12] |
| Buffalo | 2700 | 82 | T. annulata | Microscopy | Khouzestan | [13] |
| Cattle | 372 | 216 | T. annulata | Microscopy | Razavi Khorasan | [14] |
| Sheep | 300 | 29 | T. lestoquardi | Microscopy | Lorestan | [15] |
| Cattle | 390 | 15 | Theileria | Microscopy | Chaharmahal va Bakhtiari | [16] |
| Sheep | 300 | 39 | Theileria | Microscopy | Mazandaran | [17] |
| Cattle | 124 | 61 | Theileria | ELISA | West Azerbaijan | [18] |
| Sheep | 200 | 7 | Theileria spp. | Microscopy | Mazandaran | [19] |
| Sheep | 840 | 100 | Theileria spp. | Microscopy | South Khorasan | [20] |
| Cattle | 600 | 34 | T. annulata | Microscopy | Sistan and Baluchestan | [21] |
| Cattle | 252 | 26 | T. annulata | Microscopy | Sanandaj | [22] |
| Cattle | NR | NR | T. annulata | Semi-nested PCR | Tehran, Fars, Sistan, and Baluchestan | [23] |
| Sheep | 150 | 71 | Theileria spp., T. ovis, T. lestoquardi | PCR | Sistan and Baluchestan | [24] |
| Sheep | 100 | 60 | Theileria spp., T. lestoquardi | PCR | South Khorasan | [25] |
| Cattle | 160 | 110 | Theileria spp., T. annulata | Microscopy | Razavi Khorasan | [26] |
| Sheep | 100 | 56 | T. ovis, T. lestoquardi | PCR | East and South-East provinces | [27] |
| Sheep | 470 | 21 | T. lestoquardi, T. ovis | PCR | 10 various regions of Iran | [28] |
| Sheep | 220 | 181 | T. lestoquardi, T. ovis | PCR and microscopy | 5 various regions in eastern half of Iran | [24] |
| Cattle | 160 | 16 | T. annulata and T. orientalis | Semi-nested PCR | Golestan | [29] |
| Sheep | 200 | 11 | T. ovis | Microscopy | West Azerbaijan | [30] |
| Cattle | 100 | 22 | T. annulata | IFAT | West Azerbaijan | |
| Cattle | 160 | 20 | T. annulata | PCR | Golestan | [31] |
| Cattle | T. annulata | PCR-RFLP | Kurdistan and West-Azerbaijan | [32] | ||
| Cattle | 200 | 63 | T. annulata | Microscopy | Kerman | [33] |
| Sheep | 250 | 101 | T. lestoquardi and T. ovis | Nested PCR | Western half of Iran (Sari, Rasht, Urmia, Ilam, and Ahvaz) | [7] |
| Cattle | 52 | 19 | T. annulata | PCR-RFLP | Azerbaijan | [34] |
| Cattle | 52 | 30 | T. annulata | PCR-RFLP | Kurdistan and Kermanshah | |
| Cattle | 1202 | 706 | T. annulata | PCR | Isfahan, Khuzestan, Chaharmahal va Bakhtiari, Kohgiluyeh va Boyer Ahmad and Lorestan | [35] |
| Cattle | 160 | 34 | T. orientalis and T. annulata | Semi-nested PCR | Golestan | [36] |
| Sheep | 165 | 9 | T. annulata and T. ovis | PCR | Tehran | [37] |
| Sheep | 568 | 73 | Theileria spp. | Microscopy | Ilam | [38] |
| Sheep | 100 | 12 | Theileria spp. | Microscopy | Lorestan | [39] |
| Sheep | 300 | 6 | Theileria spp. | Microscopy | Tehran | [40] |
| Sheep | 100 | 46 | T. ovis and T. annulata | Semi-nested PCR | Fasrs | [41] |
| Sheep | 90 | 68 | T. ovis and T. lestoquardi | Semi-nested PCR | North Khorasan | [42] |
| Sheep | 452 | 295 | T. ovis, T. lestoquardi and T. annulata | Semi-nested PCR | Razavi Khorasan | [43] |
| Cattle | 176 | 42 | Theileria spp. | PCR | Isfahan | [44] |
| Cattle | 270 | 20 | T. annulata | PCR | Yazd, North Khorasan and Mazandaran | [45] |
| Horses | 165 | 47 | T. equi | PCR | Khuzestan | [46] |
| Horses | 240 | 41 | T. equi | PCR | West Azerbaijan | [47]{Malekifard, 2014 #2067}{Malekifard, 2014 #2067;Malekifard, 2014 #2067} |
| Horses | 100 | 53 | T. equi | IFAT | North Khorasan | [48] |
| Horses | 205 | 45 | T. equi | PCR | North Khorasan and Yazd | [49] |
| Sheep | 119 | 106 | Theileria spp. | PCR | Khuzestan | [50] |
| Cattle | 150 | 43 | Theileria spp. | PCR | Isfahan | [51] |
| Donkeys | 106 | 54 | T. equi | Multiplex-PCR | North Khorasan | [52] |
| Cattle | 138 | 13 | T. annulata | PCR | Kermanshah | [53] |
| Horses | 59 | 21 | T. equi | PCR | Khuzestan | [54] |
| Sheep | 150 | 19 | T. lestoquardi and T. ovis | PCR | Lorestan | [55] |
| Cattle | 150 | 84 | T. annulata | PCR | Kerman | [56] |
| Cattle | 100 | 4 | T. annulata | PCR | Yazd | [57] |
| Goats | 100 | 0 | Theileria | Microscopy | Razavi Khorasan | [58] |
| Sheep | 80 | 59 | Theileria | PCR | Sistan and Baluchestan | [59] |
| Cattle | 160 | 60 | T. annulata | PCR | Sistan and Baluchestan | [60] |
| Cattle | 138 | 37 | Theileria | Semi-nested PCR | West Azerbaijan | [61] |
| Goat | 400 | 39 | T. lestoquardi | PCR | West Azerbaijan | [62] |
| Cattle | 193 | 76 | T. annulata and T. orientalis | Molecular assays | West Azerbaijan | [63] |
| Buffalo | 291 | 10 | ||||
| Cattle | 51 | 2 | T. lestoquardi and T. annulata | PCR | Khuzestan | [64] |
| Horse | 90 | 10 | T. equi | PCR | Isfahan and Shahrekord | [65] |
NR: Not reported, T. lestoquardi=Theileria lestoquardi, T. annulata=Theileria annulata, T. ovis=Theileria ovis, T. orientalis=Theileria orientalis, T. equi=Theileria equi, PCR=Polymerase chain reaction, IFAT=Indirect fluorescent antibody test, ELISA=Enzyme-linked immunosorbent assays, RFLP=Restriction fragment length polymorphism
Effect measures
The outcome was the prevalence of Theileria, and this was obtained for each study by dividing the number of positive cases by the total sample size.
