Abstract
OBJECTIVES:
Although titanium dioxide (TiO2) nanostructural materials have been widely used in Biology and Medicine, very little is known about immunomodulation mechanism of these materials. Objectives of this study are to investigate in vitro immunomodulatory effects of TiO2. Immunosuppressant may lower immune responses and are helpful for the treatment of graft versus host diseases and autoimmune disorders.
MATERIALS AND METHODS:
In this study, we used H2Ti3O7 titanium dioxide nanotubes (TNT) nanotubes along with commercial TiO2 nanoparticles (TNP) and TiO2 fine particles (TFP). We investigated the in vitro immunomodulatory effects of TNP, TNT, and TFP using mixed lymphocyte reaction (MLR). Suppression was studied by 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. Cytokine profile was measured by enzyme-linked immunosorbent assay (ELISA).
RESULTS AND CONCLUSIONS:
The results from this study illustrated that the TiO2 nanostructural materials strongly suppressed splenocytes proliferation in MLR. For TNP and TNT, at 50 μg/ml suppression of 20%–25% and 30%–35%, respectively, and for TFP at 100 μg/ml suppression was 25%–30% was observed. Suppression of splenocytes proliferation in the presence of TNP, TNT, and TFP demonstrated that these nanostructural materials probably block T-cell-mediated responses in vitro. Our ELISA results confirmed that significantly lower levels of Th1 type cytokines (interleukin-2, interferon-γ) in the 48 h MLR culture supernatants. Our data suggest that TiO2 nanostructural materials suppress splenocytes proliferation by suppressing Th1 cytokines.
Keywords: Cytokines, enzyme-linked immunosorbent assay, mixed lymphocyte reaction, suppression, titanium dioxide nanostructural materials
Introduction
Titanium (Ti) is the ninth most abundant element in the earth crust. Titanium dioxide (TiO2) is a white noncombustible, odorless powder, and available at low cost. TiO2 particles have high refractive index, biocompatibility, and corrosion resistance in nature. These particles are available in nature as three crystalline polymorphs, namely, rutile, anatase, and brookite. TiO2 nanoparticle is an exceptional multifunctional material with several practical applications ranging from pigments in paints, toothpaste, ultraviolet light absorbent in sunscreen lotion to coatings on nonfogging surfaces, solar cells, food additives, and recently in biomedicine, agriculture, etc.[1] Nanoscale particles are the frontiers of nanotechnology with their application as a biomaterial. The use of nanomedicines has increased enormously and nanomaterials have the potential to offer promising strategies to optimize and improve the treatment of numerous disorders.
Immunomodulation is defined as adjustment of immune response to a desired level and immunomodulator alters the functioning of immune response through immunopotentiation, immunosuppression, or immunological tolerance. Th1 response is characterized by the production of interleukin-2 (IL-2) and interferon-γ (IFN-γ). IL-2 is known as “T-cell growth factor” and widely considered to be a key cytokine for T-cell proliferation.[2] High concentration of IL-2 favors the generation and proliferation of effector T cells, whereas low levels of IL-2 maintain generation of T regulatory (Treg) cells, which function to restrain immune responses and prevent autoimmune diseases. Effector Th1 cells produce large quantity of IFN-γ (~350 ng/ml) during infections.[3] It further promotes the cytotoxic CD8 T-cell-mediated adaptive immunity. In addition to T-cells, dendritic cells, NK cells, and B-cells produce IFN-γ which is involved in inflammatory response and acts as an important link between innate and adaptive immunity.[4] IFN-γ enhances the differentiation of CD4 T-cells to Th1 cells and inhibits the production of Th2 cells.[5] IL-4 induces the production of IgE from mast cells during allergy and also induces the production and maturation of B-cells. γδ T cells act as a link between innate and adaptive immunity because they lack precise major histocompatibility complex (MHC) restriction and seize the ability to recognize ligands that are generated during affliction.[6] In a biological medium, nanoparticles can pass through cell membranes and interact with biomolecules and even biological metabolites due to their nanosize and large surface to mass ratio. Recently, nanoparticles were used as immunomodulators for the beneficial purposes of immunomodulation.[7]
