Abstract
Aim:
The aim of this study was to evaluate the antimycobacterial activity of the Curcuma xanthorrhiza ethanolic extract in vitro.
Materials and Methods:
Ethanolic extract of C. xanthorrhiza was set by maceration method. The broth microdilution and disc diffusion method were used to determine the minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC), respectively, of C. xanthorrhiza ethanol extract on strain Mycobacterium tuberculosis H37Rv.
Results:
C. xanthorrhiza ethanol extract was found to have the antimycobacterial effects with a MIC value of 1600 μg/ml while MBC value of 3200 μg/ml for M. tuberculosis H37Rv.
Conclusion:
From these findings , it can be concluded that C. xanthorrhiza ethanol extract have an antibacterial activity against Mycobacterium tuberculosis H37Rv in vitro and its potency elevated by increasing the C. xanthorrhiza ethanol extract concentration.
Keywords: antimycobacterial, Curcuma xanthorrhiza, minimal bactericidal concentration, minimal inhibitory concentration
Introduction
Tuberculosis (TB) is a serious public health problem, one of the leading causes of mortality worldwide, infecting about 9 million people, kills approximately 2 million people annually. The global incidence rate for TB is growing each year by approximately 1.1% and the number of cases by about 2.4%. Resistance to anti-TB drugs continued to be recognized as a clinical problem through the latter part of the 21st century. As a result, multidrug-resistant and extensively drug-resistant TB are now becoming a major threat to health worldwide, accounting for almost 3% of all newly reported cases of TB [1]. Due to increased drug-resistant strains of bacteria such as Mycobacterium tuberculosis and methicillin-resistant Staphylococcus aureus, there has been renewed interest in herbal as potential sources of novel antibiotics. The World Health Organization estimated that annual global use of herbal medicines is about US $83 billion in 2008, indicating that natural products are important sources of new therapeutics and future medicines [1].
The use of herbal as medicine is well known in rural areas of many developing countries. Most herbal medicines are well tolerated by the patient, with fewer unintended consequences than synthetic medicine. Herbs typically have fewer side effects than synthetic medicine, and may be safer to use over time [2,3]. The findings of the new antibacterial compounds in herbal became one of the remarkable alternatives for treatments since they are rich in numerous varieties of secondary metabolites such as alkaloids, flavonoids, tannins, saponin, and phenolic compounds with antibacterial properties [4]. Medicinal plant products have long been used as antibacterial in traditional medicines, for the treatment of many diseases such as TB. The anti-M. tuberculosis of medicinal plant products has become subject to scientific investigations currently worldwide, and their active components provide a potential alternative to conventional anti-M. tuberculosis. In this context, the development of medicinal plant product-based drug candidates as anti-M. tuberculosis has gained momentum in research studies directed toward design and discovery of drugs [5]. Piliostigma thonningii [5], Curtisia dentata [6], Combretum zeyheri [7], Artemisia nilagirica, and Murraya koenigii [8] are among the medicinal plants claimed to possess potential antimycobacterial agent.
It has been demonstrated that the medicinal properties of Curcuma xanthorrhiza were due to the phytochemicals possessed including alkaloids, phenols, flavonoids, triterpenes, sterols, glycosides, and terpenoids. C. xanthorrhiza contain xanthorrhizol and curcumin may be used for antioxidant and anticancer [9], antibacterial [10-12], antiviral and antifungal [13], endothelial cell protection on hypercholesterolemia [14], pancreas protective on methylmercury toxicity [15], and testicular protective on lead acetate toxicity [3].
Recent research activities have shown that C. xanthorrhiza belongs to the family Zingiberaceae and the rhizome extract contains active phytochemical constituents with xanthorrhizol and curcumin as the main compounds [11,16,17]. Xanthorrhizol and curcumin isolated from the ethanolic rhizome extract of C. xanthorrhiza show potent antibacterial activity against a wide spectrum of Gram-positive and negative bacterial pathogen [9,14]. It also has been reported that xanthorrhizol showed the highest antibacterial activity against Escherichia coli, Propionibacterium acnes, Streptococcus mutans, Actinomyces viscosus, Porphyromonas gingivalis, S. aureus, Klebsiella pneumoniae, Pseudomonas spp., and Bacillus cereus [9,11], while curcumin also showed effective against Salmonella typhimurium, Pseudomonas aeruginosa, E. coli, S. aureus, B. cereus, Helicobacter pylori, and Listeria monocytogenes [16,17,18].