Statistical analysis
The prevalence of each study was collected, and according to the binomial distribution, standard error 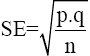 for each study was calculated and the inverse of SE each study considered as the weight of that study. The effect size (ES) for each study and pooled outcome revealed as a forest plot (reported as ES with a 95% confidence interval [95% CI]). Cochran’s heterogeneity statistics based on Chi-square test Q-test (p<0.1 as heterogeneities) and the I2 statistic which describes the percentage of variation across studies (values of 25%, 50%, and 75% indicate low, moderate, and high degrees of heterogeneity, respectively). At present heterogeneity, random effects model (Der Simonian Laird model) and otherwise applied fixed-effect model (Mantel Haenszel) were used to compute overall ES. Subgroup analyses were performed to investigate potential sources of heterogeneity from different sex and age. Egger’s tests were used to evaluate publication bias. All statistical analyses were fulfilled with the statistical software package (Stata) version 11.1. (Stata Corp LP, College Station, TX, USA). p<0.05 was measured statistically significant.
for each study was calculated and the inverse of SE each study considered as the weight of that study. The effect size (ES) for each study and pooled outcome revealed as a forest plot (reported as ES with a 95% confidence interval [95% CI]). Cochran’s heterogeneity statistics based on Chi-square test Q-test (p<0.1 as heterogeneities) and the I2 statistic which describes the percentage of variation across studies (values of 25%, 50%, and 75% indicate low, moderate, and high degrees of heterogeneity, respectively). At present heterogeneity, random effects model (Der Simonian Laird model) and otherwise applied fixed-effect model (Mantel Haenszel) were used to compute overall ES. Subgroup analyses were performed to investigate potential sources of heterogeneity from different sex and age. Egger’s tests were used to evaluate publication bias. All statistical analyses were fulfilled with the statistical software package (Stata) version 11.1. (Stata Corp LP, College Station, TX, USA). p<0.05 was measured statistically significant.
Results
Among each one of the databases investigated during 19 years from 1999 to 2017, overall 56 articles were fitting to be integrated into our systematic review and meta-analysis. All papers were assigned and assessed the prevalence of theileriosis among herbivores including sheep, goat, cattle, buffalo, horse, and donkey in Iran. Absolutely, 11,317 cattle, 9394 sheep, 2991 buffaloes, 1504 horses, 600 goats, and 212 donkeys were analyzed, respectively. In general, because of the restricted data fulfilled on camel theileriosis in Iran, we included just articles correlated to the disease among domesticated herbivores except for camel.
The mean of scores for the STROBE scale was to be found 21.73 which performed quality of these studies was moderate to height. As indicated by a random effect meta-analysis (I2=98.94%, p<0.001) the pooled event of Theileria infection in Iran was acquired 19% (95% CI: 15%, 22%). The prevalence rate of ovine (sheep and goats), bovine (cattle and buffaloes), and equine (horses and donkeys); theileriosis was 23.0% (17.0-30.0%), 14.0% (11.0-19.0%), and 20.0% (11.0-30.0%), respectively; a significant statistically difference was observed among them (z=5.80, df=2, p=0.05) (Figures-2 and 3).
Figure-2.
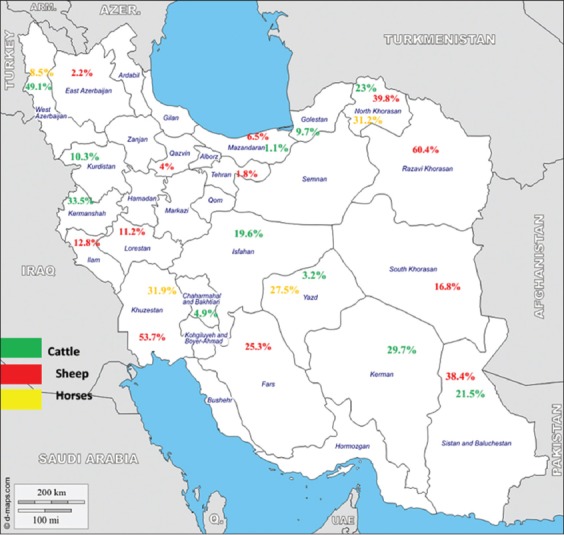
Detailed presentation of overall distribution of Theileria spp. infection in the provinces of Iran.
Figure-3.
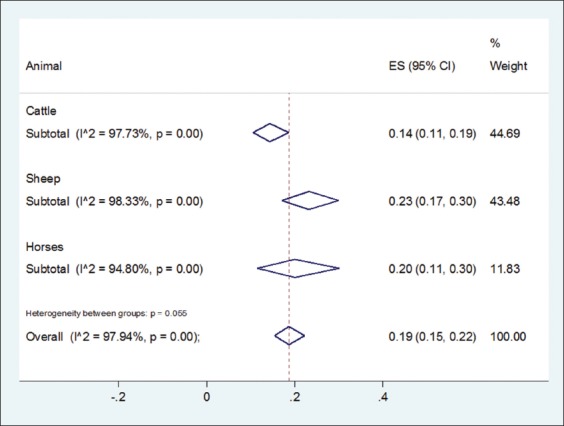
Estimate of the prevalence of Theileria infection among herbivores based on 56 studies in different years and areas in Iran. The pooled random effect size and 95% confidence interval represents by diamond, 19% (15-22%). Overall heterogeneity based on random effect model was showed by I2 (97.94%, p<0.001). The vertical dash line represents overall estimate, and the vertical solid line represents the value of null hypothesis.
