Abstract
Background
The long non-coding RNA H19 plays a crucial role in solid tumor initiation and progression. However, the potential role of H19 and its clinical significance in acute myeloid leukemia (AML) remain largely elusive.
Methods
H19 expression was detected by qPCR, and clinical significance in AML patients was further analyzed. The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) data for AML were used as validation cohorts. The roles of H19 in cell proliferation and apoptosis were determined by cell proliferation assay and flow cytometry analysis.
Results
H19 expression was significantly increased in AML patients but not associated with embedded miR-675 expression. Moreover, H19 overexpression was not dependent on the methylation pattern in H19 differentially methylated region/imprinting control region. Strong association was observed between H19 overexpression and patients’ characteristics including sex, higher white blood cells, older age, and intermediate karyotype, FLT3-ITD, and DNMT3A mutations. In addition, H19 overexpression correlated with lower complete remission (CR) rate and shorter overall survival, and further confirmed by multivariate analyses. Importantly, the prognostic effect of H19 expression was validated by TCGA and GEO data. In the follow-up of patients, H19 expression in CR phase was lower than diagnosis time and returned at relapse time. Loss-of-function experiments showed that H19 exhibited anti-proliferative and pro-apoptotic effects in leukemic cell HL60. Furthermore, H19 expression was positively correlated with potential downstream gene ID2 in AML.
Conclusions
Our findings revealed that methylation-independent H19 was a prognostic and predictive biomarker in AML, and H19/ID2 played crucial roles in leukemogenesis with potential therapeutic target value.
Electronic supplementary material
The online version of this article (10.1186/s13148-018-0486-z) contains supplementary material, which is available to authorized users.
Keywords: H19, Prognosis, Surveillance, ID2, AML
Background
Acute myeloid leukemia (AML), the most common adult leukemia, is an etiologically, clinically, cytogenetically, and molecularly heterogeneous disease characterized by uncontrolled proliferation and blocked apoptosis of immature myeloid progenitors [1]. Genetic abnormalities and epigenetic alterations played crucial roles in the pathogenesis of AML [2]. Moreover, genetic abnormalities such as chromosome aberrations and gene mutations were also seen as the most powerful prognostic information [3]. Despite recent advances in the anti-cancer or targeted drugs, clinical outcome of AML remains unsatisfactory [1]. Accordingly, progresses should be made in the mechanisms of leukemogenesis and the identification of markers that allow molecular-based stratification to risk-adapted therapies to improve the clinical outcome of AML.
Recently, long non-coding RNAs (lncRNAs) have been implicated in many human diseases especially in human cancers, and increasing studies begin to unravel the molecular mechanisms underlying lncRNA function in these pathological processes and/or carcinogenesis [4]. The human H19 gene encodes a 2.3-kb lncRNA with a crucial role in embryonal development and growth control [5]. H19 and neighboring gene IGF2 (known as IGF2/H19 locus) are reciprocally imprinted, leading to differential allelic expression of H19 from the maternal allele and IGF2 from the paternal allele [6]. Abnormal expression or loss of imprinting of H19 has also been linked to diverse human cancers including hematological malignancies [5]. Although H19 was originally seen as a tumor suppressor in Wilms’ tumors, embryonic rhabdomyosarcoma, and Beckwith-Wiedmann cancer predisposing syndrome, recent studies displayed the evidences of the oncogenic role of H19 in several human cancers, such as breast cancer, endometrial cancer, gastric cancer, and so on [5, 7]. Notably, Guo et al. reported that high expression of H19 was required for efficient tumorigenesis induced by BCR-ABL oncogene [7]. In addition, loss of imprinting (LOI) of IGF2/H19 mainly caused by “differentially methylated region” or “imprinting control region” (DMR/ICR) demethylation was shown as a frequent event in AML, adult T cell leukemia/lymphoma, and chronic myeloid leukemia (CML) [8–10]. However, the direct role and its clinical significance in AML remain poorly determined. Herein, we reported H19 as a prognostic and predictive biomarker in AML, and H19 played a crucial role in leukemogenesis with potential therapeutic target value.
Methods
Patients and treatment
A total of 161 AML patients [including 161 newly diagnosed patients, 54 patients who achieved complete remission (CR) after induction therapy and 26 relapsed patients] and 36 healthy donors were included in this study approved by the Institutional Ethics Committee of the Affiliated People’s Hospital of Jiangsu University. After written informed consents were obtained, bone marrow (BM) was collected from all participants and was extracted for the BM mononuclear cells (BMMNCs). All the patients received induction and consolidation chemotherapy as reported in our previous literature [11].
Cytogenetic analysis and gene mutation detection
Karyotypes were analyzed at the newly diagnosis time by conventional R-banding method according to the previous literature [12]. Gene mutations (such as NPM1 and DNMT3A mutations) were detected by high-resolution melting analysis (HRMA) and direct DNA sequencing (such as CEBPA and FLT3-ITD mutations) as reported [13–21].
RNA isolation, reverse transcription, and RT-qPCR
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and was transcriptionally reversed into cDNA as reported previously [22]. H19 expression was detected by real-time quantitative PCR (RT-qPCR) using the SYBR Premix Ex Taq II (TaKaRa, Tokyo, Japan) with the primers shown in Additional file 1: Table S1. The RT-qPCR reaction was carried out at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, 67 °C for 30 s, 72 °C for 30 s, and 87 °C for 30 s to collect fluorescence. ABL1 expression was detected by RT-qPCR using AceQ qPCR SYBR Green Master Mix (Vazyme Biotech Co., Piscataway, NJ, USA) as reported [22]. Relative H19 level was calculated using the following equation: NH19 = (EH19) ΔCT H19 (control − sample) ÷ (EABL) ΔCT ABL (control − sample). The parameter efficiency (E) was derived from the formula E = 10(−1/slope) (the slope referred to CT versus cDNA concentration plot).
DNA isolation, chemical modification, and RT-qMSP
Genomic DNA was isolated and modified using genomic DNA purification kit (Gentra, Minneapolis, MN, USA) and CpGenome™ DNA Modification Kit (Chemicon, Ternecula, Canada), respectively. The level of H19 DMR/ICR methylation was detected by the unmethylation primers (Additional file 1: Table S1) of real-time quantitative methylation-specific PCR (U-RT-qMSP) with SYBR Premix Ex Taq II (TaKaRa, Tokyo, Japan). U-RT-qMSP conditions were 95 °C for 30 s and 40 cycles for 5 s at 95 °C, 30 s at 57 °C, 30 s at 72 °C, and 75 °C for 30 s. The normalized ratio (NU-H19) was applied to assess the level of H19 unmethylation in samples. NU-H19 was calculated using the following formula: NU-H19 = (EU-H19) ΔCT U-H19 (control − sample) ÷ (EALU) ΔCT ALU (control − sample).