TiO2 nanoparticles (TNP) have gained preference for biological applications because of their unique property of biocompatibility. However, the properties of TiO2 nanomaterials to the immune system are not well known. Based on our published [8] and unpublished data, we hypothesized that “TiO2 has immunomodulatory properties and may lower the immune responses and would be useful for the treatment of graft versus host diseases and autoimmune disorders.” In the present work, we investigated the effect of TiO2 nanostructural materials such as synthesized H2Ti3O7 titanium dioxide nanotubes (TNT), commercial TNP, and TiO2 fine particles (TFP) on proliferation of mouse splenocytes. Spleen, is the body's largest blood filter, in addition, most important immune reactions like antifungal/antibacterial occur in it. In this study, we described the influence of TiO2 nanomaterials on T-cell proliferation using mixed lymphocyte reaction (MLR) cultures. Furthermore, to understand the mechanism of immune response in terms of cytokine production, we measured IL-2 and IFN-γ secreted by Th1-type cells and IL-4 secreted by Th2 type cells.
Materials and Methods
Material
Roswell park memorial institute (RPMI)-1640 medium, antibiotic solution (ABS), and fetal bovine serum (FBS) were purchased from Invitrogen, USA. Lipopolysaccharide (LPS), trypan blue, and mitomycin C were purchased from Sigma; 0.05% Trypsin-EDTA and EZcount™ 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl tetrazolium bromide (MTT) Cell Assay Kit from Himedia Laboratories, India; mouse enzyme-linked immunosorbent assay (ELISA) kits of IL-2, IL-4, and IFNγ were purchased from eBioscience, USA. Photocatalyst grade TiO2 (TiO2 P-25) composed of anatase 80% and rutile 20% was procured from Degussa Corporation, Germany. TiO2 (anatase 99%, TiO2) was purchased from Merck, India.
Synthesis and usage of H2Ti3O7 nanotubes
The protonic trititanate (H2Ti3O7) nanotubes were synthesized by alkaline hydrothermal method as reported earlier.[9] Briefly, TFP denoted as TFP (TiO2 LAB, Merck, India) dispersed into 10 M NaOH aqueous solution was transferred in Teflon-lined stainless steel autoclave and heated at 130°C for 20 h. The white precipitate was washed twice with distilled H2O, diluted HCl, and C2H5OH, finally dried at 80°C for 12 h, the bright white powder denoted as TNT. We used TiO2 nanomaterials at various concentrations like 6.25 μg/ml to 150 μg/ml. All our experiments were conducted at concentration close to the 30% inhibition. We did endotoxin content analyses of the TiO2 NPs using Limulus amebocyte assay as described in Sree Latha et al.[8] The amount of endotoxin detected in 1 μg of the TNP, TFP, and TNT injected into mice was 0.5, 0.4, and 0.35 pg, respectively, which did not stimulate the production of any cytokines in the mouse ligated ileal loops.
Characterization techniques
Powder wide-angle X-ray diffraction patterns of all the samples were recorded using a D8 ADVANCE X-ray diffractometer (Bruker) equipped with Ni-filtered Cu Kα(k = 1.5418 Š) radiation (30 kV, 50 mA). For transmission electron microscopy (TEM) measurements, all the samples were sonicated under acetone and the resulting dispersion of the powder samples was transferred into a holey carbon film fixed on a 3-mm copper grid (200 meshes). The specific surface area, specific pore volume, and average pore diameter of all the samples were measured by N2 adsorption-desorption isotherms at 77 K using a surface area analyzer (Micromeritics, ASAP 2020). Before the measurements, all the samples were degassed at 350 °C under vacuum (10−3 mbar) for 6 h.