Besides, our results showed the prevalence of Theileria infection among sheep (23%) which was considerably too much than other herbivores (p<0.001); and prevalence of T. ovis and T. annulata in sheep and cattle was significantly higher than other ones, respectively (p<0.001).
In addition, the highest prevalence of T. equi infection in horses 19.0% (11.0-29.0%) was detected by IFAT (51%) and the lowest by microscopic methods (7%). There was significant difference between prevalence of laboratory methods which used for diagnosing equine theileriosis for horses (Q-test=137.25, df=2, p<0.001).
The subgroup analysis showed PCR method had the most prevalence for sheep, 33.0% (95% CI: 20.0-48.0%) and a significant difference between prevalence of Theileria infection and laboratory techniques used among sheep (Q-test=9.5, df=3, p=0.02). Moreover, our meta-analysis showed a significant difference among Theileria species in sheep (Q-test=41.2, df=3, p<0.001) and cattle (Q-test=180.43, df=4, p<0.001), correspondingly. In addition, the analysis confirmed a significant difference between prevalence of Theileria infection and laboratory methods used for cattle (Q-test=699.32, df=5, p<0.001).
The results of Egger’s test for each of animal subgroups provided no evidence of publication bias in the studies (p>0.1) (Table-2). Therefore, it appears that both studies with low and high prevalence were contributed in this meta-analysis. The consequences of subgroup examination independently for type of laboratory methods and Theileria spp. infection showed that these two elements are two major sources of heterogeneity among prevalence of theileriosis for studies related to cattle and sheep, except horses.
Table-2.
The results of Egger’s test to assess publication bias.
| Animals | Number of studies | bias | p |
|---|---|---|---|
| Cattle | 25 | 1.08 | 0.21 |
| Sheep | 24 | 1.37 | 0.56 |
| Horses | 7 | 2.30 | 0.11 |
| Overall | 56 | 1.67 | 0.13 |
Discussion
Our study is the first systematic review on theileriosis in Iran. Theileriosis is an economically important protozoal disease among domesticate livestock in Iran with overall 19% infection rate, and the infection rates in sheep are 23%, which cause terrible impacts on the economy. The disease is widespread and well known in several parts of Iran, which affects animals’ husbandry and their productions in the country. The majority cases of theileriosis occurred in June-July and the lowest in March-April in northwestern and southeastern Iran [4,66]. In addition, the high prevalence of ovine theileriosis in different areas of Iran is probably identified with a few issues, for example, vector seasonal frequency, weather and environmental alterations, host susceptibility, ticks resistance to insecticide, high frequency of tick-infested in sheep versus cattle, and insufficient preventive policies. [4,67-70].
Several hard tick genera including Amblyomma, Haemaphysalis, Hyalomma, and Rhipicephalus as main vectors of theileriosis are scattered in all ruminant grazing lands, and accordingly, the chance of transmission of it is increased [48].
In our study, the pooled prevalence of livestock theileriosis is approximated to be 19% in Iran. Moreover, the prevalence rate of theileriosis in various geographical regions demonstrated that there were assorted zones in Iran with high prevalence rates in cattle with 58% in Razavi Khorasan, northeastern, and 57.6% in Kermanshah, western Iran, in sheep with 71% in Sistan and Baluchestan, Eastern, 70%, 55.6% in North Khorasan and Razavi Khorasan, respectively; in horses with 53% in North Khorasan provinces. Furthermore, the average prevalence of infection rate was notably lower in the central and northern provinces of the country.
Our findings are in symmetry with a study carried out on sheep and goats in Pakistan, eastern neighborhood of Iran, in 2012, by microscopic examination, 11.2% samples were positive for Theileria spp. The prevalence of Theileria spp. was 13.9 % and 8.2% in sheep and goat, respectively [71].
In Turkey, as western neighborhood of Iran, the prevalence of T. buffeli/T. orientalis was reported between 0.9% and 13.6% using PCR, and T. annulata was reported between 0% and 60.5% using microscopic method. The seroprevalence of T. annulata was found between 1.8% and 91.4% by IFAT. The prevalence of T. annulata by molecular techniques was between 15.4% and 61.2%. The prevalence of T. recondita/T. ovis by microscopic examination of thin blood smears was varied from 0% to 41.3% and its seroprevalence was found to be between 8.2% and 63.2% by IFAT. Weather conditions, the tick seasonal activities, and ecological conditions of Turkey and Kurdistan region of Iraq are extremely close to western and northwestern Iranian provinces, e.g., West and East Azerbaijan [32,72-75].
On the whole, our study showed that the average prevalence rate of ovine, bovine, and equine theileriosis was 23, 14, and 20%, respectively. In this study, five species of Theileria were identified which abundantly found in different livestock, would be considered as causal agents of theileriosis in the country. Our data showed that ovine theileriosis caused by T. lestoquardi infection is common in southern, southwestern, and southeastern parts of Iran and T. ovis is prevalent all over the country; even though the latest species is the main species in northern, western, and northwestern areas of Iran [7,24,43].
Regarding bovine theileriosis in Iran, our data are similar to the prior epidemiological surveys of T. orientalis and T. annulata in neighboring countries. For example, in four districts of Punjab, Pakistan, the pooled prevalence of T. orientalis was approximately 24.5, 6, and 6.1% by multiplexed tandem PCR method in the imported and native Pakistani cattle and buffaloes, respectively [76].
Equine theileriosis is caused due to T. equi and Babesia caballi and it is widespread in the most of tropical parts of countries in Europe, Africa, Asia, and countries surrounding Iran such as Turkey [77,78], Oman [79], Saudi Arabia [80], Kuwait [81], United Arab Emirates [82], and Iraq [83] as well as from other countries, found varying degrees of parasite prevalence. Overall, T. equi is most common and malignant than T. caballi in many endemic parts of the world including Iran [49].