C. xanthorrhiza has drawn the attention of researchers because of their suitable applications in the fields of material science and medicine. Therefore, the objective of the present study was to evaluate the antimycobacterial activity of the C. xanthorrhiza ethanol extract in vitro.
Materials and Methods
Ethical approval
The study was conducted in the Department of Veterinary Pharmacology, Faculty of Veterinary Medicine, Airlangga University. All procedure employed was approved by the Ethical Clearance Committee for preclinical research, Institute of Tropical Disease, Airlangga University.
Preparation of ethanol extract of C. xanthorrhiza
Plant material and extract preparation of C. xanthorrhiza were collected from Surabaya, Indonesia. C. xanthorrhiza materials were cleaned with running tap water and chopped into pieces. They were dried under shade at ambient temperature for 5 days, and the air dried C. xanthorrhiza was then ground to powder for extraction. The powdered C. xanthorrhiza (1 kg) was macerated with ethanol 96% (5 L) for 1 week at 37°C. The supernatant was then collected and filtered through Whatman No. 1 filter paper in a Buchner funnel under vacuum. The filtrate was concentrated by evaporation with a vacuum rotary evaporator at 45°C [8]. Furthermore, the extract was freeze-dried to get ethanol-free powder.
Qualitative phytochemical testing
The C. xanthorrhiza ethanol extract was subjected to the qualitative phytochemical screening for the presence of some chemical constituents. In the most active extracts, qualitative tests for terpenoids, saponins, tannins, flavonoid, phenols, and alkaloids were carried out as described by Jyoti and Rajeshwari [4].
Culture and preparation of M. tuberculosis
M. tuberculosis strains H37Rv were obtained from the Institute of Tropical Disease, Airlangga University, Surabaya, Indonesia. M. tuberculosis was cultured at 37°C in Middlebrook 7H9 broth (Becton Dickinson, Sparks, MD) supplemented with 0.2% glycerol (Sigma Chemical Co., St. Louis, MO) and 10% oleic acid albumin dextrose catalase (OADC; Becton Dickinson) until logarithmic growth was reached. Each culture was mixed with a sufficient volume of sterile supplemented Middlebrook 7H9 broth to achieve a turbidity equivalent to that of McFarland’s No. 1 standard. To obtain the test inoculum, this suspension was further diluted 1:50 with the same culture medium to approximately 6×106 colony-forming units (CFU)/mL immediately before use [19].
Minimum inhibitory concentration (MIC) determination by resazurin microtiter plate assay (REMA) method
REMA was performed with minor modifications [20]. The REMA plate method was performed in 7H9-S medium containing Middlebrook broth, 0.1% Casitone, and 0.5% glycerol and supplemented with OADC (Becton-Dickinson). Briefly, 100 μL of Middlebrook 7H9 broth was dispensed into each well of the microtiter plate. Serial two-fold dilutions of C. xanthorrhiza extract (powder-free ethanol) were performed in Middlebrook 7H9 broth to obtain final drugs concentration 0; 200; 400; 800; 1600, and 3200 μg/ml, and rifampicin (10 μg/ml) was used as a standard drug and were made in the plate. M. tuberculosis strains H37Rv suspension (100 μL) containing approximately 6×106 CFU/mL were added to all the wells. Sterility control and growth control were also included. The plate was wrapped in aluminum foil and incubated at 37°C for 7 days. After completion of the incubation period, 30 μL resazurin solution (100 μg/mL) was added to each well and plate was again wrapped in aluminum foil and incubated overnight. The plate was then observed for change in color. The color change from blue to pink or colorless indicated the growth of the bacteria. The lowest concentration of C. xanthorrhiza ethanolic extract that prevented color a change from blue to pink was taken as the upper limit for MIC range, and the highest C. xanthorrhiza ethanolic extract concentration that showed a change in color from blue to pink was considered the lower limit. All evaluations were carried out in quadruplicate.