Animals
Female C57BL/6 and BALB/c mice at 5–6 weeks of age were housed in free of murine-specific pathogens under optimal conditions of hygiene, temperature, humidity, light (cycles of 12 h dark/light), and fed with standard rodent chow and water ad libitum. Experimental animal protocols were approved by the Institutional Animal Ethics Committee, CPCSEA registration number is 1841/GO/Re/S/15/CPCSEA and all procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory.”
Preparation of splenocytes
Splenocytes were prepared aseptically from inbred 6–8-week-old BALB/c and C57BL/6 mice, cell strainers were used to obtain a homogeneous cell suspension. The erythrocytes were lysed with red blood cells lysis buffer. After centrifugation (1500 rpm for 5 min), the pelleted cells were washed three times in phosphate-buffered saline, and resuspended in complete medium [RPMI-1640 supplemented with 10% heat-inactivated FBS, 100× ABS (1 ml/100 ml)]. Cell viability was measured by trypan blue dye exclusion technique. We observed 97% of cells are viable and cultured these cells in complete media for further experiments.
Mixed lymphocyte reaction in mice splenocytes
MLR is an in vitro method for measuring the proliferative responses of allogenic responder lymphocyte. In this study, we used MLR cultures to study the effect of TiO2 nanomaterials on T-cell proliferation. Splenocytes were isolated from 6 to 8-week-old C57BL/6 mice, having MHC H-2b haplotype and incubated (1 × 106 cells/ml) with different concentrations of TNP, TNT, and TFP for 24 h at 37°C in a humidified 5% CO2 incubator. Cells were inactivated with mitomycin C for 30 min, washed, and used as stimulators. Splenocytes of BALB/c (H-2d) mice were used as responders. Stimulators (0.01 × 106 cells/ml) and responders (0.1 × 106 cells/ml) were cocultured in a flat bottom 96 well plate for 3 days. Proliferation response was measured by MTT assay using EZcount™ MTT cell assay kit.
Measuring cell proliferation by 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl tetrazolium bromide assay
Splenocytes proliferation was measured using MTT assay. It is a quantitative colorimetric assay that measures the reduction of yellow MTT to purple formazan crystals by mitochondrial succinate dehydrogenase. Briefly, MTT (5 mg/ml) was pulsed 3 h before the end of the experiment, and then 100 μl of dimethyl sulfoxide was added to each well to dissolve the crystals and the absorbance was measured using a microplate reader at 570 nm with a 650 nm reference.
Cytokine production from mouse splenocytes was measured by enzyme-linked immunosorbent assay
ELISA is the most popular way to detect cytokines or chemokines quantitatively based on the antigen and antibody specificity. Splenocytes were prepared from 6- to 8-week-old C57BL/6 mice and distributed 1 × 106 cells into each well having 1 ml of RPMI-1640 media supplemented with 10% heat-inactivated FBS, 100 × ABS (1 ml/100 ml). Cells were incubated with TNP (50 μg/ml), TNT (50 μg/ml), and TFP (100 μg/ml) for 24 h in 24 well culture plates. The culture supernatants were collected and stored at −80°C until further use. Culture supernatants were also collected from the MLR experiment at 48 h to measure the IL-2, IL-4, and IFNγ production using ELISA kit (eBiosciences, CA, USA) according to the manufacturer instructions.
Results
Characterization of titanium dioxide nanomaterials
The X-ray diffraction pattern of TNT, TNP, and TFP nanoparticles showed in the Figure 1. The TNT shows characteristic diffraction peak at 2θ = 10.2° (001) which indicates the presence of layered crystal structure. All other peaks centered at 2θ = 24.1° (202), 28.3° (112), and 48.2° (303) can be ascribed to the monoclinic H2Ti3O7 structure (JCPDS No. 47–0561). In the XRD patterns of TFP, the major diffraction peak at 2θ = 25.4°(101) is assigned to anatase phase of TiO2 particles. The TNP displays two characteristic peaks at 2θ = 25.4°(101) and 27.5° (110) demonstrating the presence of biphasic anatase-rutile TiO2 with tetragonal crystal structures (JCPDS No. 21–1272 and No. 21–1276). The surface area of TiO2 TFP powder is about 5.1 m2/g, while the BET surface area of prepared TNT is about 282 m2/ g, which is ~56 times higher. The BET surface area of TNP is 51 m2/g. Our data are in good agreement with observed structural transformations and previous data.[9]
Figure 1.