In a study from Iran, with PCR assay showed infection with T. equi in 50.9% of the donkeys [52]. The frequency of T. equi infections was recorded from 31.8% of donkeys in Brazil using PCR method [84], 0.5-12% of blood smears of donkeys in Ethiopia [85-89]. Seropositivity rates of T. equi infection were in73.8% of donkeys in Brazil [84], 55.7% in Ethiopia [87], 9.6% in China [88], 47.2% in Spain [89], and 4-13% in Turkey [90,91].
Our findings and recent data show that T. equi and T. caballi were common in horse populations in provinces of Iran including East and West Azerbaijan, North Khorasan, and Khuzestan [23,47].
Herbivores theileriosis is answerable for high mortality and morbidity of livestock and subsequently fizzling economy, stunning effects on traditional and industrial animal breeding, in addition falling control approaches [4,92].
The diagnosis of theileriosis is based on traditional methods including microscopic examination (mostly thick and thin Giemsa-stained blood smears), and clinical symptoms. On the other hand, since the microscopic method has low sensitivity, this technique is not being reliable for the detection of asymptomatic and or subclinical infections because of the low parasitemia and or low virulence [6,93]. Moreover, serological assays such as ELISA and IFAT have also many disadvantages such as cross-reactivity between various species. Recently, molecular tools have picked up notoriety and prominence for detection and characterization proof of various pathogens. Altogether, serological and molecular tools may be appropriate in mild and also asymptomatic and or subclinical infections [6,93-95].
Given that there is a large number of studies about distribution status of ruminant theileriosis contrasting babesiosis [62] and a limited number of studies regarding equine theileriosis in Iran. Moreover, the most of studies are concentrated on ovine theileriosis, so an urgent need for updated data about the prevalence of theileriosis in Iranian equine theileriosis would be required. Nonetheless, a few unpublished data concerning ovine and bovine theileriosis are available in local provincial veterinary health and management centers gathered from private veterinary clinics and diagnostic laboratories in the various parts of Iran, which even though they are not exactly noteworthy and valid for appraisal.
Conclusion
The high occurrence of Theileria infection in domestic livestock in Iran, frequently among sheep, confirm the endemic and stable situations of theileriosis in the studied regions particularly northeastern and western provinces of the country and maybe a warning for animal welfare and health economy. In brief, our data offer valuable and encouraging information as regards the current situation of theileriosis in domestic herbivores in Iran, which might be useful for active and passive surveillance and preventing plans for the disease. Further investigation and monitoring will be needed to expand the surveillance and control policies, such as quite vaccination full coverage and improvement the traditional diagnostic tools and assessment the pesticide resistance in ticks to reduce the mortality and morbidity of theileriosis among livestock and consequently decrease the risk of outbreaks and economic failure and public health hazardous in Iran.
Authors’ Contributions
MS, MMH, FE, and SA made searching strategy paper selection and wrote the manuscript draft, MF and HZH designed all steps of the study, SHT analyzed the extracted data. All authors read, revised, and approved the final manuscript.
Acknowledgments
The Vice Chancellor of Research and Technology, Mazandaran University of Medical Sciences, Iran provided funding for this systematic review and meta-analysis (grant number: 2980).
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Demessie Y, Derso S. Tick borne hemoparasitic diseases of ruminants:A review. Adv. Biol. Res. 2015;9:210–224. [Google Scholar]
- 2.Gul N, Ayaz S, Gul I, Adnan M, Shams S, ul Akbar N. Tropical theileriosis and east coast fever in cattle:Present, past and future perspective. Int. J. Curr. Microbiol. App. Sci. 2015;4:1000–1018. [Google Scholar]
- 3.Uilenberg G. International collaborative research:Significance of tick-borne hemoparasitic diseases to world animal health. Vet. Parasitol. 1995;57:19–41. doi: 10.1016/0304-4017(94)03107-8. [DOI] [PubMed] [Google Scholar]
- 4.Kalani H, Fakhar M, Pagheh A. An overview on present situation babesiosis and theileriosis and their distribution of ticks in Iran. Iran. J. Med. Microbiol. 2012;5:59–71. [Google Scholar]
- 5.Gubbels M.J, Katzer F, Hide G, Jongejan F, Shiels B.R. Generation of a mosaic pattern of diversity in the major merozoite-piroplasm surface antigen ofTheileria annulata. Mol. Biochem. Parasitol. 2000;110:23–32. doi: 10.1016/s0166-6851(00)00253-x. [DOI] [PubMed] [Google Scholar]
- 6.Mans B.J, Pienaar R, Latif A.A. A review ofTheileriadiagnostics and epidemiology. Int. J. Parasitol. 2015;4:104–118. doi: 10.1016/j.ijppaw.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaeemi M, Haddadzadeh H, Khazraiinia P, Kazemi B, Bandehpour M. Identification of differentTheileriaspecies (Theileria lestoquardi Theileria ovisandTheileria annulata) in naturally infected sheep using nested PCR–RFLP. Parasitol. Res. 2011;108:837–843. doi: 10.1007/s00436-010-2119-0. [DOI] [PubMed] [Google Scholar]
- 8.Bishop R, Musoke A, Morzaria S, Gardner M, Nene V. Theileria:Intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitol. 2004;129:271–283. doi: 10.1017/s0031182003004748. [DOI] [PubMed] [Google Scholar]
- 9.Kirvar E, Ilhan T, Katzer F, Wilkie G, Hooshmand-Rad P, Brown D. Detection ofTheileria lestoquardi(hirci) in ticks, sheep, and goats using the polymerase chain reaction. Ann. N. Y. Acad. Sci. 1998;849:52–62. doi: 10.1111/j.1749-6632.1998.tb11033.x. [DOI] [PubMed] [Google Scholar]
- 10.Shayan P, Rahbari S. Simultaneous differentiation betweenTheileriasppBabesiaspp. on stained blood smear using PCR. Parasitol. Res. 2005;97:281–286. doi: 10.1007/s00436-005-1434-3. [DOI] [PubMed] [Google Scholar]
- 11.Altay K, Dumanli N, Holman P.J, Aktas M. Detection ofTheileria ovisin naturally infected sheep by nested PCR. Vet. Parasitol. 2005;127:99–104. doi: 10.1016/j.vetpar.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Khaki Z, Rahbari S, Norouzian I. A study of theileriosis malignant hematological and biochemical findings in sheep. J. Vet. Res. 1999;54:49–52. [Google Scholar]
- 13.Navidpour S, Hashemi Fesharaki R, Goudarzi M. The Study Of Blood Protozoa in Buffaloes In Khouzestan. 2000. [Accessed on 28-10-2017]. Available from: http://www.agris.fao.org/agris-search/search.do?recordID=IR2012013493 .