Bisulfite sequencing PCR
Bisulfite sequencing PCR (BSP) reaction was carried out using TaKaRa Taq™ Hot Start Version kit (Tokyo, Japan) as reported [11]. The main conditions were 98 °C for 10 s, 55 °C for 30 s, and 72 °C for 30 s. Nine independent clones per specimen were picked out and sequenced.
Cell line and cell culture
Human leukemic cell line HL60 (American Type Culture Collection, Manassas, VA, USA) was cultured in RPMI 1640 medium (BOSTER, Wuhan, China) containing 10% fetal calf serum (ExCell Bio, Shanghai, China) and grown at 37 °C in 5% CO2 humidified atmosphere.
SiRNA transfection
SiRNA-mediated knockdown of H19 was used for loss-of-function experiments. The siH19-1 (sense strand: 5′-CCCGUCCCUUCUGAAUUUATT-3′; antisense strand: 5′-UAAAUUCAGAAGGGACGGGTT-3′) and siH19-2 (sense strand: 5′-UAAGUCAUUUGCACUGGUUTT-3′; antisense strand: 5′-AACCAGUGCAAAUGACUUATT-3′) [23] were purchased from GenePharma (Shanghai, China). SiRNA transfection was performed using the X-tremeGENE siRNA Transfection Reagent (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Cells were used for experiments in 3 days after siRNA transfection.
Cell proliferation assays
Cells (1 × 105 cells/mL) for 2 mL per well were seeded in a 6-well plate in RPMI 1640 medium containing 10% fetal calf serum. After culturing for 0, 1, 2, and 3 days, cells were counted in a counting board for three times.
Cell apoptosis assays
Cells (2 × 105 cells/mL) for 2 mL per well were seeded in a 6-well plate in RPMI 1640 medium containing 0% fetal calf serum. Annexin V-PE/7-AAD apoptosis detection (BD Pharmingen, San Diego, CA, USA) was used and then analyzed via flow cytometry (BD FACSCalibur, San Jose, CA, USA). Each experiment was repeated three times.
TCGA and GEO datasets
H19 expression (RNA Seq V2 RSEM) and H19 methylation (HM27 and HM450) data from a cohort of 200 AML patients from The Cancer Genome Atlas (TCGA) [24] were downloaded via cBioPortal (http://www.cbioportal.org) [25, 26].
Two independent cohorts of 78 and 162 cytogenetically normal AML (CN-AML) patients from Gene Expression Omnibus (GEO) data (http://www.ncbi.nlm.nih.gov/geo/; accession number GSE12417) were applied to analyze the prognostic impact of H19 expression using the online web tool Genomicscape (http://genomicscape.com/microarray/survival.php) [27, 28].
Bioinformatics analyses
H19 function prediction based on text mining was performed using the Coremine Medical online database (http://www.coremine.com/medical/).
Statistical analyses
SPSS 20.0 software package and GraphPad Prism 5 were applied to statistical analyses. Mann-Whitney’s U test was performed to compare the differences of continuous variables, whereas the differences of categorical variables were analyzed using the Pearson chi-square analysis/Fisher exact test. Spearman correlation test was conducted to evaluate the correlation between continuous variables. The ROC curve and area under the ROC curve (AUC) were carried out to assess the discriminative capacity of H19 expression between patients and controls. H19 expression for achievement of CR was evaluated via logistic regression models (univariate and multivariate). Kaplan-Meier and Cox regression (univariate and multivariate) analyses were used to analyze the impact of H19 expression on overall survival (OS) and leukemia-free survival (LFS). Statistical significance was set at P < 0.05 and all tests were two sided.
Results
H19 expression was upregulated in AML
In order to determine the role of H19 in AML pathogenesis, we first evaluated H19 expression in AML patients and controls by RT-qPCR. As presented in Fig. 1a, H19 expression was significantly upregulated in AML patients (median 0.107) than controls (median 0.014) (P = 0.003). Since microRNA-675 (miR-675) is embedded within the first exon of H19, we further assess the association of H19 with miR-675 in AML. Previously, our study reported miR-675 expression was significantly downregulated in AML patients [29]. Herein, we further found that there was no significant correlation between H19 and miR-675 expression in AML (R = 0.032, P = 0.750, n = 101).
Fig. 1.
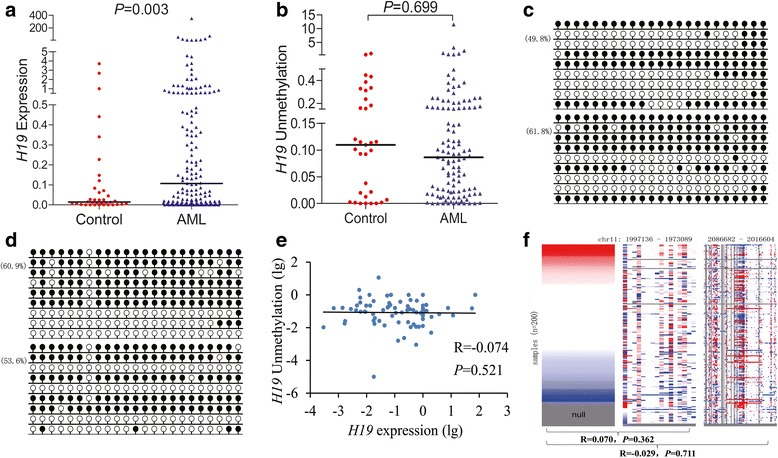
H19 expression and methylation in AML. a H19 expression level detected by real-time quantitative PCR in controls and AML patients. b H19 unmethylation level detected by real-time quantitative unmethylation-specific PCR in controls and AML patients. c, d H19 methylation density detected by bisulfite sequencing in controls and AML patients, respectively. White cycle, unmethylated CpG dinucleotide; black cycle, methylated CpG dinucleotide. e The correlations between H19 expression and unmethylation. f Relationship between H19 expression and methylation of two different regions using The Cancer Genome Atlas (TCGA) data. Left: H19 expression, value in log2(x + 1) transformation, x is the RSEM value. Middle: H19 methylation in HM27K. Right: H19 methylation in HM450K. Red color: higher expression/methylation. Blue color: lower expression/methylation. White color: intermediate level of H19 expression/methylation. Gray color: no data. Each row represents H19 expression and H19 methylation level of the same patient; each line from right to left in middle and right figures represents the methylation level at different sites
H19 overexpression was not dependent on H19 methylation in AML
Since H19 is an imprinted gene and controlled by the methylation pattern in DMR/ICR, we hypothesized that H19 overexpression was mediated by H19 DMR/ICR hypomethylation in AML. However, RT-qMSP showed that its DMR/ICR methylation level in AML patients (median 0.086) was similar to controls (median 0.109) (P = 0.699, Fig. 1b). The same result was also confirmed by BSP analysis (Fig. 1c, d). Moreover, no significant association was observed between H19 DMR/ICR methylation and expression in AML (R = − 0.074, P = 0.521, n = 77, Fig. 1e).