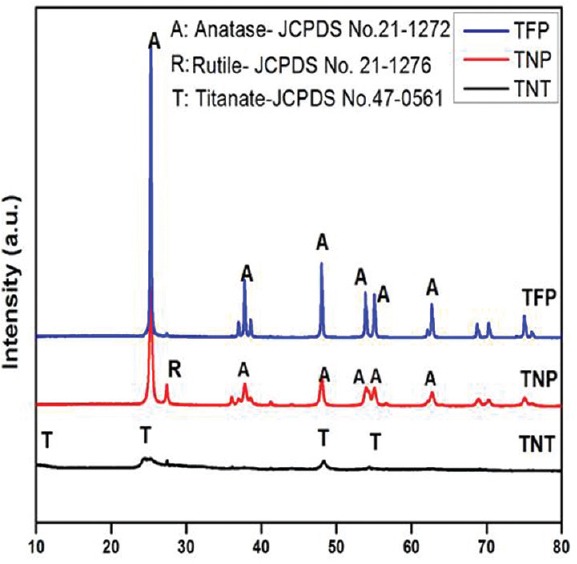
XRD pattern of titanium dioxide nanoparticles, titanium dioxide fine particles and titanium dioxide nanotubes nanoparticles
TEM was used to characterize the titania-based materials such as TNP, TNT, and TFP images are displayed in Figure 2. The TNP image showed spherical shape with agglomeration, particle size ranges from 20 to 60 nm, and the TFP image shows flakes-like fine particles with high agglomeration. The particle size ranged from 60 to 160 nm. The TEM images of TNT showed nanotubular morphology, cylindrical in shape with hollow inside, and open at both ends.
Figure 2.
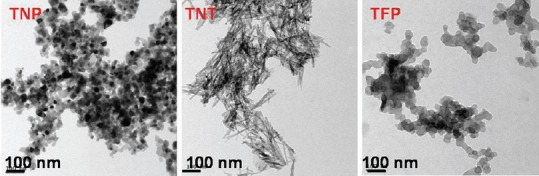
High-resolution transmission electron microscopy images of titanium dioxide nanoparticles, titanium dioxide nanotubes, and titanium dioxide fine particles
Suppressed splenocytes proliferation in mixed lymphocyte reaction cultures
MLR is a model of T-cell response to alloantigenic peptide complex with MHC proteins on antigen presenting complex. Splenocytes (H-2b) were cultured in the presence of 25, 50, and100 μg/ml concentration of TNP; 12.5, 25, 50 μg/ml of TNT; and 50, 100, 150 μg/ml of TFP for 24 h. Mitomycin-fixed splenocytes were used as stimulators. BALB/c mice splenocytes (H-2d) were used as responders. In MLR, stimulators were co-cultured with responders at a ratio of 1:10 in a flat bottom 96 well plate for 72 h. Proliferation of responder splenocytes in the presence of TNP, TNT, and TFP-treated stimulators was measured by MTT assay. Figure 3 showed that TNP, TNT, and TFP inhibited proliferation of responder splenocytes. For TNP and TNT at 50 μg/ml, we observed suppression of 20%–25% and 30%–35%, respectively, and for TFP at 100 μg/ml, suppression was 25%–30%. MLR results suggest that TNP, TNT, and TFP probably block T-cell-mediated responses in vitro.
Figure 3.