- 14.Sakha M, Radfar M, Janbaz M. evaluation of bovine theileriosis in 372 cases during spring and summer 1377, Gonabad-Iran. Iranian. J. Vet. Res. 2001;2:187–192. [Google Scholar]
- 15.Maleki S. Case study ofTheileriacontamination in liver of diseased sheep perished and slaughtered in the slaughterhouse of Khorramabad. J. Vet. Res. 2002;57:99–101. [Google Scholar]
- 16.Meshgi B, Shahgolian L, Momtaz H, Samiapour V. A prevalence of theileriosis in cattle in Shahr-e-Kord township in Iran. Pajouhesh. Sazandegi. 2003;2:41–43. [Google Scholar]
- 17.Haji K.M.R, Lotf Elahzadeh S, Marzban K. Investigation of prevalance ofTheileriasp. infection and interrelationship with clinical signs at Ghaemshahr abattoir. J. Vet. Res. 2003;58:101–103. [Google Scholar]
- 18.Morshedi A, Horr-yadollahi M.R, Tavassoli M, Dalir N.B. A seroprevalence survey ofTheileriainfection by ELISA, compare with blood-smear observation in cattle. J. Vet. Res. 2003;58:319–322. [Google Scholar]
- 19.Ranjbar-Bahadori S, Lotfolahzadeh S, Tavasoli A. Survey the contamination of sheep slaughtered in the municipal city of Sari blood protozoa. Anim. Sci. 2004;64:1–3. [Google Scholar]
- 20.Razmi G.R, Eshrati H, Rashtibaf M. Prevalence ofTheileriaspp. infection in sheep in South Khorasan province, Iran. Vet. Parasitol. 2006;140:239–243. doi: 10.1016/j.vetpar.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Mozafari A, Nourolahi F.S.R, Mohamadi V. Frequency ofTheileriain cattle in farms of Zahedan. Iranian. J. Vet. Res. 2007;3:67–70. [Google Scholar]
- 22.Akradi L. A survey of bovine theileriosis in carcasses of Sanandaj slaughterhouse. J. Vet. Med. 2007;2:44–48. [Google Scholar]
- 23.Habibi G, Esmaeil-Nia K, Bozorgi S, Najjar E, Hashemi-Fesharki R, Bordbar N. PCR-based detection ofTheileriainfection and molecular characterization of Tams1T. annulatavaccine strain. Arch. Razi. Instit. 2007;62:83–89. [Google Scholar]
- 24.Hadadzadeh H, Khazrainia P, Heidarpour M, Zaeemi M. Geographic distribution of differentTheileriaspecies in sheep in Iran. Fourth Asean Congress of Tropical Medicine and Parasitology. 2010;2010 [Google Scholar]
- 25.Khazrainia P, Haddadzadeh H, Kazemi B, Heidarpour M, Aktas M. Molecular identification of ovineTheileriaspecies in Ferdos (South Khorasan province) East of Iran, Paper presented at the VI International Conference on Ticks and Tick Borne Pathogens. 2008 [Google Scholar]
- 26.Razmi G.R, Barati F, Aslani M. Prevalence ofTheileria annulatain dairy cattle in Mashhad area, Iran. J. Vet. Parasitol. 2009;23:81–83. [Google Scholar]
- 27.Bami M.H, Haddadzadeh H, Kazemi B, Khazraiinia P, Bandehpour M, Aktas M. Molecular identification of ovineTheileriaspecies by a new PCR–RFLP method. Vet. Parasitol. 2009;161:171–177. doi: 10.1016/j.vetpar.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 28.Heidarpour B.M, Khazraiinia P, Haddadzadeh H, Kazemi B. Identification ofTheileriaspecies in sheep in the eastern half of Iran using nested PCR-RFLP and microscopic techniques. Iranian. J. Vet. Res. 2010;11:262–266. [Google Scholar]
- 29.Ghaemi P, Hoghooghi R.N, Shayan P, Eckert B. Study on the protozoal infection ofTheileriaspecies in traditional animal husbandry in two ecological regions of Golestan province, Iran. Vet. Res. 2011;1:51–59. [Google Scholar]
- 30.Hashemzadeh F.H, Shahbazi P, Fard M.R.F. The infestation rate of heamoparasite in slaughtered sheep and goats of Tabriz abattoir 2009. J. Food. Hyg. 2011;1:17–21. [Google Scholar]
- 31.Hoghooghi-Rad N, Ghaemi P, Shayan P, Eckert B, Sadr-Shirazi N. Detection of native carrier cattle infected withTheileria annulataby semi-nested PCR and smear method in Golestan Province of Iran. World. Appl. Sci. J. 2011;12:317–323. [Google Scholar]
- 32.Tavassoli M, Tabatabaei M, Nejad B, Tabatabaei M, Najafabadi A, Pourseyed S. Detection ofTheileria annulataby the PCR-RFLP in ticks (Acari Ixodidae) collected from cattle in West and North-West Iran. Acta. Parasitol. 2011;56:8–13. [Google Scholar]
- 33.Zadeh S.S, Fathi S, Dehaghi M.M, Asl E.N, Nezhad H.A. Survey ofTheileria annulataandAnaplasma marginalein cattle in Kerman area, Southeast of Iran. Astrocytosis as a Biomarker for Late Stage Human African Trypanosomiasis in the Vervet Monkey Model, Southeast of Iran. 2011:61. [Google Scholar]
- 34.Akbari JJ.J, Tavassouli M, Tabatabai M, Shafiei R. Molecular survey ofTheileria annulatain cattle by PCR-RFLP method in Iran. J. Bacteriol. Parasitol. 2012;3:2. [Google Scholar]
- 35.Dehkordi F.S, Parsaei P, Saberian S, Moshkelani S, Hajshafiei P, Hoseini S. Prevalence study ofTheileria annulataby comparison of four diagnositcs Bulgarian. J. Vet. Med. 2012;15:123–130. [Google Scholar]