In order to verify our results, we further implemented an independent assessment of H19 methylation and expression in AML from TCGA database. As expected, no significant negative correlation was observed between H19 methylation and expression in AML (R = 0.070, P = 0.362, n = 170 and R = − 0.029, P = 0.711, n = 170, respectively, Fig. 1f).
H19 overexpression correlated with clinical characteristics and genetic events in AML
ROC curve analysis revealed that the sensitivity and the specificity were 49.1 and 80.6% (sensitivity + specificity − 1 was the highest value) when H19 expression was at the value of 0.121 (Additional file 2: Figure S1). By the cutoff value, we classified the whole-cohort AML patients into two groups (high and low) in order to further analyze the clinical significance of H19 expression in AML. High H19 expression was found to be associated with sex (P = 0.075), higher white blood cells (P = 0.009), and older age (P = 0.004, Table 1). Moreover, significant differences were observed among both karyotype and karyotypic classifications (P = 0.048 and 0.010, respectively). H19 overexpression had the highest frequency in intermediate karyotype [70% (55/79), P = 0.002] and much lower in favorable karyotype [18% (14/79), P = 0.013] especially in t(15;17) [6% (5/79), P = 0.008].
Table 1.
Comparison of clinical manifestations and laboratory features between H19low and H19high AML patients
| Patient’s parameters | Low (n = 82) | High (n = 79) | P value |
|---|---|---|---|
| Sex, male/female | 44/38 | 54/25 | 0.075 |
| Median age, years (range) | 51.5 (10–87) | 61 (17–93) | 0.009 |
| Median WBC, × 109/L (range) | 7.7 (1.0–185.4) | 31.1 (0.3–528.0) | 0.004 |
| Median hemoglobin, g/L (range) | 75 (40–133) | 78.5 (32–144) | 0.144 |
| Median platelets, × 109/L (range) | 42 (5–447) | 33 (3–399) | 0.262 |
| Median BM blasts, % (range) | 44 (3.0–94.5) | 43 (1–99) | 0.339 |
| Karyotype classification | 0.010 | ||
| Favorable | 29 (35%) | 14 (18%) | |
| Intermediate | 37 (45%) | 55 (70%) | |
| Poor | 12 (15%) | 9 (11%) | |
| No data | 4 (5%) | 1 (1%) | |
| Karyotype | 0.048 | ||
| Normal | 28 (34%) | 42 (54%) | |
| t(8;21) | 7 (9%) | 5 (6%) | |
| t(16;16) | 0 (0%) | 1 (1%) | |
| t(15;17) | 22 (27%) | 8 (10%) | |
| t(9;22) | 0 (0%) | 1 (1%) | |
| + 8 | 3 (4%) | 4 (5%) | |
| − 5/5q− | 1 (1%) | 2 (3%) | |
| − 7/7q− | 1 (1%) | 0 (0%) | |
| Complex | 10 (12%) | 6 (8%) | |
| Others | 6 (7%) | 9 (11%) | |
| No data | 4 (5%) | 1 (1%) | |
| Gene mutation | |||
| CEBPA (+/−) | 10/66 | 7/61 | 0.617 |
| NPM1 (+/−) | 6/70 | 11/57 | 0.195 |
| FLT3-ITD (+/−) | 6/70 | 13/55 | 0.053 |
| c-KIT (+/−) | 4/72 | 1/67 | 0.370 |
| N/K-RAS (+/−) | 4/72 | 8/60 | 0.228 |
| IDH1/2 (+/−) | 2/74 | 6/62 | 0.149 |
| DNMT3A (+/−) | 2/74 | 9/59 | 0.025 |
| U2AF1 (+/−) | 3/73 | 3/65 | 1.000 |
| SRSF2 (+/−) | 3/75 | 4/66 | 0.708 |
| SETBP1 (+/−) | 1/77 | 1/69 | 1.000 |
| CR (+/−) | 40/35 | 22/52 | 0.005 |
AML acute myeloid leukemia, WBC white blood cells, CR complete remission
We further assessed the association of H19 expression with gene mutations in AML. A total of 12 common gene mutations were screened in 144 AML patients. Patients with high H19 expression harbored higher incidence of FLT3-ITD and DNMT3A mutations than those with low H19 expression (P = 0.053 and 0.025, respectively, Table 1). No significant differences were observed in other gene mutations among the two groups (P > 0.05, Table 1).
H19 overexpression correlated with poor chemotherapy response and OS in AML
Follow-up data was available for 149 AML patients including 121 non-APL-AML patients and 64 CN-AML patients. As shown in Table 1, whole-cohort AML patients with high H19 expression had a significantly lower CR rate than those with low H19 expression (P = 0.005). The similar results also existed among non-APL-AML and CN-AML patients (P = 0.012 and 0.036, respectively). Moreover, multivariate analysis revealed that high H19 expression taken as a dichotomous variable was an independent prognostic predictor for poor CR rate among both whole-cohort and non-APL-AML patients (P = 0.034 and 0.011, respectively, Table 2) but not CN-AML patients (data not shown).
Table 2.