Cytotoxicity of titanium dioxide nanoparticles/titanium dioxide nanotubes/titanium dioxide fine particles in mixed lymphocyte reaction of mice splenocytes, measured using 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl tetrazolium bromide assay on a 72h culture. titanium dioxide nanoparticles/titanium dioxide nanotubes/titanium dioxide fine particles inhibited MLR in a dose-dependent manner for a 72 h co-culture
Titanium dioxide nanoparticles, titanium dioxide nanotubes, and titanium dioxide fine particles inhibited production of inflammatory cytokines in mixed lymphocyte reaction cultures
The production of Th1 type cytokines (IL-2, IFNγ) and Th2 type cytokine (IL-4) were examined in the 24h culture supernatant of TNP (50 μg/ml), TNT (50 μg/ml), and TFP (100 μg/ml)-treated splenocytes of C57BL/6 mice (H-2b) shown in the Figure 4. The production of pro-inflammatory cytokines IL-2 and IL-4 was slightly elevated in TNP/TNT/TFP-treated splenocytes compared with the untreated cells (cells alone). We observed decreased level of IFN-γ in culture supernatants of splenocytes treated with TNP/TNT/TFP for 24 h when compared to untreated splenocytes.
Figure 4.

Influence of titanium dioxide nanoparticles, titanium dioxide nanotubes, and titanium dioxide fine particles on cytokine production in 24h supernatants of C57BL/6 mice splenocytes culture. Cytokines interleukin-2 (a), interleukin-4 (b) are slightly elevated and interferon γ (c) significantly suppressed by the titanium dioxide nanomaterials. Cells alone (untreated cells) taken as control. Data are presented as mean ± standard deviation of three independent experiments. Statistical significance was defined as *P < 0.05, **P < 0.01
Consistent with cytotoxic results, we observed significantly lower levels of Th1 type cytokines (IL-2, IFNγ) in the 48 h culture supernatants of MLR i.e.; TNP (50 μg/ml)/TNT (50 μg/ml)/TFP (100 μg/ml) treated and fixed stimulators co-cultured with responders at a ratio of 1:10. However, slightly elevated levels of Th2 type cytokine IL-4 were observed in TiO2 nanomaterials-treated MLR culture supernatants compared with the culture of untreated stimulators with responders (S X R). We used LPS (10 μg/ml)-treated stimulators co-cultured with responders as a positive control ([S + LPS] X R) [Figure 5].
Figure 5.
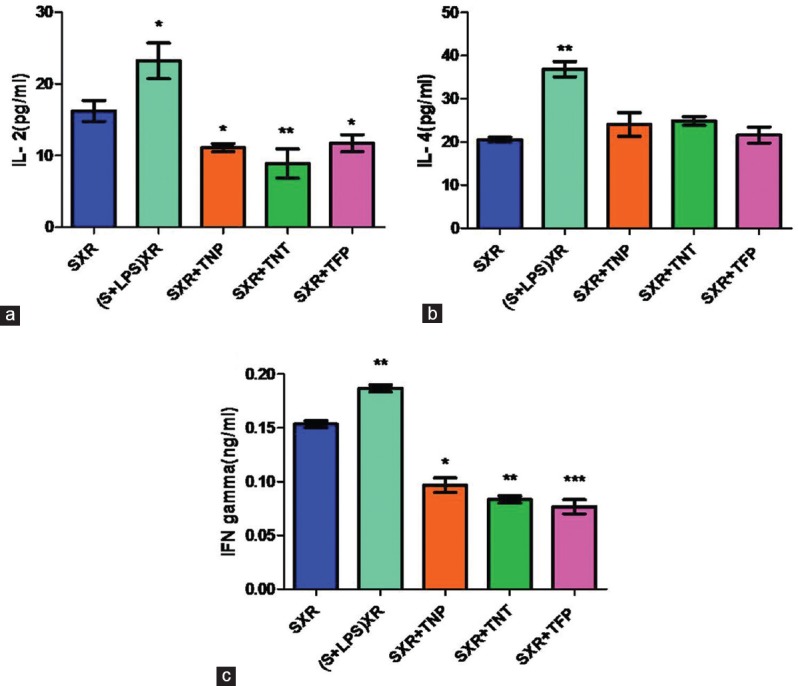
Effect of titanium dioxide nanoparticles, titanium dioxide nanotubes, and titanium dioxide fine particles on cytokine production in 48h MLR culture supernatants. C57BL/6 mice splenocytes treated with titanium dioxide nanoparticles/titanium dioxide nanotubes/titanium dioxide fine particles for 24h, fixed with mitomycin C and co-culture with BALB/c mice splenocytes for 72h. Supernatant was collected from 48h MLR and cytokine levels were measured by ELISA. Th1 cytokines interleukin-2 (a) and interferon γ (c) levels suppressed, whereas interleukin-4 (b) levels slightly elevated in the presence of titanium dioxide nanomaterials-treated stimulators. Data are presented as mean ± standard deviation of three independent experiments. Statistical significance was defined as *P < 0.05, **P < 0.01