- 36.Ghaemi P, Hoghooghi-Rad N, Shayan P, Eckert B. Detection ofTheileria orientalisin Iran by semi-nested PCR. Parasitol. Res. 2012;110:527–531. doi: 10.1007/s00436-011-2517-y. [DOI] [PubMed] [Google Scholar]
- 37.Shemshad M, Shemshad K, Sedaghat M.M, Rafinejad J. Prevalence of ovine and bovine theileriosis in domestic ruminants based on 18s rRNA gene and microscopic techniques in Qazvin Province, Iran. J. Pure. Appl. Microbiol. 2012;6:627–632. [Google Scholar]
- 38.Bahrami A, Hosseini E, Razmjo M. Theileriosis in grazing sheep and its interrelation with the reptiles ticks. Global. Vet. 2013;10:599–606. [Google Scholar]
- 39.Hoghooghi R.N, Hashemi S, Abdigoudarzi M. Detection ofTheileria ovisin vector ticks by polymerase chain reaction method (PCR) in lorestan province. Vet. Clin. Pathol. 2013;2:1828–1834. [Google Scholar]
- 40.Tahamtan M.H, Nabian S, Khodaveisi M, Ronaghi H, Sadeghian A.G. Study on prevalence of blood parasites of sheep and detection of their vectors using methyl green pyronin in Varamin, Iran. Eur. J. Exp. Biol. 2013;3:11–15. [Google Scholar]
- 41.Yaghfoori S, Razmi G, Heidarpour M. Molecular detection ofTheileriaspp. in sheep and vector ticks in Fasa and Kazeroun areas, Fars province, Iran. Arch. Razi. Instit. 2013;68:159–164. [Google Scholar]
- 42.Rashidi A, Razmi G. Molecular detection ofTheileriaspp. in sheep and vector ticks in the North Khorasan Province, Iran. Trop. Anim. Health Prod. 2012;45:299–303. doi: 10.1007/s11250-012-0218-x. [DOI] [PubMed] [Google Scholar]
- 43.Razmi G, Pourhosseini M, Yaghfouri S, Rashidi A, Seidabadi M. Molecular detection ofTheileriasppBabesiaspp. in sheep and ixodid ticks from the northeast of Iran. J. Parasitol. 2013;99:77–81. doi: 10.1645/GE-3202.1. [DOI] [PubMed] [Google Scholar]
- 44.Noaman V. A molecular study onTheileriaandBabesiain cattle from Isfahan province, Central Iran. J. Parasit. Dis. 2013;37:208–210. doi: 10.1007/s12639-012-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheshti B, Razmi G, NaghibI A. A comparative study on haemoprotozoa infection in apparently healthy cattle in different geographical areas of Iran using PCR method. J. Vet. Microbiol. 2013;9:139–145. [Google Scholar]
- 46.Bahrami S, Ghadrdan A, Pourmahdi B.M, Vafayi S.M. b epidemiology ofTheileriaequi in Persian Arab horses from Iran. Vet. Med. 2014;59:409–414. [Google Scholar]
- 47.Malekifard F, Tavassoli M, Yakhchali M, Darvishzadeh R. Detection ofTheileriaequi andBabesia caballiusing microscopic and molecular methods in horses in suburb of Urmia, Iran. Vet. Res. Forum. 2014;5:129–133. [PMC free article] [PubMed] [Google Scholar]
- 48.Abedi V, Razmi G, Seifi H, Naghibi A. Molecular and serological detection ofTheileria equiandBabesia caballiinfection in horses and ixodid ticks in Iran. Tick. Tick. Born. Dis. 2014;5:239–244. doi: 10.1016/j.ttbdis.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Bahrami S, Ghadrdan A, Mirabdollahi S, Fayed M. diagnosis of subclinical equine theileriosis in center of Iran using parasitological and molecular methods. Trop. Biomed. 2014a;31:110–117. [PubMed] [Google Scholar]
- 50.Jalali S.M, Khaki Z, Kazemi B, Rahbari S, Shayan P, Bandehpour M, Yasini S.P. Molecular detection and identification ofTheileriaspecies by PCR-RFLP method in sheep from Ahvaz, Southern Iran. Iran. J. Parasitol. 2014;9:99. [PMC free article] [PubMed] [Google Scholar]
- 51.Noaman V. Comparison of molecular and microscopic technique for detection ofTheileriaspp. in carrier cattle. J. Parasit. Dis. 2014;38:64–67. doi: 10.1007/s12639-012-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abedi V, Razmi G, Seifi H, Naghibi A. Molecular detection of equine piroplasms in donkeys (Equus asinus) in North Khorasan province, Iran. Iran. J. Vet. Res. 2015;16:202. [PMC free article] [PubMed] [Google Scholar]
- 53.Ghashgai O, Yakhchali M, Sohrabi S. PCR-RELP for detecting ofTheileria annulatainfection in cattle andHyalommaspecies in Kermanshah Province, Iran. Arch. Razi. Institut. 2015;70:7–12. [Google Scholar]
- 54.Jalali M, Raki A, Shahriari A, Ghadrdan M.A, Hamidi N.H, Jelodar M. study of hematology and clinical signs piroplasmosis in Arab thoroughbreds in Ahwaz. Iran. J. Vet. Res. 2015;11:65–75. [Google Scholar]
- 55.Hashemi S, Kh E. Molecular identification ofTheileria ovisandT. lestoquardiin vector ticks ofIxodidaefamily in Lorestan province. Iranian. Vet. J. 2015;3:97–104. [Google Scholar]
- 56.Nourollahi-Fard S.R, Khalili M, Ghalekhani N. Detection ofTheileria annulatain blood samples of native cattle by PCR and smear method in Southeast of Iran. J. Parasit. Dis. 2015;39:249–252. doi: 10.1007/s12639-013-0333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khodabandeh S, Razmi G. Molecular detection ofTheileriaspecies and its vectors in cattle in Yazd area by Semi-nested PCR method. J. Vet. Res. 2015;70:249–253. [Google Scholar]