Univariate and multivariate analyses of prognostic factors for complete remission in whole-cohort and non-APL-AML patients
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Whole-cohort AML | ||||
| Age | 0.119 (0.054–0.262) | < 0.001 | 0.162 (0.069–0.379) | < 0.001 |
| WBC | 0.269 (0.126–0.575) | 0.001 | 0.505 (0.204–1.253) | 0.140 |
| Karyotype classifications | 0.214 (0.112–0.408) | < 0.001 | 0.269 (0.139–0.519) | < 0.001 |
| H19 expression | 0.370 (0.189–0.726) | 0.004 | 0.416 (0.185–0.935) | 0.034 |
| Non-APL AML | ||||
| Age | 0.164 (0.069–0.392) | < 0.001 | 0.199 (0.079–0.502) | 0.001 |
| WBC | 0.353 (0.153–0.819) | 0.015 | 0.522 (0.198–1.378) | 0.189 |
| Karyotype classifications | 0.316 (0.145–0.691) | 0.004 | 0.297 (0.129–0.687) | 0.005 |
| H19 expression | 0.361 (0.164–0.791) | 0.011 | 0.306 (0.123–0.762) | 0.011 |
Variables were composed of age (≤ 60 vs. > 60 years), WBC (≥ 30 × 109 vs. < 30 × 109/L), karyotype classifications (favorable vs. intermediate vs. poor), and H19 expression (low vs. high). The multivariate analysis included variables with P < 0.100 in univariate analysis for complete remission
AML acute myeloid leukemia, APL acute promyelocytic leukemia, WBC white blood cells, CI confidence interval
Kaplan-Meier analysis revealed that whole-cohort AML patients with H19 overexpression had a significantly shorter OS than those without H19 overexpression (P = 0.020, Fig. 2a). Among non-APL-AML and CN-AML, patients with high H19 expression were also associated with shorter OS (P = 0.041 and 0.018, Fig. 2b, c, respectively). However, there was no significant association between H19 expression and LFS among either AML sub-groups (all P > 0.05, Fig. 2d–f, respectively). Since H19 expression closely correlated with several well-established prognostic factors such as age, WBC, karyotypic classifications, and gene mutations, we further conducted a Cox regression model adjusting for prognosis-related factors. Multivariate analysis revealed that high H19 expression might act as an independent prognostic biomarker for poor OS in non-APL-AML patients (HR = 1.554, P = 0.063, Table 3) but not whole-cohort AML (HR = 1.355, P = 0.169) or CN-AML patients (HR = 1.393, P = 0.313).
Fig. 2.
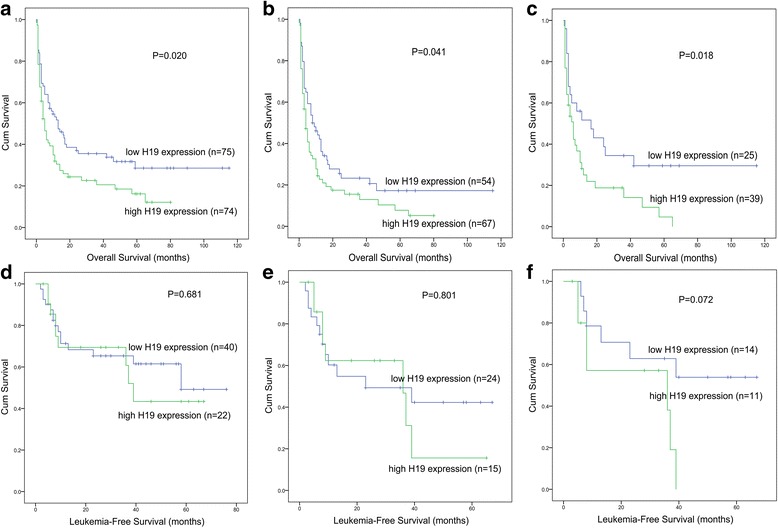
The impact of H19 expression on survival in AML. a Overall survival (OS) among whole-cohort AML patients. b OS among non-APL-AML patients. c OS among cytogenetically normal AML (CN-AML) patients. d Leukemia-free survival (LFS) among whole-cohort AML patients. e LFS among non-APL-AML patients. f LFS among CN-AML patients
Table 3.
Univariate and multivariate analyses of prognostic factors for overall survival in non-APL-AML patients
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Age | 2.294 (1.528–3.446) | < 0.001 | 1.651 (1.067–2.555) | 0.024 |
| WBC | 1.856 (1.247–2.764) | 0.002 | 1.447 (0.943–2.220) | 0.091 |
| Karyotype classifications | 1.756 (1.314–2.346) | < 0.001 | 1.858 (1.320–2.616) | < 0.001 |
| H19 expression | 1.486 (0.997–2.216) | 0.052 | 1.554 (0.977–2.472) | 0.063 |
| CEBPA mutation | 0.793 (0.410–1.533) | 0.491 | ||
| NPM1 mutation | 1.142 (0.606–2.152) | 0.681 | ||
| FLT3-ITD mutation | 1.005 (0.532–1.898) | 0.987 | ||
| c-KIT mutation | 1.043 (0.255–4.263) | 0.953 | ||
| N/K-RAS mutation | 1.070 (0.533–2.149) | 0.849 | ||
| IDH1/2 mutation | 4.246 (1.964–9.179) | < 0.001 | 3.781 (1.593–8.978) | 0.003 |
| DNMT3A mutation | 1.256 (0.630–2.506) | 0.518 | ||
| U2AF1 mutation | 2.756 (1.177–6.455) | 0.020 | 2.499 (1.050–5.950) | 0.038 |
| SRSF2 mutation | 2.005 (0.914–4.399) | 0.083 | 1.590 (0.673–3.758) | 0.291 |
| SETBP1 mutation | 0.497 (0.069–3.583) | 0.488 | ||
Variables were composed of age (≤ 60 vs. > 60 years), WBC (≥ 30 × 109 vs. < 30 × 109/L), karyotype classifications (favorable vs. intermediate vs. poor), H19 expression (low vs. high), and gene mutations (mutant vs. wild-type). The multivariate analysis included variables with P < 0.100 in univariate analysis for overall survival
AML acute myeloid leukemia, APL acute promyelocytic leukemia, WBC white blood cells, CI confidence interval
The prognostic value of H19 expression validated by TCGA and GEO data
In order to validate the prognostic value of H19 expression in AML, we searched and analyzed an independent assessment in AML patients from TCGA databases. By the median level of H19 expression set as the cut-off value, patients with higher H19 expression showed a significantly shorter OS among both whole-cohort AML (P = 0.062, Fig. 3a) and non-APL-AML (P = 0.004, Fig. 3b). Nevertheless, no significant difference was observed between the two groups for OS among CN-AML (P = 0.147, Fig. 3c).
Fig. 3.