Minimum bactericidal concentration (MBC) determination using the paper disc diffusion method
Screening of C. xanthorrhiza ethanolic extract and its solvents for antimycobacterial activity against M. tuberculosis strain H37Rv was done using the paper disc diffusion method [18]. Serial two-fold dilutions of C. xanthorrhiza extract (powder-free ethanol) were performed in a distilled water solution (0, 200, 400, 800, 1600, and 3200 μg/ml) and were slowly absorbed into the sterilized paper disc (diameter: 8 mm, Watman, England) and adhered to the surface of the plate on which M. tuberculosis strains H37Rv at a concentration of 106 CFU/ml had been inoculated in Middlebrook 7H9 broth. Sterilized distilled water was used as a control. After culturing for 24 h in an incubator at 370C, antibacterial activity was defined as the diameter (mm) of the clear inhibitory zone formed around the discs. MBC was defined as the lowest concentration that induced the clear inhibitory zone formed around the discs [19].
Results
Phytochemical of C. xanthorrhiza ethanolic extract
The preliminary phytochemical analysis of C. xanthorrhiza ethanol extract (Table-1) showed the presence of alkaloids, saponins, flavonoids, triterpenoids, and tannins of phytochemicals. Any of these phytochemicals, either singly or in a combination with each other could be responsible for the antibacterial activity of the C. xanthorrhiza ethanol extract.
Table-1.
Phytochemical analysis of extracts of Curcuma xanthorrhiza ethanol extract.
| Curcuma xanthorrhiza ethanol extract | |
|---|---|
| Phytochemical | Level |
| Alkaloids | ++ |
| Phenols | +++ |
| Saponins | ++ |
| Flavonoids | ++ |
| Triterpenoids | +++ |
| Tannins | |
+: Low, ++: Immediate, +++: High
The MIC of the C. xanthorrhiza ethanol extract against M. tuberculosis on REMA method
The MIC of the C. xanthorrhiza ethanolic extract was determined for their antimycobacterial activity using resazurin as an indicator of M. tuberculosis viability in 96-well microplates. The investigation showed that C. xanthorrhiza ethanolic extract was active against M. tuberculosis. In this study, MIC of C. xanthorrhiza ethanol extract against M. tuberculosis strains H37Rv was 1600 μg/mL, while rifampicin was 10 μg/mL (Figure-1).
Figure-1.
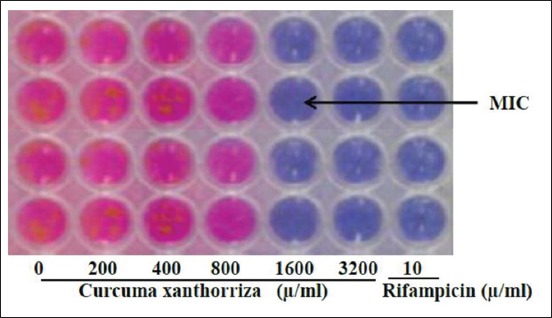
Minimal inhibitory concentration of Curcuma xanthorrhiza ethanol extract against Mycobacterium tuberculosis H37Rv on resazurin microtiter plate assay method was 1600 μg/ml (Black arrows). Serial two-fold dilutions C. xanthorrhiza ethanol extract (0 μg/ml, 200 μg/ml, 400 μg/ml, 800 μg/ml, 1600 μg/ml, and 3200 μg/ml) and rifampicin (10 μg/ml).
The MBC of the C. xanthorrhiza ethanolic extract against M. tuberculosis
The antimycobacterial activity of C. xanthorrhiza ethanolic extract against M. tuberculosis strains H37Rv was done using the plates of Middle brook 7H9 broth. MBC was defined as the lowest concentration that produced no growth M. tuberculosis strains H37Rv at Middle brook 7H9 broth. The MBC C. xanthorrhiza ethanolic extract against M. tuberculosis strains H37Rv was 3200 μg/mL, which produced no growth M. tuberculosis strains H37Rv at Middle brook 7H9 broth (Figure-2).
Figure-2.