- 58.Azghandi M, Razmi G. Identification ofBabesiaandTheileriaspecies in goats and ticks with smear observation and molecular examination in Mashhad, Khorasan Razavi province, Iran. J. Vet. Res. 2015;70:e1–.e5. [Google Scholar]
- 59.Sharifi N, Ganjali M, Nabavi R, Saadati D. A study on prevalence and identification of ovineTheileriaandBabesiainfection in Zabol using PCR method. J. Parasit. Dis. 2016;40:1535–1539. doi: 10.1007/s12639-015-0722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Majidiani H, Nabavi R, Ganjali M, Saadati D. Detection ofTheileria annulatacarriers in Holstein-Friesian (Bos taurustaurus) and Sistani (Bos taurusindicus) cattle breeds by polymerase chain reaction in Sistan region, Iran. J. Parasit. Dis. 2016;40:1184–1188. doi: 10.1007/s12639-015-0646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamchi J.A, Tavassoli M. Survey on infection rate, vectors and molecular identification ofTheileria annulatain cattle from North West, Iran. J. Parasit. Dis. 2016;40:1071–1076. doi: 10.1007/s12639-014-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohammadi S.M, Esmaeilnejad B, Jalilzadeh-Amin G. Molecular detection, infection rate and vectors ofTheileria lestoquardiin goats from West Azerbaijan province, Iran. Vet. Res. Forum. 2017;8:139–144. [PMC free article] [PubMed] [Google Scholar]
- 63.Narimani B, Rad N.H, Shayan P, Rahbari S. Molecular and microscopic detection ofTheileriaspp. among cattle and buffaloes in West Azarbaijan, Iran. Arch. Razi. Inst. 2017;72:189–195. doi: 10.22092/ari.2017.111605. [DOI] [PubMed] [Google Scholar]
- 64.Jalali S.M, Jolodar A, Rasooli A, Darabifard A. Detection ofTheileria lestoquardicross infection in cattle with clinical theileriosis in Iran. Acta Parasitol. 2016;61:756–761. doi: 10.1515/ap-2016-0104. [DOI] [PubMed] [Google Scholar]
- 65.Taktaz-hafshejani T, Khamesipour F. Molecular detection ofTheileriaequi andBabesia caballiinfections in horses by PCR method in Iran. Kafkas. Univ. Vet. Fak. Derg. 2017;23:161–164. [Google Scholar]
- 66.Haghi S.M.M, Fakhar M, Sharif M, Keighobadi M. An overview on different diagnostic methods for babesiosis. J. Mazandaran. Univ. Med. Sci. 2014;23:283–295. [Google Scholar]
- 67.Pipano E. Observations on the seasonal distribution of blood parasites in sheep in Israel. Israel. J. Vet. Med. 1991;46:37–38. [Google Scholar]
- 68.Yeruham I, Handani A, Galker F, Rosen S, Schlien J. A field study of haemoparasites in two flocks of sheep in Israel. Israel. J. Vet. Med. 1992;47:107–111. [Google Scholar]
- 69.Nabian S, Rahbari S. Occurrence of soft and hard ticks on ruminants in Zagros mountainous areas of Iran. J. Arthr. Dis. 2008;2:16–20. [Google Scholar]
- 70.Rahbari S, Nabian S, Shayan P. Primary report on distribution of tick fauna in Iran. Parasitol. Res. 2007;101:175–177. doi: 10.1007/s00436-007-0692-7. [DOI] [PubMed] [Google Scholar]
- 71.Naz S, Maqbool A, Ahmed S, Ashraf K, Ahmed N, Saeed K. Prevalence of theileriosis in small ruminants in Lahore-Pakistan. J. Vet. Anim. Sci. 2012;2:16–20. [Google Scholar]
- 72.Vatansever Z, İça A, Deniz A, Nalbantoğlu S, Karaer Z, Çakmak A. Ankara yöresinde sığırlarda kene kaynaklıprotozoon enfeksiyonlarının yayılışının reverse lineblotting (RLB) ve indirek floresan antikor testi (IFAT) ile saptanmas. Ulusal. Parazitol. Kong. 2003;194:1465–1469. [Google Scholar]
- 73.Ica A, Inci A, Yildirim A. Parasitological and molecular prevalence of bovineTheileriaandBabesiaspecies in the vicinity of Kayseri. Turk. J. Vet. Anim. Sci. 2007;31:33–38. [Google Scholar]
- 74.Çiçek H, Cicek H, Eser M, Tandogan M. Current status of ruminant theileriosis and its economical impact in Turkey. Türk. Parazitol. Derg. 2009;33:273–279. [PubMed] [Google Scholar]
- 75.Al-Saeed A.T.M, Omer L.T, Abdo J, Habibi G, Salih D.A, Seitzer U. Epidemiological studies on tropical theileriosis (Theileria annulatainfection of cattle) in Kurdistan Region, Iraq. Parasitol. Res. 2010;106:403. doi: 10.1007/s00436-009-1675-7. [DOI] [PubMed] [Google Scholar]
- 76.Gebrekidan H, Abbas T, Wajid M, Ali A, Gasser R.B, Jabbar A. Molecular characterisation ofTheileriaorientalis in imported and native bovines from Pakistan. Infect. Genet. Evol. 2017;47:19–25. doi: 10.1016/j.meegid.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Sevinc F, Maden M, Kumas C, Sevinc M, Ekici O.D. A comparative study on the prevalence ofTheileriaequi andBabesia caballiinfections in horse sub-populations in Turkey. Vet. Parasitol. 2008;156:173–177. doi: 10.1016/j.vetpar.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 78.Karatepe B, Karatepe M, Çakmak A, Karaer Z, Ergün G. Investigation of seroprevalence ofTheileriaequi andBabesia caballiin horses in Nigde province, Turkey. Trop. Anim. Health. Prod. 2009;41:109–113. doi: 10.1007/s11250-008-9164-z. [DOI] [PubMed] [Google Scholar]