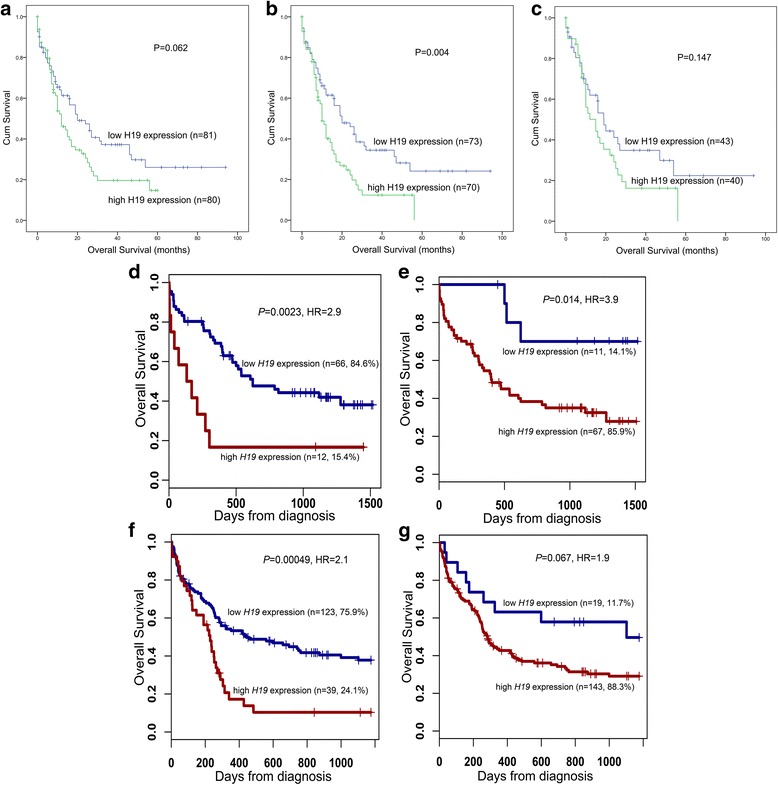
Prognostic value of H19 expression on overall survival in AML using TCGA and GEO data. a–c The impact of H19 expression on overall survival (OS) in a cohort of 200 AML patients from The Cancer Genome Atlas (TCGA) databases. The patients were classified into H19 low-expressed and high-expressed groups by the median level of ID4 expression. a OS among whole-cohort AML. b OS among non-APL-AML. c OS among cytogenetically normal AML (CN-AML). d–g The impact of H19 expression on OS in two independent cohorts of 78 and 162 CN-AML patients were obtained from Gene Expression Omnibus (GEO) data. Survival analysis was performed through the online web tool Genomicscape. d Probe 224646_at among a cohort of 78 CN-AML patients. e Probe 224997_at among a cohort of 78 CN-AML patients. f Probe 224646_at a cohort of 162 CN-AML patients. g Probe 224997_at among a cohort of 162 CN-AML patients
Moreover, the published data from two cohorts of CN-AML patients available in GEO databases were set as the independent validation cohort. Through the online tool GenomicScape, high H19 expression was significantly correlated with shorter OS among both two cohorts (P = 0.002, 0.014, < 0.001, and 0.067, respectively, Fig. 3d–g).
H19 expression was a predictive biomarker in the surveillance of AML
To identify whether H19 expression could act as a potential biomarker in the surveillance of AML, we next assessed H19 expression in AML patients of different clinical stages including 54 patients who achieved CR after induction therapy and 26 relapsed patients. Our data indicated that H19 expression in CR phase was lower to diagnosis time and was returned to primary level when in relapse time (Fig. 4a). Moreover, the dynamic changes of H19 expression in seven paired patients with available follow-up data were also shown in Fig. 4b.
Fig. 4.
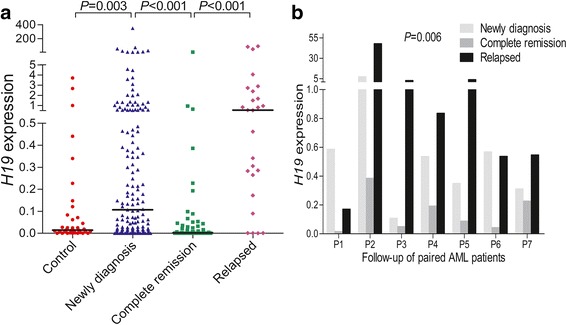
H19 expression in the surveillance of AML. a H19 expression in different clinical stages (newly diagnosis, complete remission, and relapse time) of AML patients. b Dynamic change of H19 expression in the follow-up of seven paired AML patients during newly diagnosis, complete remission, to relapse time
H19 exhibited pro-proliferative and anti-apoptotic effects in leukemia cells
We first identified the potential biological role of H19 in leukemia by bioinformatics analysis on the basis of Coremine Medical mining. As shown in Fig. 5a, the associations of H19 with proliferation, division, differentiation, apoptotic process, and hemopoiesis were comprehensively analyzed. Next, we performed in vitro experiments to validate the leukemia-promoting effects of H19 in AML. Since all the leukemic cells showed an increased H19 expression, we conducted loss-of-function assays in H19 relatively high-expressed cells (Fig. 5b, c). As a result, knockdown of H19 in HL60 cells by two different siRNAs resulted in a significantly reduced proliferation and elevated apoptosis (Fig. 5d–g). In addition, similar results were also observed in K562 cells and had been published in our previous study [30].
Fig. 5.
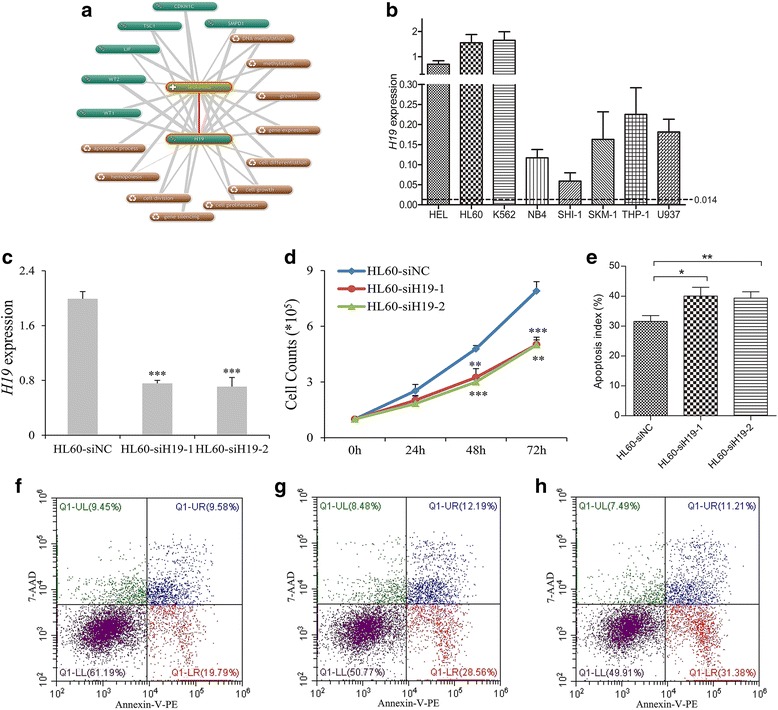
The biological role of H19 on leukemic cell line HL60. a The underlying role of H19 in leukemogenesis determined by Coremine Medical online database (http://www.coremine.com/medical/). b H19 expression in eight common leukemic cell lines. The dotted line indicated the cutoff value to define H19 overexpression. c H19 expression mediated by siRNA-based H19 knockdown. H19 expression was significantly downregulated in siRNA-based H19 knockdown (siH19-1 and siH19-2) and control (siNC) groups. d The effect of H19 knockdown on cell proliferation. The siH19 groups (siH19-1 and siH19-2) showed significantly lower proliferation capacity than the siNC group at 48 and 72 h. e The effect of H19 knockdown on cell apoptosis. The siH19 groups (siH19-1 and siH19-2) showed significantly higher apoptosis rate than the siNC group at 48 h. f–h Flow-type apoptosis figures for siNC, siH19-1, and siH19-2, respectively. *P < 0.05, **P < 0.01, ***P < 0.001
H19 expression positively correlated with potential downstream gene ID2 in AML
As is well-known, lncRNAs function directly or indirectly through the protein-encoding gene. A previous study showed that H19 was positively associated with ID2 expression in bladder cancer [31]. Moreover, ID2 overexpression was a frequent event and predicted poor chemotherapy response and adverse prognosis in AML [32]. Herein, we also found knockdown of H19 also induced decreased ID2 expression in HL60 cells (P = 0.006, Fig. 6a). Moreover, significant positive association was also observed between H19 expression and ID2 transcript level in clinical AML patients (R = 0.262, P = 0.002, n = 135, Fig. 6b). All these suggested that ID2 might be a potential downstream gene of H19 in AML.