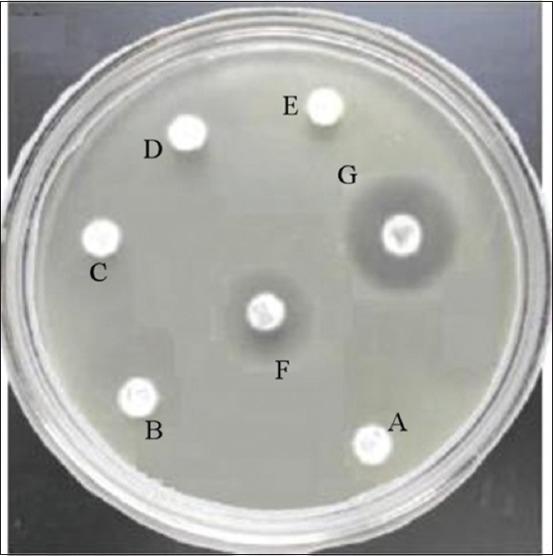
Minimal bactericidal concentration of Curcuma xanthorrhiza ethanol extract against M. tuberculosis H37Rv on the plates of middle brook 7H9 broth was 3200 μg/ml. C. xanthorrhiza ethanol extract at dose 0 μg/ml (a), 200 μg/ml (b), 400 μg/ml (c), 800 μg/ml (d), 1600 μg/ml (e), and 3200 μg/ml (f) and rifampicin 10 μg/ml (g).
Discussion
TB is a chronic disease caused by M. tuberculosis. The emergence of antibiotic-resistant strains of this species underscores the need for novel effective drugs against resistant mycobacteria as first-line anti-TB medications [6]. The uses of natural product as traditional medicines are accepted, particularly in developing countries. Medicinal plants have been used for centuries as remedies for human diseases because they contain components of therapeutic value [2]. Extraction of bioactive compounds from medicinal plants permits the demonstration of their physiological activity. It also facilitates pharmacology study leading to the synthesis of more potent drugs for meeting demand for effective and safe use [8]. This led us to the investigation of the effects of C. xanthorrhiza ethanolic extract on antimycobacterial activity. Phytochemical analysis of C. xanthorrhiza ethanolic extract showing antibacterial activity revealed C. xanthorrhiza ethanolic extract contained alkaloids, tannin, flavonoid, phenol, and saponin. There are reports showing that phenol, alkaloids, and flavonoids are the responsible compounds for the antibacterial activities in higher plants [17]. In vitro and in vivo toxicity shown that the C. xanthorrhiza extract non-toxic to peripheral blood mononuclear cells and in mice (data not shown).
The MIC is defined as the lowest concentration of an antimicrobial agent that prevents the visible growth of test microorganism in the 96-well microtiter plates. The first well with no visible growth after the incubation period was taken as the MIC. The MBC is the minimal concentration of the antimicrobial that kills the inoculums and can be determined by broth dilution MIC test [19].
Investigation of MIC and MBC plays an important role during the process of screening, prioritizing, and optimizing a chemical series during early antibacterial drug discovery. The MIC of the C. xanthorrhiza ethanolic extract was determined for their antimycobacterial activity using REMA method. Many researchers have used the REMA method to screen test substances for antimycobacterial activity against M. tuberculosis. Resazurin, an oxidation-reduction indicator, has been used to assess viability and bacterial contamination and to test for antimicrobial activity. Results obtained using the REMA assay is faster and less expensive. Bearing in mind considerations of rapidity, low technology requirements, and low cost, microplate assays that use resazurin type compounds have the potential of becoming the methods of choice for drug susceptibility testing of M. tuberculosis in places where TB is a major problem [20]. The MBC of the C. xanthorrhiza ethanol extract against M. tuberculosis was conducted on the plates of Middle brook 7H9 broth. In this study, MIC of C. xanthorrhiza ethanol extract against M. tuberculosis strains H37Rv was 1600 μg/mL, while MBC of C. xanthorrhiza ethanol extract against M. tuberculosis strains H37Rv was 3200 μg/mL. This suggests that C. xanthorrhiza ethanol extract has potent activity as antimycobacterial and this has been confirmed experimentally. These results are in agreement with reports in the literature that have documented the antibacterial activity of C. xanthorrhiza ethanol extract against a large number of Gram-positive and Gram-negative bacteria [10,17]. Some research has also shown that C. xanthorrhiza ethanol extract generally showed stronger effects for Gram-positive bacteria (e.g., Streptococcus mutans, L. monocytogenes, Bacillus megaterium, B. cereus, S. aureus, Staphylococcus epidermidis, and Lactobacillus plantarum) and for Gram-negative bacteria (e.g., E. coli, Pseudomonas fluorescens, P. aeruginosa, Salmonella typhimurium, Vibrio parahaemolyticus, and Klebsiella pneumoniae) [11-13,16,17]. Previous studies have shown that phenolic compounds of C. xanthorrhiza may act on microbial cell walls or membranes. They inhibit microbial growth by changing microbial cell permeability, which leads to the loss of intracellular molecules such as protein, DNA, RNA, and ATP [12]. Phenolic compounds could also affect cellular wall, membrane integrity, and microbial physiological responses [18].