Discussion
Nanotechnology has gained increasing attention for researchers due to its applications in various fields including food industry and nanomedicine. TiO2 nanotube (TNT) surface structuring significantly improved bioactivity at the implant interface and for enhanced cell adhesion. TNT arrays may be used as drug delivery capsules and a drug-eluting coating on biomaterial implant materials.[10] However, a few in vivo studies demonstrated that intragastric and intravenous injection of TNP at high doses in mice causes acute toxicity effects in the brain, lung, liver, and kidney.[11]
Previous studies reported that cytotoxicity of TiO2 nanomaterials is due to the production of reactive oxygen species (ROS) from dendritic cells, ROS intern activates DC maturation result in the generation of pro-inflammatory cytokines.[12] Yin et al. observed that decreased proliferation and differentiation of keratinocytes treated with TiO2 NPs, due to the internalization of TiO2 NPs in cells disrupts the calcium homeostasis and generation of ROS production and further stated that this decreased proliferation is dependent on the incubation time and dosage of TiO2 NPs.[13] Recently, we showed that TiO2-based nanomaterials ameliorate experimental autoimmune encephalomyelitis and collagen-induced arthritis and demonstrated that lowering HMGB1 levels is possible mechanism for this amelioration.[14]
Lymphocyte proliferation is a crucial event in the activation cascade of both cellular and humoral immune responses. In this study, we used MLR cultures to study the effect of TiO2 nanomaterials on proliferation. Furthermore, the suppression of MLR by immunosuppressant also helps to improve the success of transplantation.[15]
Our data demonstrated that, stimulators treated with TNP, TNT, and TFP nanomaterials suppressed the responder T-cell proliferation. Cytokines are important modulators and effectors in the immune system. Our ELISA results showed significantly lower levels of Th1 type cytokines (IL-2, IFNγ) in the 48 h MLR culture supernatants. IL-2 is involved in T-cell proliferation in MLR cultures. Decreased levels of IL2 in the presence of TiO2 nanomaterials suggest that these nanomaterials suppress T-cell proliferation by reducing IL2 levels in MLR cultures. Th2 response is characterized by the production of IL-4 cytokine and is associated with diminished ability to induce graft versus host response. Our results showed that slightly enhanced IL-4 level in response to TiO2 nanomaterials. These results suggest that TiO2 nanomaterials influence a differential balance between Th1 and Th2 responses, in which the Th2 immune response is slightly more compared to Th1-mediated immune response.