- 79.Donelli J, Joyner L, Graham-Jones O, Ellis C. A comparison of the complement fixation and immunofluorescence antibody tests in a survey of the prevalence ofBabesia equiandBabesia caballiin horses in the Sultanate of Oman. Trop. Anim. Hlt. Prod. 1980;12:50–60. doi: 10.1007/BF02242631. [DOI] [PubMed] [Google Scholar]
- 80.Alanazi A.D, Alyousif M.S, Hassieb M.M. Seroprevalence study onTheileriaequi andBabesia caballiantibodies in horses from central province of Saudi Arabia. J. Parasitol. 2012;98:1015–1017. doi: 10.1645/GE-2997.1. [DOI] [PubMed] [Google Scholar]
- 81.Donnelly J, Joyner L.P, Frank C. Quantitative epidemiological studies on the prevalence of babesiosis in horses in Kuwait. Trop. Anim. Health. Prod. 1980;12:253–258. doi: 10.1007/BF02236625. [DOI] [PubMed] [Google Scholar]
- 82.Jaffer O, Abdishakur F, Hakimuddin F, Riya A, Wernery U, Schuster R.K. A comparative study of serological tests and PCR for the diagnosis of equine piroplasmosis. Parasitol. Res. 2010;106:709–713. doi: 10.1007/s00436-009-1669-5. [DOI] [PubMed] [Google Scholar]
- 83.Alsaad K.M. Evaluation of hemogram, acute phase response, acid-base balance and blood gas analysis in newborn foals infected with babesiosis. J. Anim. Plant. Sci. 2014;24:738–742. [Google Scholar]
- 84.Machado R.Z, Toledo C.Z.P, Teixeira M.C.A, André M.R, Freschi C.R, Sampaio P.H. Molecular and serological detection ofTheileria equiandBabesia caballiin donkeys (Equus asinus) in Brazil. Vet. Parasitol. 2012;186:461–465. doi: 10.1016/j.vetpar.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 85.Mekibib B, Manegerew M, Tadesse A, Abuna F, Megersa B, Regassa A, Mekuria S, Abebe R. Prevalence of haemoparasites and associated risk factors in working donkeys in Adigudem and Kwiha districts of Tigray region, Northern Ethiopia. J. Anim. Vet. Adv. 2010;9:2249–2255. [Google Scholar]
- 86.Tefera M, Worku A, Tolosa T, Bitew M. Prevalence and risk factors for donkey babesiosis in and around Debre Zeit, Central Ethiopia. Vet. Res. 2011;4:56–60. [Google Scholar]
- 87.Gizachew A, Schuster R.K, Joseph S, Wernery R, Georgy N.A, Elizabeth S.K. Piroplasmosis in Donkeys-A Hematological and Serological Study in Central Ethiopia. J. Equine Vet. Sci. 2013;33:18–21. [Google Scholar]
- 88.Chahan B, Zhang S, Seo J.Y, Nakamura C, Zhang G, Bannai H, Jian Z, Inokuma H, Tuchiya K, Sato Y, Kabeya H, Maruyama S, Mikami T, Xuan X. Seroepidemiological evidence for the possible presence ofBabesia(Theileria) equi andBabesia caballiinfections in donkeys in western Xinjiang, China. J. Vet. Med. Sci. 2006;68:753–755. doi: 10.1292/jvms.68.753. [DOI] [PubMed] [Google Scholar]
- 89.García-Bocanegra I, Arenas-Montes A, Hernández E, Adaszek Ł, Carbonero A, Almería S, Jaen-Tellez J.A, Gutierrez-Palomino P, Arenas A. Seroprevalence and risk factors associated withBabesia caballiandTheileriaequi infection in equids. Vet. J. 2013;195:172–178. doi: 10.1016/j.tvjl.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 90.Acici M, Umur S, Guvenc T, Arslan H.H, Kurt M. Seroprevalence of equine babesiosis in the black sea region of Turkey. Parasitol. Int. 2008;57:198–200. doi: 10.1016/j.parint.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 91.Balkaya I, Utuk A.E, Piskin F.C. Prevalance ofTheileriaequi andBabesia caballiin donkeys from Eastern Turkey in winter season. Pak. Vet. J. 2010;30:245–246. [Google Scholar]
- 92.Ahmed J, Yin H, Schnittger L, Jongejan F. Ticks and tick-borne diseases in Asia with special emphasis on China. Parasitol. Res. 2002;88:51–55. doi: 10.1007/s00436-001-0574-3. [DOI] [PubMed] [Google Scholar]
- 93.Nayel M, El-Dakhly K.M, Aboulaila M, Elsify A, Hassan H, Ibrahim E, Salama A, Yanai T. The use of different diagnostic tools forBabesiaandTheileriaparasites in cattle in Menofia, Egypt. Parasitol. Res. 2012;111:1019–1024. doi: 10.1007/s00436-012-2926-6. [DOI] [PubMed] [Google Scholar]
- 94.d' Oliveira C, Van der Weide M, Jacquiet P, Jongejan F. Detection ofTheileria annulataby the PCR in ticks (Acari Ixodidae) collected from cattle in Mauritania. Exp. Appl. Acarol. 1997;21:279–291. doi: 10.1023/a:1018455223462. [DOI] [PubMed] [Google Scholar]
- 95.Sparagano O. Molecular diagnosis of Theileria and Babesia species. J. Vet. Parasitol. 1999;13:83–92. [Google Scholar]