Recent research activities have shown that C. xanthorrhiza extract contains active phytochemical constituents with xanthorrhizol and curcumin as the main compounds [17]. It has been demonstrated that the antibacterial activity of curcumin against Bacillus subtilis occurs through the inhibition of bacterial cell proliferation by blocking the assembly dynamics of FtsZ in the Z ring [18] and caused membrane damage of Gram-positive (S. aureus and Enterococcus faecalis) and Gram-negative (E. coli and P. aeruginosa) [12]. While xanthorrhizol strongly inhibited the growth of B. cereus, C. perfringens, L. monocytogenes, S. aureus, Salmonella Typhimurium, and V. parahaemolyticus with MICs of 8, 16, 8, 8, 16, and 8 µg/ml, respectively. These strains were killed by xanthorrhizol at MBCs of 16, 32, 16, 16, 16, and 16 µg/ml, respectively [9-11].
The potency of the extracts of C. xanthorrhiza as compared to the standard antimycobacterial drugs (rifampicin) used was still very low. This is understandable considering the fact that rifampicin was pure compounds known to be the most active against M. tuberculosis compared with the activity of C. xanthorrhiza that was crude extracts. If the active compounds could be isolated and purified of C. xanthorrhiza, it is possible that they could show a comparable activity or even more activity than some of the antimycobacterial drugs currently in use.
Conclusion
Thus, from the results obtained, it can be concluded that C. xanthorrhiza ethanol extract has promising anti-TB activity by preliminary in vitro techniques. Therefore, it has the definite potential as a source of compounds that may be developed further into antimycobacterial drugs.
Authors’ Contributions
N, S, K, and E equally contributed in conception and design of the experiment, data collection, data analysis, and interpretation. SAS helped in critical revision of the article and final approval of the version to be published. All the authors read and approved of the final manuscript.
Acknowledgments
The authors would like to acknowledge the support of The Ministry of Health Republic of Indonesia through the Health Polytechnic Surabaya Indonesia in conducting this research work (Grant No: 018/HB/Poltekkes/Menkes/II/2017).
Competing Interests
The authors declare that they have no competing interests.