Conclusion
Our findings suggest that TiO2 nanomaterials suppressed splenocytes proliferation by reducing IL-2 and IFNγ cytokine levels in MLR cultures. Based on our published data [14] and current suppression and cytokine data, we hypothesized that TiO2 nanomaterials may lower the immune responses and could be useful for the treatment of graft versus host diseases and autoimmune disorders.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This work was supported by grants from Science and Engineering ResearchBoard (SERB), India (Grant #: SR/FT/LS-149/2010) and Council of Scientific and Industrial Research (CSIR), India, (Grant# 37(1517)/11/EMR-II) to Dakshayani Lomada and University Grants Commission, New Delhi (UGC F. No: 42-176/2013(SR)) to Madhava C Reddy.
References
- 1.Zhou W, Liu H, Boughton RI, Du G, Lin J, Wang J, et al. One-dimensional single-crystalline Ti–O based nanostructures: Properties, synthesis, modifications and applications. J Mater Chem. 2010;20:5993–6008. [Google Scholar]
- 2.Gillis S, Smith KA. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977;268:154–6. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- 3.Tau GZ, von der Weid T, Lu B, Cowan S, Kvatyuk M, Pernis A, et al. Interferon gamma signaling alters the function of T helper type 1 cells. J Exp Med. 2000;192:977–86. doi: 10.1084/jem.192.7.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T (H) 1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Botran R, Sanders VM, Mosmann TR, Vitetta ES. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med. 1988;168:543–58. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latha TS, Reddy MC, Durbaka PV, Rachamallu A, Pallu R, Lomada D, et al. Γδ T cell-mediated immune responses in disease and therapy. Front Immunol. 2014;5:571. doi: 10.3389/fimmu.2014.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao Q, Li L, Mu Q, Zhang Q. Immunomodulation of nanoparticles in nanomedicine applications. Biomed Res Int 2014. 2014:426028. doi: 10.1155/2014/426028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sree Latha T, Reddy MC, Muthukonda SV, Srikanth VV, Lomada D. In vitro and in vivo evaluation of anti-cancer activity: Shape-dependent properties of tiO2 nanostructures. Mater Sci Eng C Mater Biol Appl. 2017;78:969–77. doi: 10.1016/j.msec.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Praveen Kumar D, Shankar MV, Kumari MM, Sadanandam G, Srinivas B, Durgakumari V, et al. Nano-size effects on cuO/TiO2 catalysts for highly efficient H2 production under solar light irradiation. Chem Commun (Camb) 2013;49:9443–5. doi: 10.1039/c3cc44742a. [DOI] [PubMed] [Google Scholar]
- 10.Aw MS, Gulati K, Losic D. Controlling drug release from titania nanotube arrays using polymer nanocarriers and biopolymer coating. J Biomater Nanobiotechnol. 2011;05:8. [Google Scholar]
- 11.Xu J, Shi H, Ruth M, Yu H, Lazar L, Zou B, et al. Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice. PLoS One. 2013;8:e70618. doi: 10.1371/journal.pone.0070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schanen BC, Karakoti AS, Seal S, Drake DR, 3rd, Warren WL, Self WT, et al. Exposure to titanium dioxide nanomaterials provokes inflammation of an in vitro human immune construct. ACS Nano. 2009;3:2523–32. doi: 10.1021/nn900403h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin JJ, Liu J, Ehrenshaft M, Roberts JE, Fu PP, Mason RP, et al. Phototoxicity of nano titanium dioxides in haCaT keratinocytes – Generation of reactive oxygen species and cell damage. Toxicol Appl Pharmacol. 2012;263:81–8. doi: 10.1016/j.taap.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latha TS, Lomada D, Dharani PK, Muthukonda SV, Reddy MC. Ti–O based nanomaterials ameliorate experimental autoimmune encephalomyelitis and collagen-induced arthritis. RSC Adv. 2016;6:8870–80. [Google Scholar]
- 15.Goes N, Chandraker A. Human leukocyte antigen matching in renal transplantation: An update. Curr Opin Nephrol Hypertens. 2000;9:683–7. doi: 10.1097/00041552-200011000-00015. [DOI] [PubMed] [Google Scholar]