Fig. 6.
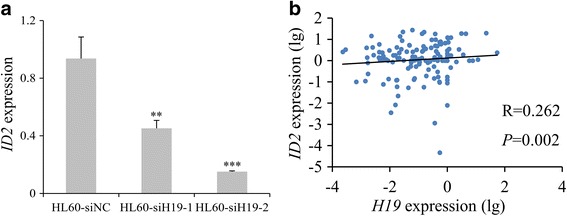
Relationship between H19 and ID2 in AML. a ID2 mRNA expression in after siRNA-based H19 knockdown in HL60 cell line. ID2 mRNA expression was significantly downregulated after siRNA-based H19 knockdown (siH19-1 and siH19-2) and control (siNC) groups. **P < 0.01, ***P < 0.001. b Correlation between H19 and ID2 expressions in AML patients. Correlation analysis was performed by Spearman test
Discussion
Oncogenic role of lncRNA H19 has been demonstrated in diverse human solid tumors, and H19 expression was significantly upregulated in these cancer patients [5]. In this study, we first quantified H19 expression in BMMNCs of AML patients and showed that H19 overexpression was a frequent event in AML. We next performed functional experiments in vitro to investigate the potential role of H19 in AML. Loss-of-function of H19 by siRNA in human HL60 cells exhibited anti-proliferative and pro-apoptotic effects in accordance with previous literatures showing the role of H19 in solid tumors [5]. In addition, a recent study showed the functional involvement of H19 in BCR-ABL-mediated leukemogenesis [7]. Taken together, all these data implicated that H19 might also act as a proto-oncogene during leukemogenesis. However, Tessema et al. showed H19/IGF2 was frequently downregulated in AML, CML, and chronic myelomonocytic leukemia (CMML) [33]. One explanation for the differing results may be attributed to the limited cases of AML, CML, and CMML in the previous study.
As is well known, lncRNAs function often through promoting the strength of specific enhancer-promoter looping and thus contributing to gene activation, regulating protein activities, sequestering microRNAs, and serving as precursors of small RNAs during the pathological processes [4]. In addition to H19, it can be dissected into two major functions: one is a reservoir of miR-675 that suppresses its targets, and the other is a modulator of micro-RNAs or proteins via their binding [5]. However, our study showed that miR-675 expression was significantly downregulated in AML patients [29] and was not correlated with H19 expression, which indicated that the function of H19 during leukemogenesis was not mediated by miR-675. Notably, our study further confirmed H19 expression was positively associated with ID2 expression in AML. Coincidentally, a recent study reported H19 regulated ID2 expression through competitive binding to hsa-miR-19a/b in AML [34]. All these suggested that the function of H19 may be mediated by ID2 during leukemogenesis.
DNA methylation, one of the most common epigenetic modifications, has been related to various regulatory processes, such as transcriptional regulation, LOI, chromatin structure, and genome integrity [35]. Strong evidence has proved that aberrant H19 DMR/ICR methylation by controlling CTCF6 binding sites led to LOI of IGF2/H19 and finally resulted in abnormal expression of IGF2/H19 in diverse human cancers [36–38]. Moreover, our previous study showed that H19 DMR/ICR demethylation resulted in upregulation of H19 expression in leukemic cell line K562 [39]. Herein, we also investigated the pattern of H19 DMR/ICR methylation in AML patients and determined the association with H19 expression. However, our data found that H19 DMR/ICR methylation level was similar to controls and was not associated with H19 expression. These results suggested that H19 overexpression in AML was not dependent on H19 DMR/ICR methylation. Therefore, other mechanisms were involved in the regulation of H19 expression in AML, and further studies were urged to identify the underlying mechanism.
Clinical significance of H19 expression was increasingly investigated in solid tumors. A recent meta-analysis showed that H19 expression might be a novel molecular marker for predicting prognosis and could also be a predictive factor of clinicopathological features in various cancers [40]. Herein, we found that H19 overexpression was also associated with age, WBC, karyotypic classifications, and several common gene mutations in AML patients. Moreover, H19 overexpression also acted as an independent prognostic biomarker for OS in non-APL-AML patients, and the similar results were also confirmed by TCGA and GEO data. In addition, we further identified that H19 expression was changed in response to chemotherapy in AML. Significantly, H19 expression in relapsed AML patients was markedly higher than AML patients who achieved CR and newly diagnosed AML patients, which implicated that H19 also played a role in AML recurrence. All these results indicated that H19 was a potential therapeutic target in AML and using H19-based targeted therapy could improve the clinical outcome for AML patients.
Conclusions
Our findings revealed that methylation-independent H19 is a prognostic and predictive biomarker in AML, and H19/ID2 played crucial roles in leukemogenesis with potential therapeutic target value.