References
- 1.World Health Organization. Global Tuberculosis Report. Geneva, Switzerland: WHO; 2015. pp. 1–145. [Google Scholar]
- 2.Cragg G.M, Newman D.J. Natural products:A continuing source of novel drug. Biochim. Biophys. Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudjarwo S.A, Koerniasari G.W. Protective effect of curcumin on lead acetate-induced testicular toxicity in Wistar rats. Res. Pharm. Sci. 2017;12:381–390. doi: 10.4103/1735-5362.213983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jyoti S, Rajeshwari S. Evaluation of phytochemical constituents in conventional and non-conventional species of curcuma. Int. Res. J. Pharm. 2012;3:203–204. [Google Scholar]
- 5.Njeru S.N, Obonyo M.A, Ngari S.M, Njeru J.M, Kamweru P.P. Antituberculous, antimicrobial, cytotoxicity and phytochemical activity study ofPiliostigma thonningiiextract fractions. J. Med. Plants Res. 2015;9:655–663. [Google Scholar]
- 6.Fadipe V.O, Opoku A.R, Dikhoba P.M, Makhafola T.J. Isolation of anti-mycobacterial compounds fromCurtisia dentata(Burm.f.) C.A.Sm (Curtisiaceae) BMC Complement. Altern. Med. 2017;17:306–313. doi: 10.1186/s12906-017-1818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyambuya T, Mautsa R, Mukanganyama S. Alkaloid extracts fromCombretum zeyheriinhibit the growth ofMycobacterium smegmatis. BMC Complement. Altern. Med. 2017;17:124–130. doi: 10.1186/s12906-017-1636-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naik S.K, Mohanty S, Padhi A, Pati R, Sonawane A. Evaluation of antibacterial and cytotoxic activity ofArtemisia nilagiricaandMurraya koenigiileaf extracts against mycobacteria and macrophages. BMC Complement. Altern. Med. 2014;14:1–6. doi: 10.1186/1472-6882-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oon S.F, Nallappan M, Tee T.T, Shohaimi S, Kassim N.K, Cheah Y.H. Xanthorrhizol:A review of its pharmacological activities and anticancer properties. Cancer. Cell. Int. 2015;15:100. doi: 10.1186/s12935-015-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sylvester W.S, Son R, Lew K.F, Rukayadi Y. Antibacterial activity of java turmeric (Curcuma xanthorrhizaRoxb.) extract againstKlebsiella pneumoniaeisolated from several vegetables. Int. Food Res. J. 2015;22:1770–1776. [Google Scholar]
- 11.Lee L.Y, Shim J.S, Rukayadi Y, Hwang J.K. Antibacterial activity of xanthorrhizol isolatedCurcuma xanthorrhiza Roxb. against food borne pathogens. J Food Prot. 2008;71:1926–1930. doi: 10.4315/0362-028x-71.9.1926. [DOI] [PubMed] [Google Scholar]
- 12.Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K. Bactericida activity of curcumin is associated with damaging of bacterial membrane. PLoS One. 2015;10:e0121313–e0121328. doi: 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moghadamtousi S.Z, Kadir H.A, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014;2014:186864–186875. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudjarwo S.A, Eraiko K, Koerniasari G.W. Mechanisms of endothelial cell protection by curcumin in hypercholesterolemia. J. Appl. Pharm. Sci. 2011;1:32–35. [Google Scholar]
- 15.Sudjarwo S.A, Eraiko K, Koerniasari G.W. Antioxidant activity of curcumin as protector on methylmercury induced-pancreas damage in mice. J. Chin. Pharm. Sci. 2017;26:196–201. [Google Scholar]
- 16.Anjusha S, Gangaprasad A. Phytochemical and antibacterial analysis of two importantCurcumaspeciesCurcuma aromaticaSalisb andCurcuma xanthorrhizaRoxb. (Zingiberaceae) J. Pharm. Phytochem. 2014;3:50–53. [Google Scholar]
- 17.Mary Helen P.A, Susheela G.K, Jayasree S, Nizzy A.M, Rajagopal B, Jeeva S. Phytochemical characterization and antimicrobial activity ofCurcuma xanthorrhizaRoxb. Asian. Pac. J. Trop. Biomed. 2012;2:S637–S640. [Google Scholar]
- 18.Rai D, Singh J.K, Roy N, Panda D. Curcumin inhibits FtsZ assembly:An attractive mechanism for its antibacterial activity. Biochem. J. 2008;410:147–155. doi: 10.1042/BJ20070891. [DOI] [PubMed] [Google Scholar]
- 19.Primm T.P, Franzblau S.G. Recent advances in methodologies for the discovery of anti-mycobacterial Drugs. Curr. Bioact. Compd. 2007;3:1–8. [Google Scholar]
- 20.Ang C.F, Mendoza M.T, Bulatao W.C. Evaluation of the resazurin microtiter assay for drug susceptibility testing of clinical isolates ofMycobacterium tuberculosis. J. Microbiol. Infect. Dis. 2010;39:59–65. [Google Scholar]