Additional files
Table S1. Primers used for RQ-PCR, RQ-MSP, and BSP. (DOCX 16 kb)
Figure S1. ROC curve analysis using H19 expression for discriminating AML patients from controls. (DOCX 126 kb)
Acknowledgments
Funding
This work was supported by the National Natural Science foundation of China (81270630), Medical Innovation Team of Jiangsu Province (CXTDB2017002), 333 Project of Jiangsu Province (BRA2016131), Six Talent Peaks Project in Jiangsu Province (2015-WSN-115), Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX17_1821), China Postdoctoral Science Foundation funded project (2016M601748), Social Development Foundation of Zhenjiang (SH2016045, SH2016046), and Key Medical Talent Program of Zhenjiang City.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AML
Acute myeloid leukemia
- AUC
Area under the ROC
- BM
Bone marrow
- BMMNCs
BM mononuclear cells
- BSP
Bisulfite sequencing PCR
- CML
Chronic myeloid leukemia
- CMML
Chronic myelomonocytic leukemia
- CN-AML
Cytogenetically normal AML
- CR
Complete remission
- DMR/ICR
Differentially methylated region/imprinting control region
- GEO
Gene Expression Omnibus
- HRMA
High-resolution melting analysis
- LFS
Leukemia-free survival
- lncRNAs
Long non-coding RNAs
- LOI
Loss of imprinting
- OS
Overall survival
- ROC
Receiver operating characteristic
- RT-qMSP
Real-time quantitative methylation-specific PCR
- RT-qPCR
Real-time quantitative PCR
- TCGA
The Cancer Genome Atlas
Authors’ contributions
JQ and JDZ conceived and designed the experiments. TJZ and WZ performed the experiments. TJZ and JL analyzed the data. JCM, XMW, QY, XXL, and ZJX contributed clinical data collection. TJZ and JDZ wrote the paper. All authors read and approved the final manuscript. TJZ and WZ contributed equally.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee and Institutional Review Board of the Affiliated People’s Hospital of Jiangsu University.
Consent for publication
Written informed consents were obtained from all enrolled individuals prior to their participation.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13148-018-0486-z) contains supplementary material, which is available to authorized users.
Contributor Information
Ting-juan Zhang, Email: 1162004751@qq.com.
Jing-dong Zhou, Email: zhoujingdong1989@qq.com.
Wei Zhang, Email: zhangweizwmz@126.com.
Jiang Lin, Email: linjiangmail@sina.com.
Ji-chun Ma, Email: majichun606@sohu.com.
Xiang-mei Wen, Email: wenxiangmei@126.com.
Qian Yuan, Email: 1197596549@qq.com.
Xi-xi Li, Email: 781743121@qq.com.
Zi-jun Xu, Email: 972416919@qq.com.
Jun Qian, Email: qianjun0007@hotmail.com.
References
- 1.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Mason CE, Melnick A. Genetic and epigenetic heterogeneity in acute myeloid leukemia. Curr Opin Genet Dev. 2016;36:100–106. doi: 10.1016/j.gde.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara F, Palmieri S, Leoni F. Clinically useful prognostic factors in acute myeloid leukemia. Crit Rev Oncol Hematol. 2008;66:181–193. doi: 10.1016/j.critrevonc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Wei S, Wang K. Long noncoding RNAs: pivotal regulators in acute myeloid leukemia. Exp Hematol Oncol. 2016;5:30. doi: 10.1186/s40164-016-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raveh E, Matouk IJ, Gilon M, Hochberg A. The H19 long non-coding RNA in cancer initiation, progression and metastasis—a proposed unifying theory. Mol Cancer. 2015;14:184. doi: 10.1186/s12943-015-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordin M, Bergman D, Halje M, Engström W, Ward A. Epigenetic regulation of the Igf2/H19 gene cluster. Cell Prolif. 2014;47:189–199. doi: 10.1111/cpr.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo G, Kang Q, Chen Q, Chen Z, Wang J, Tan L, Chen JL. High expression of long non-coding RNA H19 is required for efficient tumorigenesis induced by Bcr-Abl oncogene. FEBS Lett. 2014;588:1780–1786. doi: 10.1016/j.febslet.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Wu HK, Weksberg R, Minden MD, Squire JA. Loss of imprinting of human insulin-like growth factor II gene, IGF2, in acute myeloid leukemia. Biochem Biophys Res Commun. 1997;231:466–472. doi: 10.1006/bbrc.1997.6127. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi S, Hofmann WK, Tsukasaki K, Takeuchi N, Ikezoe T, Matsushita M, Uehara Y, Phillip Koeffler H. Loss of H19 imprinting in adult T-cell leukaemia/lymphoma. Br J Haematol. 2007;137:380–381. doi: 10.1111/j.1365-2141.2007.06581.x. [DOI] [PubMed] [Google Scholar]
- 10.Randhawa GS, Cui H, Barletta JA, Strichman-Almashanu LZ, Talpaz M, Kantarjian H, Deisseroth AB, Champlin RC, Feinberg AP. Loss of imprinting in disease progression in chronic myelogenous leukemia. Blood. 1998;91:3144–3147. [PubMed] [Google Scholar]
- 11.Zhou JD, Zhang TJ, Li XX, Ma JC, Guo H, Wen XM, Zhang W, Yang L, Yan Y, Lin J, Qian J. Epigenetic dysregulation of ID4 predicts disease progression and treatment outcome in myeloid malignancies. J Cell Mol Med. 2017;21:1468–1481. doi: 10.1111/jcmm.13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ, Burnett AK; National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010;116:354–365. [DOI] [PubMed]
- 13.Lin J, Yao DM, Qian J, Chen Q, Qian W, Li Y, Yang J, Wang CZ, Chai HY, Qian Z, Xiao GF, Xu WR. Recurrent DNMT3A R882 mutations in Chinese patients with acute myeloid leukemia and myelodysplastic syndrome. PLoS One. 2011;6:e26906. doi: 10.1371/journal.pone.0026906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Qian J, Sun A, Lin J, Xiao G, Yin J, Chen S, Wu D. RAS mutation analysis in a large cohort of Chinese patients with acute myeloid leukemia. Clin Biochem. 2013;46:579–583. doi: 10.1016/j.clinbiochem.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Qian J, Yao DM, Lin J, Qian W, Wang CZ, Chai HY, Yang J, Li Y, Deng ZQ, Ma JC, Chen XX. U2AF1 mutations in Chinese patients with acute myeloid leukemia and myelodysplastic syndrome. PLoS One. 2012;7:e45760. doi: 10.1371/journal.pone.0045760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Yang J, Wen XM, Yang L, Deng ZQ, Qian Z, Ma JC, Guo H, Zhang YY, Qian W, Qian J. Detection of SRSF2-P95 mutation by high-resolution melting curve analysis and its effect on prognosis in myelodysplastic syndrome. PLoS One. 2014;9:e115693. doi: 10.1371/journal.pone.0115693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Yao DM, Ma JC, Yang L, Guo H, Wen XM, Xiao GF, Qian Z, Lin J, Qian J. The prognostic implication of SRSF2 mutations in Chinese patients with acute myeloid leukemia. Tumour Biol. 2016;37:10107–10114. doi: 10.1007/s13277-015-4716-0. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Yao DM, Qian J, Chen Q, Qian W, Li Y, Yang J, Wang CZ, Chai HY, Qian Z, Xiao GF, Xu WR. IDH1 and IDH2 mutation analysis in Chinese patients with acute myeloid leukemia and myelodysplastic syndrome. Ann Hematol. 2012;91:519–525. doi: 10.1007/s00277-011-1352-7. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Qian J, Yao DM, Li Y, Yang J, Chen Q, Chai HY, Xiao GF, Xu WR. Rapid and reliable detection of IDH1 R132 mutations in acute myeloid leukemia using high-resolution melting curve analysis. Clin Biochem. 2011;44:779–783. doi: 10.1016/j.clinbiochem.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Wen XM, Lin J, Yang J, Yao DM, Deng ZQ, Tang CY, Xiao GF, Yang L, Ma JC, Hu JB, Qian W, Qian J. Double CEBPA mutations are prognostically favorable in non-M3 acute myeloid leukemia patients with wild-type NPM1 and FLT3-ITD. Int J Clin Exp Pathol. 2014;7:6832–6840. [PMC free article] [PubMed] [Google Scholar]
- 21.Wen XM, Hu JB, Yang J, Qian W, Yao DM, Deng ZQ, Zhang YY, Zhu XW, Guo H, Lin J, Qian J. CEBPA methylation and mutation in myelodysplastic syndrome. Med Oncol. 2015;32:192. doi: 10.1007/s12032-015-0605-z. [DOI] [PubMed] [Google Scholar]
- 22.Zhou JD, Yao DM, Han L, Xiao GF, Guo H, Zhang TJ, Li XX, Yuan Q, Yang L, Lin J, Qian J. Low NKD1 expression predicts adverse prognosis in cytogenetically normal acute myeloid leukemia. Tumour Biol. 2017;39:1010428317699123. doi: 10.1177/1010428317699123. [DOI] [PubMed] [Google Scholar]
- 23.Dey BK, Pfeifer K, Dutta A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev. 2014;28:491–501. doi: 10.1101/gad.234419.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research Network Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kassambara A, Rème T, Jourdan M, Fest T, Hose D, Tarte K, Klein B. GenomicScape: an easy-to-use web tool for gene expression data analysis. Application to investigate the molecular events in the differentiation of B cells into plasma cells. PLoS Comput Biol. 2015;11:e1004077. doi: 10.1371/journal.pcbi.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metzeler KH, Hummel M, Bloomfield CD, Spiekermann K, Braess J, Sauerland MC, et al. An 86-probe-set gene-expression signature predicts survival in cytogenetically normal acute myeloid leukemia. Blood. 2008;112:4193–4201. doi: 10.1182/blood-2008-02-134411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang T, Wang Y, Zhou J, Li X, Qian W, Ma J, Yang L, Yao D, Chen Q, Lin J, Qian J. MiR-675 downregulation correlates with favorable/intermediate karyotypes in de novo acute myeloid leukemia. Int J Clin Exp Pathol. 2016;9:1684–1691. [Google Scholar]
- 30.Lin J, Ma JC, Yang J, Yin JY, Chen XX, Guo H, Wen XM, Zhang TJ, Qian W, Qian J, Deng ZQ. Arresting of miR-186 and releasing of H19 by DDX43 facilitate tumorigenesis and CML progression. Oncogene. 2018; 10.1038/s41388-018-0146-y. [DOI] [PMC free article] [PubMed]
- 31.Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Upregulated H19 contributes to bladder cancer cell proliferation by regulating ID2 expression. FEBS J. 2013;280:1709–1716. doi: 10.1111/febs.12185. [DOI] [PubMed] [Google Scholar]
- 32.Zhou JD, Ma JC, Zhang TJ, Li XX, Zhang W, Wu DH, Wen XM, Xu ZJ, Lin J, Qian J. High bone marrow ID2 expression predicts poor chemotherapy response and prognosis in acute myeloid leukemia. Oncotarget. 2017;8:91979–91989. doi: 10.18632/oncotarget.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tessema M, Länger F, Bock O, Seltsam A, Metzig K, Hasemeier B, Kreipe H, Lehmann U. Down-regulation of the IGF-2/H19 locus during normal and malignant hematopoiesis is independent of the imprinting pattern. Int J Oncol. 2005;26:499–507. [PubMed] [Google Scholar]
- 34.Zhao TF, Jia HZ, Zhang ZZ, Zhao XS, Zou YF, Zhang W, Wan J, Chen XF. LncRNA H19 regulates ID2 expression through competitive binding to hsa-miR-19a/b in acute myelocytic leukemia. Mol Med Rep. 2017;16:3687–3693. doi: 10.3892/mmr.2017.7029. [DOI] [PubMed] [Google Scholar]
- 35.Taby R, Issa JP. Cancer epigenetics. CA Cancer J Clin. 2010;60:376–392. doi: 10.3322/caac.20085. [DOI] [PubMed] [Google Scholar]
- 36.Ulaner GA, Vu TH, Li T, Hu JF, Yao XM, Yang Y, Gorlick R, Meyers P, Healey J, Ladanyi M, Hoffman AR. Loss of imprinting of IGF2 and H19 in osteosarcoma is accompanied by reciprocal methylation changes of a CTCF-binding site. Hum Mol Genet. 2003;12:535–549. doi: 10.1093/hmg/ddg034. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Qin Y, Li B, He WZ, Sun ZL. Hypomethylated and hypermethylated profiles of H19DMR are associated with the aberrant imprinting of IGF2 and H19 in human hepatocellular carcinoma. Genomics. 2008;91:443–450. doi: 10.1016/j.ygeno.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 38.De Castro Valente Esteves LI, De Karla Cervigne N, Do Carmo Javaroni A, Magrin J, Kowalski LP, Rainho CA, Rogatto SR. H19-DMR allele-specific methylation analysis reveals epigenetic heterogeneity of CTCF binding site 6 but not of site 5 in head-and-neck carcinomas: a pilot case-control analysis. Int J Mol Med. 2006;17:397–404. [PubMed] [Google Scholar]
- 39.Zhou JD, Lin J, Zhang TJ, Ma JC, Li XX, Wen XM, Guo H, Xu ZJ, Deng ZQ, Zhang W, Qian J. Hypomethylation-mediated H19 overexpression increases the risk of disease evolution through the association with BCR-ABL transcript in chronic myeloid leukemia. J Cell Physiol. 2018;233:2444–2450. doi: 10.1002/jcp.26119. [DOI] [PubMed] [Google Scholar]
- 40.Liu FT, Pan H, Xia GF, Qiu C, Zhu ZM. Prognostic and clinicopathological significance of long noncoding RNA H19 overexpression in human solid tumors: evidence from a meta-analysis. Oncotarget. 2016;7:83177–83186. doi: 10.18632/oncotarget.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used for RQ-PCR, RQ-MSP, and BSP. (DOCX 16 kb)
Figure S1. ROC curve analysis using H19 expression for discriminating AML patients from controls. (DOCX 126 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


